Abstract
Our group and others have determined that immune effector cells from patients with advanced cancers exhibit reduced activation of IFN signaling pathways. We hypothesized that increases in immune regulatory cells termed myeloid derived suppressor cells (MDSC) could interfere with the host immune response to tumors by inhibiting immune cell responsiveness to interferons. The C26 murine adenocarcinoma model was employed to study immune function in advanced malignancy. C26 bearing mice had significantly elevated levels of GR1+CD11b+ MDSC as compared to control mice, and splenocytes from tumor bearing mice exhibited reduced phosphorylation of STAT1 (P-STAT1) on Tyr 701 in response to IFN alpha or IFN gamma. This inhibition was seen in splenic CD4+ and CD8+ T cells as well as NK cells. In vitro co culture experiments revealed that MDSC inhibited the IFN responsiveness of splenocytes from normal mice. Treatment of C26 bearing mice with gemcitabine or an anti-GR1 antibody led to depletion of MDSC and restored splenocyte IFN responsiveness. Spleens from C26 bearing animals displayed elevated levels of iNOS protein and nitric oxide (NO). In vitro treatment of splenocytes with a nitric oxide donor led to a decreased STAT1 IFN response. The elevation in NO in C26 bearing mice was associated with increased levels of nitration on STAT1. Finally, splenocytes from iNOS knockout mice bearing C26 tumors exhibited a significantly elevated IFN-response as compared to control C26 tumor bearing mice. These data suggest that NO produced by MDSC can lead to reduced interferon responsiveness in immune cells.
Keywords: myeloid derived suppressor cell, interferon
Introduction
Previous studies demonstrated that the endogenous production of interferon is essential for immunosurveillance against developing tumors (1, 2). It has been determined that mice lacking either the IFNγ receptor (IFNγR1) or the major IFN signal transducer STAT1, developed tumors at a faster rate than wild type mice following exposure to the carcinogen 3-methylcholanthrene (MCA). In addition, mice lacking IFNγR1 or STAT1 as well as the tumor suppressor p53 developed a wider range of spontaneous tumors as compared to wild type mice lacking p53 only (1). Studies by Dunn et al. have also demonstrated the distinct importance of IFN-α/β sensitivity in cells of the hematopoietic lineage for immunoediting and anti-tumor immunity in vivo (3). Interferons are now accepted as critical mediators of immunosurveillance and are important in both innate and adaptive anti-tumor immune responses. A functional immune system in patients with cancer is also critical for the success of immune-based therapies, such as exogenously administered cytokines, vaccines and targeted antibodies via the induction of type I interferons (e.g. IFN-α, IFN-β) and type II IFNs (IFN-γ). Our group and others have determined that immune effector cells from patients with advanced cancers exhibit reduced activation of IFN-induced signaling pathways (4, 5). One potential mechanism for the immune inhibition seen in tumor bearing hosts is the presence of increased numbers of immune suppressor cells.
Myeloid-derived suppressor cells (MDSC) are a heterogenous population of early myeloid cells that accumulate in the blood and tumors of patients with cancer. Their numbers correlate with tumor burden (6). These cells arise from myeloid precursors in response to tumor-derived growth factors and pro-inflammatory cytokines (6, 7). MDSC are described phenotypically in murine models as GR1+CD11b+, with subsets expressing IL-4Rα (7, 8). MDSC have been shown to reside in the peripheral blood, lymphoid tissue, and tumor tissue of mice in a number of experimental models (6, 9–13). Prior studies have demonstrated that MDSC can inhibit the effector function of NK and T cells in tumor-bearing animals through multiple mechanisms, including the release of immune-suppressive cytokines, the generation of nitric oxide and reactive oxygen species, and the depletion of arginine or cystine from the tumor microenvironment (6, 14, 15). Studies in murine models indicate that disruption of MDSC function can reverse immune tolerance to tumor antigens, stimulate anti-tumor immune responses and markedly inhibit tumor growth (6, 7).
We hypothesized that elevated numbers of MDSC present in the setting of advanced malignancy would inhibit the response of immune cells to type I and II interferons. The results of our experiments demonstrated that mice bearing C26 adenocarcinoma tumors exhibit elevated numbers of MDSC which led to increased nitration on STAT1 and impaired responsiveness of immune effector cells to interferons.
Methods
Cytokines and Reagents
Recombinant murine IL-6 and IFN-γ were purchased from R & D Systems, Inc. (Minneapolis, MN). Recombinant Universal Type I Interferon (IFN-A/D) was purchased from PBL Biomedical Sciences (Piscataway, NJ). S-nitroso-N-acetylpenicillamine (SNAP) was purchased from Molecular Probes (Eugene, OR).
Murine Tumor Models
Male 4–6 week old BALB/c X DBA/2F1 (CD2F1) mice were used (Harlan, Indianapolis, IN). Colon 26 (C26) tumor cells (106 cells) were injected subcutaneously into the flank of CD2F1 mice (16). Male Balb/c By and iNOS knockout mice were injected with C26 tumor cells (106 cells) for nitric oxide studies (4–6 weeks, Jackson Labs). Female BALB/c mice (4–6 weeks, Harlan) were injected in the mammary fat pad with 5×104 4T1 tumor cells.
Intracellular Flow Cytometry
Levels of P-STAT1 and STAT1 were measured via flow cytometry in cells derived from murine splenocytes or lymph nodes following in vitro stimulation with PBS, IFN-A/D or IFN-γ as described previously (4). Data were expressed as specific fluorescence (Fsp = Ft – Fb), where Ft represents the median value of total staining and Fb represents the median value of background staining with an isotype control antibody (4, 17, 18). CD4, CD8, and CD49b antibodies were used for surface staining of immune subsets (BD Biosciences). For nitration flow cytometry, splenocytes were co-labeled with anti-STAT1-PE and anti-nitrotyrosine-alexafluor-488 antibody (BD Biosciences and Millipore).
Real Time PCR
Following TRIzol extraction (Invitrogen) and RNeasy purification (Qiagen), total RNA was quantitated and reverse transcribed as previously described (19). The resulting cDNA was used to measure gene expression by Real-Time PCR using pre-designed primer/probe sets and 2× TaqMan Universal PCR Master Mix with18s rRNA as an internal control (Applied Biosystems).
Immunoblot Analysis
Lysates were prepared from splenocytes following in vitro stimulation with PBS or IFN-A/D and assayed for the expression of proteins by immunoblot (19). For the detection of nitrated STAT1, samples were immunoprecipitated with STAT1 (BD Biosciences) and then probed with an anti-nitrotyrosine antibody (Invitrogen).
Flow Cytometric Analysis of Myeloid Derived Suppressor Cells
Analysis of the MDSC in splenocytes was conducted as previously described (8). Briefly, 106 splenocytes were labeled with fluorochrome-labeled Ab targeting murine CD11b, GR1, IL-4Rα or appropriate isotype control Ab (BD Biosciences) for 1hr at 4°C, washed, resuspended in 1% formalin, and analyzed via flow cytometry.
Immunofluorescent staining of MDSC
Tumors were fixed in 4% paraformaldehyde overnight, dehydrated in sucrose, and embedded in OCT and frozen. Frozen sections (5 microns) were probed with anti-GR1 alexafluor 488 (Invitrogen) and anti-CD11b alexafluor 647 (BD Bioscience) antibodies, or appropriate isotype control. Sections were also labeled with DAPI nuclear stain. Slides were analyzed on an Olympus FV1000 Spectral Confocal microscope.
Isolation of MDSC from C26-bearing mice
Spleens were harvested aseptically from tumor-bearing mice (18), filtered through 70 µM cell strainers, washed with PBS, and resuspended in media. GR1+/CD11b+ MDSC were isolated using anti-GR1 biotinylated beads (Miltenyi Biotec, Auburn, CA) with purity > 95% by flow cytometry. Isolated MDSC were co-cultured with splenocytes at a 1:3 ratio. This ratio recapitulates the number of MDSC present within the spleen of a C26-bearing mouse. After 24 hours, splenocytes were harvested from culture and stimulated with IFN-A/D (104 U/mL) for 15 minutes. IFN-induced levels of P-STAT1 were measured by flow cytometry as described.
MDSC depletion with GR1 antibody
On day 20 post-tumor inoculation with C26, mice were treated with either anti-GR1 (BD Biosciences) or isotype control antibody (Sigma) at a concentration of 0.25mg/mouse in 200 µl PBS. MDSC depletion was verified in the spleen and tumor by flow cytometry and immunofluorescent staining of frozen tumor sections
Gemcitabine treatment of C26-bearing mice
Two days after C26 tumor inoculation, mice received twice weekly i.p. injections of gemcitabine (Gemzar®, Eli Lilly) at a dose of 75µg/g of body weight. This agent has been previously validated as an effective means of reducing MDSC (20–22). For 24 hour depletion studies, mice were injected with 75µg/g gemcitabine 24 hours prior to animal harvest. MDSC depletion was also verified in the tumor by immunofluorescent staining of frozen tumor sections.
Measurement of nitric oxide by DAF-FM
Spleens were washed with an ice-cold PBS buffer and embedded in OCT. Frozen segments (6 µm) were incubated with 10 µM DAF-FM diacetate (Molecular probes, D-23842) for 30 min at 37°C. The images were obtained using a fluorescence microscope (Nikon), and intensity was quantitatively determined using MetaMorph image analysis software (Molecular devices, CA).
Immunohistochemistry of tumors
Tumors were fixed in formalin, washed with PBS, paraffin embedded, sectioned into 4-µm slices, attached to lysine-coated slides, and stained with H&E as previously described (23). Briefly, replicate sections were de-paraffinized in xylene, rehydrated, and endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 5 min, followed by rinses in diH2O. Ag retrieval was achieved in Dako's target retrieval solution (Dako S1699) by heating slides at 94°C for 30 min and cooling at room temperature for 15 min. Slides were then incubated for 60 min with Abs specific for CD34 (Abcam; clone MEC14.7), and Ki67 (Thermo; clone SP6). Detection was achieved with the Vectastain Elite ABC system and Novared Chromogen (Vector, Burlingame, CA). All samples were examined in a blinded fashion by an experienced pathologist (K.M.L.) using an Olympus BX45 light microscope with an attached DP25 digital camera (B & B Microscopes limited, Center Valley, PA). Automatic quantification was performed on whole slide images scanned at 40× magnification (ScanScope XT, Aperio Technologies, Vista, CA) using the Positive Pixel Count Algorithm in the ImageScope software (Aperio Technologies).
Statistical Analysis
Two-sample t-tests were used to compare outcomes (e.g. IL-6, Ifit2, P-STAT1) between groups. If necessary, outcomes were log-transformed to meet the assumptions of normality and constant variance for the test. Pearson correlation coefficients were used to quantify the relationship between outcomes. Tumor growth was assumed to be log-linear and a mixed effects model with a random intercept and slope for each animal was used to estimate the rate of increase. P-values less than 0.05 were considered statistically significant.
Results
Decreased interferon response of immune cells from tumor-bearing mice
We hypothesized that immune effector cells in tumor-bearing mice would have an altered response to cytokines that promote anti-tumor immunity as is seen in patients with advanced malignancy. Of particular interest were the type I and type II IFNs, as these cytokines mediate tumor immunosurveillance, and reductions in immune cell IFN responsiveness have been shown to exist in cancer patients (1–3, 24). The C26 model was chosen for this study due to its rapid tumor growth and ability to mimic advanced clinical disease. The ability of IFN-α or IFN-γ to activate the STAT1 transcription factor (by phosphorylation at Tyr701) was measured by intracellular flow cytometry in splenocytes from normal and C26-bearing mice (Figure 1 and representative histogram Supplemental Figure 1A). Induction of P-STAT1 following a 15 minute stimulation of splenocytes with IFN-A/D (104 U/mL) was significantly reduced at day 22 in C26-bearing mice (mean MFI P-STAT1 = 18.8; range = 4.0 – 33.4) as compared to control mice (mean MFI P-STAT1 = 37.9; range = 27.0 – 50.0; Figure 1A; p<0.0001). Induction of P-STAT1 was also significantly decreased in splenocytes from C26-bearing mice following stimulation with 10 ng/ml IFN-γ (Figure 1B; p = 0.003). MDSC (approximately 15–20% of total splenocytes) were excluded from this analysis by gating to ensure that the lymphocyte populations of normal and tumor-bearing animals were of comparable composition. These results were validated by immunoblot analysis (Supplemental Figure 1B). The decrease in IFN responsiveness was also observed in cells derived from lymph nodes (Supplemental Figure 1C). To evaluate the effect of the C26 tumor on IFN on responsiveness in vivo, normal or C26 tumor-bearing animals were injected with IFN-A/D (2×104 U) and splenocytes were harvested 2 hours later for analysis. The level of P-STAT1 was evaluated by intracellular flow cytometry as described previously (Figure 1C). As expected the P-STAT1 response was reduced in C26-tumor bearing mice both at baseline and in response to exogenous administration of IFN. An analysis of IFN responsiveness in lymphocyte subsets by dual parameter intracellular flow cytometry revealed that the induction of P-STAT1 was inhibited in CD4, CD8, and CD49b (NK cells) (Figure 1D, CD4 p = .009; CD8 p = .002; NK, p = .02). We next analyzed the induction of the IFN-α stimulated gene (ISG), Ifit2 and the IFN-γ stimulated gene, interferon-regulatory factor (Irf1) by Real Time PCR following a 4 hour in vitro treatment of splenocytes from C26-bearing or normal mice with IFN-A/D (102 U/mL or 104 U/mL). The expression of Ifit2 was significantly reduced in splenocytes obtained from C26-bearing mice as compared to controls (n = 5 mice per group) at both doses of IFN-A/D (Normal = 92.7 ± 24.5 vs. C26 36.4 ± 12.6, p = 0.0018 at 102 U/mL; Normal = 202.2 ± 21.7 vs. C26 99.9 ± 17.2, p = .0059 at 104 U/mL; Figure 2A). The expression of Irf1 was also significantly reduced in splenocytes from C26-bearing mice (n = 5–6 mice per group) versus controls following a 4 hr stimulation with IFN-γ (Figure 2B; p<0.05). We also evaluated expression of the interferon-inducible genes ISG15, OAS1, and IRF8 and observed similar trends (Supplemental Figure 3A – B and data not shown).
Figure 1. Decreased interferon response in tumor-bearing mice.
P-STAT1 was measured in splenocytes from control (Control; n = 19) or day 22 C26 tumor-bearing mice (C26 Tumor; n = 19) by intracellular flow cytometry following stimulation with (A) IFN-α (104 U/mL) or (B) IFN-γ (10 ng/mL). Y-axis is specific fluorescence (Fsp) of staining for P-STAT1. (C) P-STAT1 was measured in splenocytes from untreated normal mice and mice bearing C26 tumors, or from mice stimulated with 2×104 U IFN-A/D in vivo and harvested 2 hours later. (D) P-STAT1 was measured following IFN stimulation in splenocytes that were co-labeled with antibodies against CD4, CD8, and CD49b.
Figure 2. Attenuated interferon-stimulated gene expression in splenocytes from C26-bearing mice.
(A) Total RNA was isolated from splenocytes of control or C26-tumor bearing mice following stimulation of the cells for 4 hours with IFN-A/D or PBS, converted to cDNA and analyzed by real-time RT-PCR for Ifit2. (B) Irf1 expression was evaluated in a similar manner following stimulation of splenocytes with PBS or IFN-γ (10 ng/mL). Real-time RT-PCR data were obtained in triplicate and are expressed as the mean fold increase relative to the level of 18s mRNA. Each symbol represents the fold change of an individual animal.
MDSC levels are elevated in tumor-bearing mice, and are associated with decreased IFN response in vitro
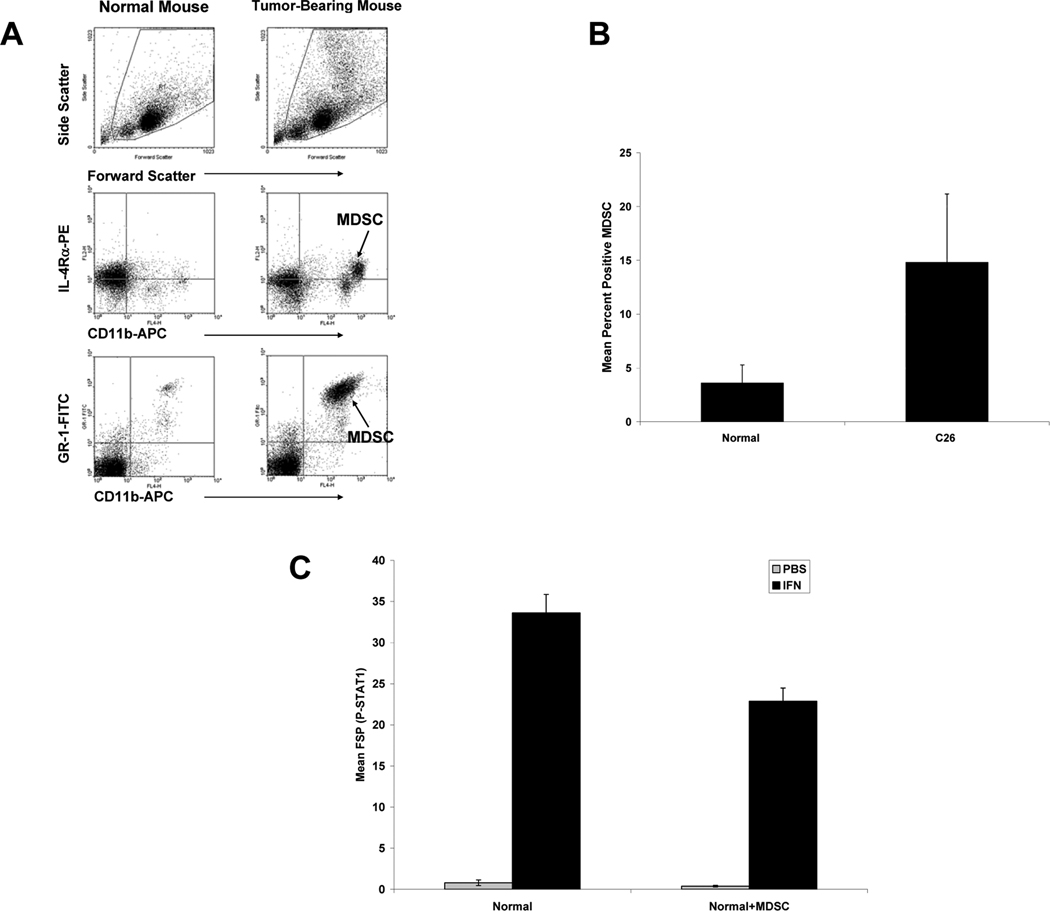
Flow cytometric analysis demonstrated a significantly higher percentage of MDSC in splenocytes from C26-bearing mice as compared to splenocytes from control animals (Figure 3A–B) (25). Histologic analysis of splenic tissue revealed extramedullary hematopoesis and abundant myeloid precursors resulting in extensive splenomegaly (data not shown). Time course studies revealed that an increase in MDSC occurred as early as 7 days post-tumor inoculation (Supplemental Figure 2A). To test whether MDSC can directly inhibit IFN responsiveness of immune cells, a series of in vitro studies was initiated in which GR1+CD11b+ MDSC were isolated from splenocytes of C26-bearing mice and co-cultured with immune cells from tumor-naïve animals. Splenocytes isolated from normal mice and co-cultured with MDSC had a reduced level of IFN-stimulated P-STAT1 as compared to normal splenocytes cultured for the same time period (Figure 3C; mean MFI P-STAT1 = 22.87 vs. 33.61; p = 0.0004). P-STAT1 was measured in the lymphocyte population only, excluding MDSC from analysis by gating. We also showed that elevated MDSC levels were associated with concomitant decreases in IFN responsiveness in the 4T1 murine epithelial cancer model (Supplemental Figure 2B) which supported the association of MDSC with reduced generation of P-STAT1 in response to IFN-α.
Figure 3. MDSC are elevated in tumor-bearing mice and are associated with decreased IFN responsiveness in vitro.
(A) Splenocytes from day 22 C26 mice were evaluated for MDSC using antibodies targeting GR1, IL-4Rα, and CD11b. (B) Splenocytes from day 22 C26 mice were evaluated for MDSC using antibodies targeting GR1 and CD11b. MDSC percentage in splenocytes from day 22 C26 tumor-bearing mice (C26; n = 19) or controls (Normal; n = 22) as described above. (C) MDSC were isolated from C26 splenocytes by magnetic labeling with anti-GR1 beads. After 24 hours, P-STAT1 was measured in splenocytes obtained from control mice (n = 10) or normal splenocytes that had been co-cultured with MDSC at a 1:3 ratio (n = 10).
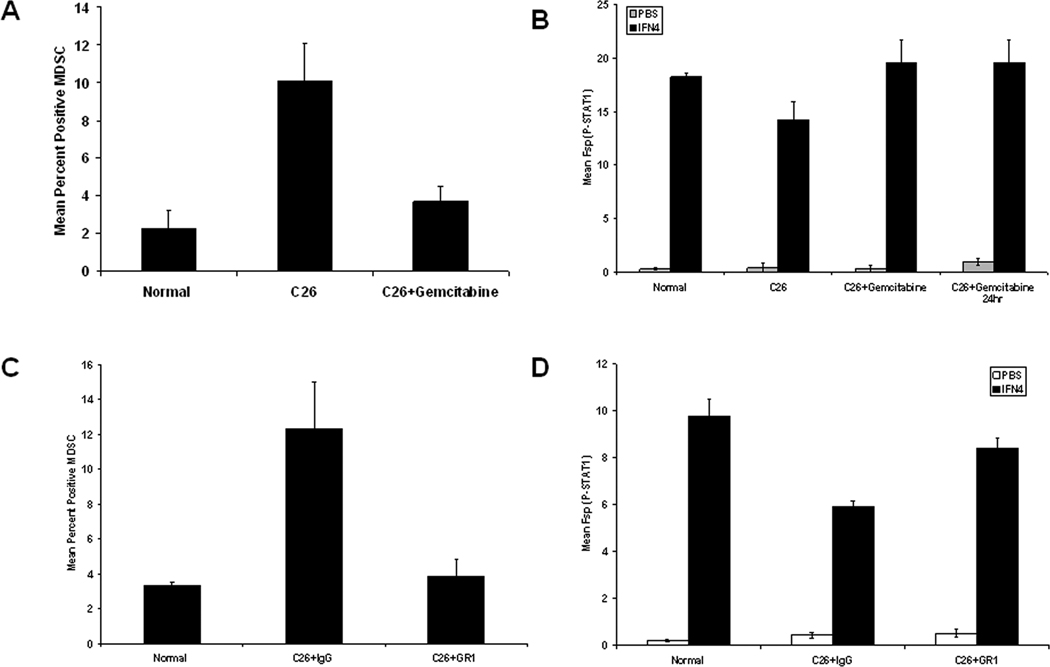
Reduction of MDSC leads to restored IFN-responsiveness in C26-bearing mice
C26-bearing animals were treated with the drug gemcitabine, a nucleoside analog, which has been previously employed by other groups to deplete MDSC (20–22, 26). This treatment regimen led to a decrease in the percentage of MDSC present within splenic tissues (Figure 4A; 10.1% vs. 3.7%; p = 0.0010). We also treated mice with a single dose of gemcitabine 24-hours prior to spleen harvest, and saw comparable levels of MDSC depletion in splenocytes from C26 tumor-bearing animals. As predicted, long-term gemcitabine treatment reduced tumor growth by approximately 90%, while the 24-hour treatment resulted in no change in tumor volume. Splenocytes obtained from C26-bearing mice treated both long term and for 24-hours with gemcitabine exhibited a restoration of interferon responsiveness as measured by intracellular P-STAT1 flow cytometry and western blot analysis (Figure 4B and Supplemental Figure 4). Notably, when we evaluated the effects of gemcitabine directly on C26 tumor cells in vitro, only 15% of C26 cells were apoptotic when using an equivalent dose of gemcitabine (data not shown). C26-bearing animals were also treated with an anti-GR1 antibody 24-hours prior to spleen harvest (day 20 post-tumor inoculation). This short-term depletion strategy was utilized to evaluate the direct effects of MDSC on IFN response in the presence of fully developed tumors. The reduction in MDSC levels (77% decrease) was verified by flow cytometry (Figure 4C). Splenocytes from tumor-bearing mice treated with one dose of anti-GR1 exhibited a significantly higher IFN response compared to the splenocytes of tumor-bearing mice treated with isotype control as evaluated by intracellular P-STAT1 flow cytometry and western blot analysis (Figure 4D, 42% increase in mean MFI P-STAT1 at 104 U/mL p = 0.002; Supplemental Figure 4). GR1+CD11b+ MDSC were also evaluated in the tumor after treatment with gemcitabine or anti-GR1. All treatments led to a reduction in intra-tumor MDSC as compared to tumor-bearing control animals (Supplemental Figure 5).
Figure 4. Reduction in MDSC with gemcitabine or anti-GR1 restores IFN responsiveness.
(A) Splenocytes obtained from day 22 C26 tumor-bearing were evaluated by flow cytometry for the presence of MDSC. (B) P-STAT1 was measured in splenocytes obtained from control mice (Normal; n = 5), Day 22 mice bearing C26 tumors and treated with PBS (C26; n = 5), Day 22 mice bearing C26 tumors and treated with gemcitabine (C26+gemcitabine; n = 6), and Day 22 mice bearing C26 tumors and treated with gemcitabine 24-hours prior to harvest (C26+gemcitabine 24hr; n=5) by intracellular flow cytometry following stimulation with IFN-A/D (104 U/mL). (C) Splenocytes obtained from Day 21 control mice (Normal), mice bearing C26 tumors and treated with isotype control (C26+IgG; n = 6), or mice bearing C26 tumors and treated with anti-GR1 (C26+GR1; n = 7) were evaluated for MDSC by flow cytometry and (D) P-STAT1 was measured in splenocytes obtained from normal or day 21 tumor-bearing mice and treated with isotype control (C26+IgG; n = 6), or mice bearing C26 tumors and treated with anti-GR1 (C26+GR1; n = 7) by intracellular flow cytometry following a 15 minute stimulation with IFN-A/D (104 U/mL).
Elevated nitric oxide and IFN-responsiveness
We hypothesized that soluble factors produced from the abundant MDSC present in the splenic tissue could contribute to the reduced IFN-response in tumor-bearing mice. We evaluated two of the major mechanisms through which MDSC are known to exert their suppressive effects, and discovered that while arginase I transcript levels were not significantly different in tumor-bearing splenocytes as compared to splenocytes from normal mice, iNOS mRNA levels were dramatically elevated (data not shown and Supplemental Figure 3C). DAF-FM and iNOS staining and fluorescent microscopic analysis were employed to test whether decreased IFN-responsiveness might be associated with increased levels of NO in the splenic tissue. These data confirmed a dramatic increase in the level of iNOS protein and NO in splenocytes from C26-bearing mice as compared to spleens from control animals (Figure 5A). Pre-treatment of splenocytes from normal mice with the NO donor S-nitroso-N-acetylpenicillamine (SNAP; Molecular Probes, Eugene, OR) led to a decreased phosphorylation of STAT1 in response to IFN-α stimulation (Figure 5B; mean MFI P-STAT1 = 35.7 vs. 16.4). STAT1 was immunoprecipitated from equal numbers of splenocytes from normal and C26 tumor-bearing mice and probed with an anti-nitrotyrosine antibody to detect nitration of STAT1 protein. We performed a Student’s t-test analysis on the densitometry results and determined that the ratio of nitrated STAT1 to total STAT1 in tumor bearing mice is statistically higher than in normal mice, demonstrating that C26-bearing animals exhibited significantly elevated nitration of STAT1 as compared to control mice (p=0.03; Figure 5C). In addition, we validated the level of nitrated tyrosine on STAT1 by flow cytometry. Splenocytes from normal and C26 tumor-bearing mice were labeled with antibodies against total STAT1 and nitrotyrosine. Splenocytes from tumor bearing and normal mice had equal levels of total STAT1. There was significantly more nitrated STAT1 in splenocytes from tumor-bearing animals as compared to splenoctyes from normal mice (36 ± 2.0% vs. 17 ± 0.3%; p<0.001; Supplemental Figure 3D). This result provides direct evidence that nitration on STAT1 was enhanced in C26-bearing mice. These data support a role for NO in dampening the response of immune effector cells to stimulation with IFN in tumor bearing animals. To determine the role of nitric oxide in interferon responsiveness in vivo, iNOS knockout mice were inoculated with C26 tumor cells and tumors were allowed to grow for 21 days. At the end of the study, tumor sizes were comparable between iNOS knockout mice and wild type control mice bearing C26 tumors. Splenocytes from iNOS knockout mice exhibited a significantly elevated induction of P-STAT1 in response to IFN stimulation as compared to immune cells from wild type mice (IFN 104 U/mL p = 0.01; Figure 5D). Quantitative histological analysis of iNOS-deficient tumors and tumors from WT mice revealed a significant decrease in Ki67 staining (34.24 ± 0.4% vs. 11.2 ± 2.85%; p = 0.01) as well as CD34 expression (29.93 ± 2.25% vs. 6.55 ± 2.77%; p = 0.02) expression in iNOS-deficient mice, indicating a reduced proliferation rate as well as decreased vascularity (Figure 6).
Figure 5. Elevated levels of nitric oxide (NO) in tumor bearing mice inhibits IFN responsiveness in immune cells.
(A) IF staining for iNOS protein (Fitc) and DAPI nuclear counterstain (left two panels). DAF-FM Fitc staining for nitric oxide and DAPI nuclear counterstain. (B) Splenocytes from normal mice were pre-treated with the nitrogen donor SNAP for 16 hours, stimulated for 15 minutes with PBS or IFN-A/D (104 U/mL) and evaluated for P-STAT1 by flow cytometry. (C) Protein lysates from splenocytes of normal or C26-bearing mice were immunoprecipitated with STAT1 antibody and then probed for nitrotyrosine. Lysates were also run on a separate gel and probed with an anti-STAT1 antibody (lower panel). Numbers represent densitometry values for nitrotyrosine and STAT1, and the ratio of nitrotyrosine/STAT1 (p=0.03). (D) P-STAT1 was measured in splenocytes obtained from day 21 iNOS knockout and control mice with and without C26 tumors by intracellular flow cytometry following stimulation with IFN-A/D (104 U/mL).
Figure 6. Reduced CD34 and Ki67 expression in tumors from iNOS knockout mice.
Formalin-fixed, paraffin-embedded tumor tissue from C26 tumors in wild type mice as compared to iNOS KO mice. Each panel represents an individual mouse tumor section stained with (A) CD34, a vascular endothelial marker (p=.02) or (B) Ki67, a proliferative marker (p=0.01).
Discussion
In the present report, we have demonstrated that immune cells from tumor-bearing mice display a significantly reduced capacity for IFN-induced signal transduction and gene induction via the STAT1 pathway. This inhibitory effect was observed in CD8+ and CD4+ T cells, as well as in NK cells and occurred following both in vivo and ex vivo IFN stimulation. C26 tumor-bearing mice exhibited enlarged spleens as compared to normal controls and displayed a concomitant increase in the level of GR1+CD11b+ myeloid derived suppressor cells, and depletion of MDSC in tumor-bearing mice led to a restoration of IFN-responsiveness in immune cells. Consistent with these observations, in vitro co-culture of splenocytes with MDSC reduced their ability to respond to IFNs. Splenocytes from C26-bearing mice had elevated levels of iNOS protein, NO production, and STAT1 tyrosine nitration. Importantly, splenocytes from iNOS-deficient mice exhibited improved IFN responsiveness as compared to splenocytes from tumor-bearing wild type mice. These results indicate that MDSC contribute to the blunted responsiveness of immune cells to IFN-α and IFN-γ via their ability to produce reactive nitrogen species, which leads to reduced IFN-induced signal transduction in immune cells.
Our group and others have determined that immune cells from patients with advanced cancers exhibit reduced activation of IFN-induced signaling pathways, although the mechanisms underlying this observation are still under investigation. We utilized the C26 model to recapitulate the complex interactions that take place between immune cells and cancer cells in vivo. The IFN response of lymphocytes from C26 tumor-bearing mice (as measured by STAT1 phosphorylation and regulation of IFN-stimulated genes) was significantly reduced when compared to normal mice. It was also noted that the spleens of these mice were enlarged and filled with myeloid precursor cells. Phenotypic characterization revealed that these precursor cells were MDSC, and we hypothesized that they were responsible for the reduced activation of STAT1 in response to IFN-α. Previous work by Mazzoni et al. showed that co-culture of immortalized murine MDSC with T cells in an in vitro non-tumor model led to an inhibition of the T cell response to IL-2 (27). We subsequently demonstrated that the presence of primary MDSC in tumor-bearing animals was associated with altered IFN signal transduction and that removal of this cell population led to restoration of IFN responsiveness. Thus, MDSC appear to be an important regulator of the downstream response to IFNs in tumor-bearing mice. This is the first report in which MDSC have been implicated as a mechanism for the reduced activation of immune cells following IFN stimulation.
While other tumor or MDSC-derived factors may contribute to reduced IFN responsiveness, our data suggests that the production of NO by MDSC is a major mechanism in this model. Increased levels of iNOS and NO in the spleen lead to the nitration of STAT1 on immune cells, which is the proposed mechanism of inhibition of IFN signaling. Importantly, splenocytes from iNOS deficient tumor-bearing mice had a significantly improved interferon response as compared to splenocytes from wild type tumor-bearing mice. This finding provides a direct in vivo link between excess nitric oxide and decreased interferon responsiveness in the setting of malignancy. In support of these observations, Brito et al. have demonstrated that peroxynitrite treatment of PBMCs led to increased nitration of tyrosine residues, which inhibited transcription factor phosphorylation following CD3 stimulation, while Llovera et al. showed that LPS-treated macrophages had an impaired in vitro immune cell response to IFN-γ due to an increase in nitric oxide in the culture (28, 29). We observed that tumor-bearing mice exhibited elevated splenic levels of iNOS protein and treatment of normal splenocytes with SNAP, a nitric oxide donor, suppressed the P-STAT1 response of the splenocytes to IFN-α stimulation. Nitration on STAT1 in primary lymphocytes from tumor-bearing mice has not previously been demonstrated in an in vivo murine model. These findings support a role for nitric oxide in the inhibition of IFN signal transduction.
In the studies with iNOS-deficient mice, interferon responsiveness in splenocytes from tumor-bearing animals was not fully restored to levels observed in normal animals. This indicates that other mechanisms may exert an effect on the interferon response in the tumor microenvironment. We evaluated alternative mechanisms that could lead to this inhibition, such as increased levels of arginase I or IL-6. Up-regulation of arginase I has been identified as a mechanism of immune suppression that is employed by MDSC (30–32). While previous studies in macrophages have demonstrated an inverse relationship between iNOS and arginase I levels, it has been shown that in a tumor setting it is possible for iNOS and Arg I to be co-expressed (8, 20, 33, 34). However, splenic levels of this enzyme were not significantly elevated in the C26 model. While arginase could still be playing a role in the tumor environment, our data in iNOS-deficient mice suggests that iNOS is a driving factor for the decreased IFN response in immune cells in this model. Despite the observation that immune cells from iNOS-deficient mice exhibited improved responsiveness to IFN, it is important to note that C26 tumors from these knockout mice were similar in size those from control mice. We anticipated that enhancement of the immune cell IFN response might lead to improved growth control of tumor cells in iNOS-deficient mice and reduced overall tumor volume. However, there was no significant reduction in external tumor size at the end of the study. It is important to note that in this model, the nitric oxide deficiency was present in all host tissues, rather than just the MDSC compartment, and nitric oxide has effects on multiple biologic processes (tumor cell invasion/proliferation, and angiogenesis). A careful quantitative histologic analysis of tumors revealed significantly decreased levels of Ki67 (proliferative marker) and CD34 (expressed on vascular endothelium) in iNOS-deficient mice as compared to those from WT mice (Figure 6). These findings indicate that the iNOS deficiency resulted in biological differences at the site of the tumor. Additional studies would be needed to directly assess the long-term impact of improved IFN response and iNOS-deficiency on survival and anti-tumor immunity.
The presence of tumor can also induce elevations in pro-inflammatory cytokines that have been associated with MDSC generation or function. In the C26 model, IL-6 levels were elevated in tumor-bearing mice as compared to normal mice. However, in vitro treatment of splenocytes with IL-6 did not alter the P-STAT1 response to IFN-α and administration of exogenous IL-6 was similarly incapable of inhibiting the splenocyte response to this cytokine (Supplemental Figure 6). Another potential explanation for our results relates to possible changes in levels of Jak-STAT signaling intermediates. However, a careful analysis revealed no significant difference in the protein expression of STAT1, IFNAR, or Jak1 in splenocytes obtained from tumor-bearing as compared to normal control mice (data not shown). As seen in Figure 5C, there was some variability in total STAT1 protein between individual mice, but this variability was not associated with the presence or absence of tumor. Previous work by Brito et al demonstrated that increased NO did not lead to changes in total STAT1 levels in immune cells, and this was confirmed in the present model when it was shown that STAT1 protein levels were similar in splenocytes from normal and tumor-bearing mice despite high levels of NO (28).
The observation of decreased IFN-responsiveness in immune cells has important implications for the clinical course of disease in cancer patients. Type I and II IFNs play a critical role in mediating the response to immune-based therapies (24). Therefore, conditions that inhibit IFN-induced signal transduction and gene transcription are likely to dampen the responsiveness of the host immune system to immune-based treatments and the host’s ability to recognize and eliminate established tumors (35–39). Reports have also demonstrated that type I and II IFNs are functionally important in mediating tumor immunosurveillance against methylcholanthrene-induced sarcomas and the progression of tumors in p53 null mice (1, 2). Previous studies from our group and others have shown that lymphocytes from patients with metastatic melanoma and other solid malignancies have an inherent reduction in basal and/or IFN-induced STAT1 phosphorylation as compared to normal donors (4, 5, 40). It is important to note that these studies focused on the IFN response in T cells and NK cells, but did not evaluate the responsiveness of dendritic cells or other cell subsets that have been previously shown to play a role in IFN mediated anti-tumor immunity. These subsets could potentially be impaired by nitrated residues as was observed in other lymphocyte populations. Reduced immune surveillance resulting from NO-dependent inactivation may therefore be important in the process of tumorigenesis and/or tumor progression.
To our knowledge this is the first in vivo report to demonstrate that elevated numbers of MDSC are associated with a decreased cellular response to IFN-stimulation in the setting of cancer. Further experiments suggest that increased nitration of STAT1 on tyrosine residues is responsible for the diminished IFN response in splenocytes. Therefore, therapeutic strategies targeted at reducing MDSC or upstream factors could potentially serve to restore IFN-responsiveness in tumor-bearing hosts.
Supplementary Material
Acknowledgments
Grant Support: The Valvano Foundation for Cancer Research Award (to G.B. Lesinski), National Institutes of Health (NIH) Grants CA84402, K24-CA93670 (to W.E. Carson), P01 CA95426 (M. Caligiuri), P30 CA134551 (to M. Caligiuri), K22 CA134551 (to G.B. Lesinski), T32 GM068412 (to B. Mundy-Bosse), T32 GM068412 (to A. Jaime-Ramirez), T32 CA009338 (to K. Guenterberg).
References
- 1.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 4.Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker M, Carson WE., 3rd Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon Alfa immunotherapy. J Natl Cancer Inst. 2004;96:1331–1342. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 5.Critchley-Thorne RJ, Simons DL, Yan L, Miyahira AK, Dirbas FM, Johnson DL, Swetter SM, Carlson RW, Fisher GA, Koong A, Holmes S, Lee PP. Impaired interferon signaling is a common immune defect in human cancer. PNAS. 2009 doi: 10.1073/pnas.0901329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 10.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 13.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 14.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strassmann G, Jacob CO, Evans R, Beall D, Fong M. Mechanisms of experimental cancer cachexia. Interaction between mononuclear phagocytes and colon-26 carcinoma and its relevance to IL-6-mediated cancer cachexia. J Immunol. 1992;148:3674–3678. [PubMed] [Google Scholar]
- 17.Lesinski GB, Trefry J, Brasdovich M, Kondadasula SV, Sackey K, Zimmerer JM, Chaudhury AR, Yu L, Zhang X, Crespin TR, Walker MJ, Carson WE., 3rd Melanoma cells exhibit variable signal transducer and activator of transcription 1 phosphorylation and a reduced response to IFN-alpha compared with immune effector cells. Clin Cancer Res. 2007;13:5010–5019. doi: 10.1158/1078-0432.CCR-06-3092. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerer JM, Lesinski GB, Kondadasula SV, Karpa VI, Lehman A, Raychaudhury A, Becknell B, Carson WE., 3rd IFN-alpha-induced signal transduction, gene expression, and antitumor activity of immune effector cells are negatively regulated by suppressor of cytokine signaling proteins. J Immunol. 2007;178:4832–4845. doi: 10.4049/jimmunol.178.8.4832. [DOI] [PubMed] [Google Scholar]
- 19.Lesinski GB, Anghelina M, Zimmerer J, Bakalakos T, Badgwell B, Parihar R, Hu Y, Becknell B, Abood G, Chaudhury AR, Magro C, Durbin J, Carson WE., 3rd The antitumor effects of IFN-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112:170–180. doi: 10.1172/JCI16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009 doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 23.Jaime-Ramirez AC, Mundy-Bosse BL, Kondadasula S, Jones NB, Roda JM, Mani A, Parihar R, Karpa V, Papenfuss TL, LaPerle KM, Biller E, Lehman A, Chaudhury AR, Jarjoura D, Burry RW, Carson WE., 3rd IL-12 enhances the antitumor actions of trastuzumab via NK cell IFN-gamma production. J Immunol. 2010;186:3401–3409. doi: 10.4049/jimmunol.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 25.Capuano G, Rigamonti N, Grioni M, Freschi M, Bellone M. Modulators of arginine metabolism support cancer immunosurveillance. BMC Immunol. 2009;10:1. doi: 10.1186/1471-2172-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 28.Brito C, Naviliat M, Tiscornia AC, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota AM. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 29.Llovera M, Pearson JD, Moreno C, Riveros-Moreno V. Impaired response to interferon-gamma in activated macrophages due to tyrosine nitration of STAT1 by endogenous nitric oxide. Br J Pharmacol. 2001;132:419–426. doi: 10.1038/sj.bjp.0703838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 31.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 35.Eisenbeis CF, Lesinski GB, Anghelina M, Parihar R, Valentino D, Liu J, Nadella P, Sundaram P, Young DC, Sznol M, Walker MJ, Carson WE., 3rd Phase I study of the sequential combination of interleukin-12 and interferon alfa-2b in advanced cancer: evidence for modulation of interferon signaling pathways by interleukin-12. J Clin Oncol. 2005;23:8835–8844. doi: 10.1200/JCO.2005.02.1691. [DOI] [PubMed] [Google Scholar]
- 36.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119–134. doi: 10.1016/s1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 37.Davis ID, Brady B, Kefford RF, Millward M, Cebon J, Skrumsager BK, Mouritzen U, Hansen LT, Skak K, Lundsgaard D, Frederiksen KS, Kristjansen PE, McArthur G. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 38.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, Hanaoka H, Shimizu N, Suzuki M, Yoshino I, Taniguchi M, Fujisawa T, Nakayama T. A phase I–II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 39.Murad YM, Clay TM, Lyerly HK, Morse MA. CPG-7909 (PF-3512676, ProMune): toll-like receptor-9 agonist in cancer therapy. Expert Opin Biol Ther. 2007;7:1257–1266. doi: 10.1517/14712598.7.8.1257. [DOI] [PubMed] [Google Scholar]
- 40.Critchley-Thorne RJ, Yan N, Nacu S, Weber J, Holmes SP, Lee PP. Down-regulation of the interferon signaling pathway in T lymphocytes from patients with metastatic melanoma. PLoS Med. 2007;4:e176. doi: 10.1371/journal.pmed.0040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.