Abstract
Objective
To evaluate the age-related changes in pumping of mesenteric lymphatic vessels (MLV) in male 9-mo and 24-mo old Fisher-344 rats.
Methods
Lymphatic diameters, contraction amplitude, contraction frequency and fractional pump flow were determined in isolated MLV before and after L-NAME application.
Results
The data demonstrate a severe weakening of the lymphatic pump in aged MLV including diminished lymphatic contraction amplitude, contraction frequency and as a result, - lymphatic pump activity. The data also suggest that the imposed flow gradient-generated shear-dependent relaxation does not exist in aged rat MLV, and the sensitivity of both adult and aged MLV to such shear cannot be eliminated by nitric oxide synthases blockade.
Conclusions
These data provide new evidence of lymphatic regional heterogeneity for both adult and aged MLV. In MLV, a constant interplay between the tonic and phasic components of the myogenic response and the shear-dependent release of nitric oxide predominantly determines the level of contractile activity; the existence of another shear-dependent but NO-independent regulatory mechanism is likely present. Aging remarkably weakens MLV contractility, which would predispose this lymphatic network to lower total lymph flow in resting conditions and limit the ability it’s to respond to an edemagenic challenge in the elderly.
Keywords: mesenteric lymphatic vessels, stretch, shear, myogenic response, nitric oxide, aging
INTRODUCTION
Spontaneous contractions of muscle cells in the walls of lymphatic vessels are necessary to maintain effective lymph flow whereas proper functioning of lymphatic endothelial cells is vital to regulate lymphatic contractility (16). The functional status of lymphatic muscle and endothelial cells is an important factor that supports fluid and macromolecule exchange and immune cell trafficking through the body and is also crucial to the transport of lipids adsorbed in small intestine. The basic self-regulatory mechanisms controlling lymph flow in lymphatic vessels is realized through the sensitivity of their muscle cells to levels of stretch and of their endothelial cells to levels of the shear stress (see recent reviews in (15,16,20)). While the understanding of the regulatory mechanisms controlling lymphatic muscle contraction is far from complete, recent studies have demonstrated important functional roles of nitric oxide in coordinating the lymphatic contractile cycle (4,22) and in fine tuning lymphatic contractions to different levels of basal luminal flow (17,18,25).
Knowledge of how aging influences the regulatory mechanisms of lymphatic contractility is limited. There are only a few reports demonstrating the measurements of reduced lymph flow in aged animals in vivo (6,7,24). In particular, it was reported (7) that aging significantly decreased lymph flow from the main mesenteric lymph duct in rats by ~60% between ages of 3 and 22 mo. Recently, we performed the first detailed evaluation of aging-induced alterations in lymphatic contractility in isolated rat thoracic duct (TD) (21). We found that aging severely alters contractility of the TD through weakening of lymphatic contractions and complete depletion of their shear/nitric oxide (NO)-dependent regulation. Our detailed functional tests of aged TD clearly demonstrated (21) that it is too simplistic to attribute the diminished contractility only to the sclerosis of aged TD (34) and/or to atrophy of muscle cells in its wall (33,34). We found that aging severely alters NO-dependent regulation of TD contractions with signs of depleted eNOS (endothelial nitric oxide synthase) function and permanent aging-associated shear-independent NO release in the duct linked to potential iNOS (inducible nitric oxide synthase) activation (21). Non-specific nitric oxide synthase (NOS) blockade restored levels of tone and contraction frequency in aged TD to nearly the levels observed in adult vessels whereas minute productivity in aged TD after removal of the shear-independent NO production was only slightly lower than in non-aged group. Our findings provided the first evidence of complicated functional consequences of aging in lymphatic vessels and a pivotal role of functional disturbances in aged lymphatic endothelium for the aging-induced alterations of lymphatic contractility (21).
While these studies (21) demonstrate the necessity of considering the “aging factor” during investigations of lymphatic functions, we have also confirmed the heterogeneity of lymphatic contractile function in different tissue beds (17). These latter data suggest that complexity of the lymphatic system may also lead to the differential aging of various regional lymphatic networks. In this study, we investigated the aging-associated changes in stretch-/shear-/NO-dependent regulation of contractile activity of isolated rat mesenteric lymphatic vessels (MLV). These vessels are an integral component of a lymphatic bed responsible for 90% of the daily lymph formation (2) and are vital for water/lipid adsorption and immunity; and thus, the aging-associated alterations in MLV contractility may dramatically affect systemic lymphatic function.
METHODS
Animals and Surgery
For the current studies, we chose Fischer-344 (F-344) rats, a commonly used rat strain in aging-related research (30,36) (animals obtained from aged rat colony maintained by NIH National Institute of Aging), from age groups representing adulthood and senility (9- and 24-months old males). MLV were isolated from the animals following animal procedures that were reviewed and approved by our Institutional Animal Care and Use Committee.
To isolate MLV, rats were anesthetized with a solution containing a combination of Fentanyl/Droperidol (0.3ml/kg IM) and Diazepam (2.5mg/kg IM). A 4-cm long midline abdominal incision was made through the skin, underlying fascia and muscle layers. A small loop of intestine, 6–7 cm in length was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was positioned in a dissection chamber within the field of view of a dissecting microscope and continuously suffused with standard Dulbecco’s phosphate-buffered saline (DPBS) (Invitrogen Corp., Cat. # 14040-133). Suitable mesenteric lymphatic vessels were identified and cleared of all surrounding tissue. Sections of mesenteric lymphatic vessels 1–1.5 cm in length were carefully dissected and used for experiments. After isolating mesenteric lymphatic vessels, the rat was euthanized with pentobarbital (120 mg/kg body weight IP). When pressurized to a transmural pressure of 5 cm H2O, the outer diastolic diameters of the MLV segments ranged from 102 to 118 µm in 9-mo rats and 118 to 130 µm in 24-mo rats.
Isolated Lymphatic Vessel Procedures for Functional Tests
Once exteriorized, the isolated MLV segments were transferred to an isolated vessel chamber (modified Living Systems Instrumentation single vessel chamber model CH/1) filled with pre-warmed 38°C albumin-physiological salt solution (APSS) (in mM: 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 Dextrose, 2.0 Sodium Pyruvate, 0.02 EDTA, 3.0 MOPS, and 10g/l bovine serum albumin) pH adjusted to 7.36 at 38°C. The isolated MLV segments were cannulated and tied onto two carefully matched glass pipettes (100–110 µm). Great care was used to prepare and select pairs of resistance-matched pipettes for these experiments as described in our previous studies (17,22). For each experiment we chose pairs of pipettes with matching diameters and tip lengths, and tested them to ensure that the difference between their measured electrical resistances did not exceed 10% (26). The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with APSS. Care was taken to ensure that there were no air bubbles in the tubing or the pipettes. Once the vessels were cannulated, a slight positive transmural pressure 3 cm H2O was applied to detect leaks and to ensure that the vessels were undamaged and untwisted. The vessels were set to their approximate in situ length and positioned just above the glass coverslip comprising the chamber bottom. The chamber was transferred to the stage of a microscope. The vessels were set to an equilibration transmural pressure of 3 cm H2O at 38°C for 15–20 minutes. Once tone and spontaneous contractions were observed, the vessels were allowed to equilibrate at 3 cm H2O for another 30 minutes prior to beginning the experiment. During all experiments, MLV segments were constantly superfused with pre-warmed 38°C APSS, and the isolated vessel chamber was constantly warmed to maintain a temperature of 38±0.1°C in the APSS surrounding the vessel. A CCD video camera, monitor, Windows-operated computer supplied by National Instruments data acquisition card NI PCI-1410 and DVD/HDD recorder were used to observe and record the lymphatic segments and to track their diameter continuously in all experiments.
At the beginning of every experiment, we evaluated the pressure-induced contractile responses of MLV segments obtained from 9-mo and 24-mo old animals. The MLV segments were exposed to a range of transmural pressures: 1, 3, 5 and 7 cm H2O for 5 minutes at each pressure. We chose this set of transmural pressures because we have shown that the isolated MLV displays maximal active pumping at 5 cm H2O (17–19). Since we have previously shown that imposed-flow inhibits the active mesenteric lymph pump (17–19), we constantly monitored the levels of input and output pressure to prevent imposed flow during this period. Thus, flow and shear were only generated by phasic contractions of the MLV during this experimental period.
To determine the imposed flow-induced responses of MLV, vessel segments were exposed to sets of imposed flow gradients, which were generated using techniques already employed (17–19). Because MLV are very sensitive to changes in transmural pressure, it is important to maintain a constant net transmural pressure at any given imposed flow gradient. As described before, this was accomplished by raising the pressure on the inflow end of the isolated vessel segment and lowering the pressure on the outflow end of the isolated vessel segment by identical amounts so as to create an axial intraluminal pressure gradient of 1, 3 and 5 cm H2O in MLV segment without altering the effective transmural pressure in the vessel (14,17–19). These imposed flow pressure gradients have been recently verified to be consistent with those that exist in situ in rat MLV (5).
After completion of the transmural pressure and imposed flow ranges in APSS (control), the APSS in chamber was replaced with pre-warmed 38°C APSS containing the nitric oxide synthases (NOS) inhibitor L-NAME (Sigma N 5751) at 100 µM (1,8,28,31,35,37,38). The effectiveness of NOS blockade in rat lymphatic vessels induced by application of L-NAME at this concentration has been numerously demonstrated by us in previous reports (4,16,20–22). The above transmural pressure and imposed flow ranges were repeated in the presence of L-NAME in all vessels.
Data Analysis and Statistics for Isolated Vessel Experiments
Lymphatic diameters were tracked continuously during experiments using “Vessel Track” software developed previously (9,11). We used cardiac pump analogies to define systole and diastole in reference to the lymphatic contractile cycle (3,17,23,39), and the end-diastolic and end-systolic points in the diameter tracings were recorded for each 5-minute interval for each transmural pressure: 1, 3, 5 and 7 cm H2O. To minimize animal-to-animal variability in vessels sizes from vessel to vessel (which exist even with similar vessels within each age group of weight and sex (males) matched animals), diastolic and systolic diameters were normalized to the passive lymphatic diameter in Ca-free PSS at the corresponding transmural pressures. From the lymphatic end-diastolic and end-systolic diameters, the following lymph pump parameters were calculated: lymphatic tone index (the difference between the passive lymphatic diameter in Ca-free PSS and end-diastolic diameter expressed as a percentage of the passive lymphatic diameter in Ca-free PSS), contraction amplitude (the difference between the normalized diastolic and systolic diameters), contraction frequency (number of contractions per minute), ejection fraction (the fraction of end-diastolic volume ejected during a single phasic lymphatic contraction) and fractional pump flow (an index of minute lymph pump flow, calculated as the ejection fraction times the contraction frequency).
Statistical differences were determined by two-way ANOVA, regression analysis and paired Student’s t-test (JMP software version 9.0.0. for Windows) as appropriate and considered significant at p≤0.05. In the Results section, the numbers of lymphatic vessels used in the reported data are shown separately for each group of experiments, where n depicts the number of lymphatic segments used for each experimental protocol for each age group. Only one vessel segment was used from one animal.
RESULTS
Effects of aging on pressure/stretch-dependent and phasic contractions-generated shear-dependent regulation of contractility of the mesenteric lymphatic vessels
In this study, we performed for the first time a detailed evaluation of the parameters of the active lymph pump in MLV isolated from 9- and 24-mo old Fischer-344 rats. In the first part of this study we compared the pressure/stretch-induced changes in contractility of isolated segments of MLV obtained from 9-mo (n=17) and 24-mo (n=17) old F-344 rats. Additionally, we used NOS blockade by 100 µM L-NAME to evaluate the importance of phasic contraction-generated, shear-dependent NO release by MLV in modulating these contractile responses in vessels obtained from both age groups. Figure 1 demonstrates the results of these studies.
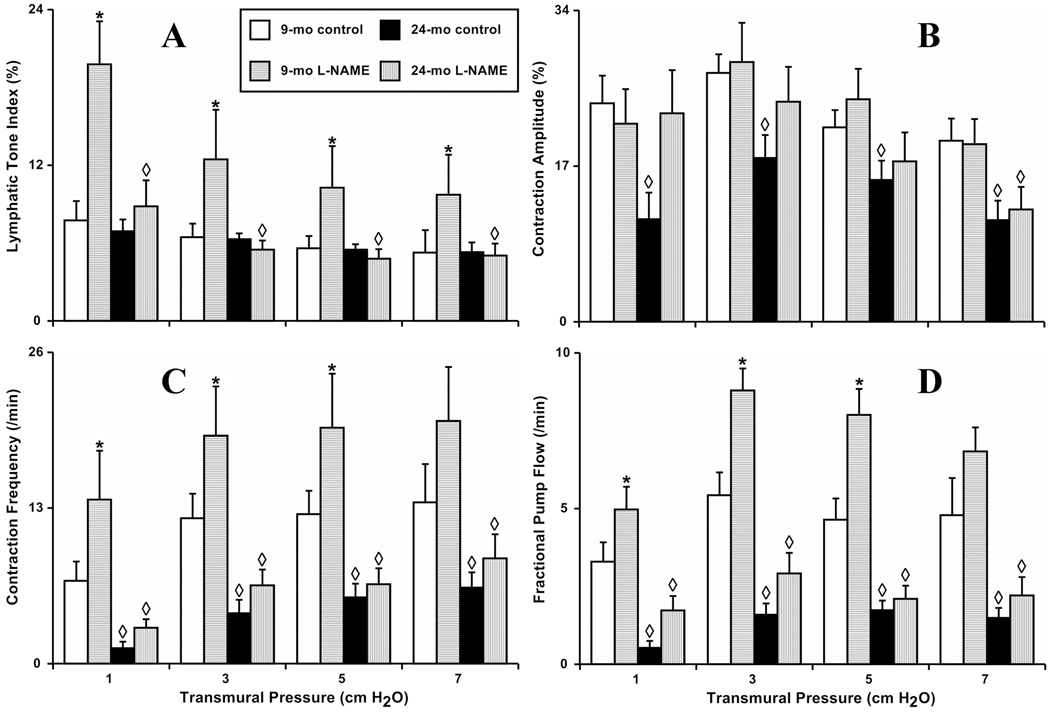
Figure 1. Aging-associated alterations in the contractile response of isolated segments of rat mesenteric lymphatic vessels to increases in transmural pressure.
A, B, C and D: 9-mo control and 24-mo control – segments isolated from 9 and 24 months old rats, respectively; 9-mo L-NAME and 24-mo L-NAME – L-NAME (100 µM) administration to the segments from 9 and 24 months old rats. Significant differences (P ≤ 0.05) between active lymph pump parameters indicated by * - 9-mo control versus 9-mo L-NAME conditions; by ◊-9-mo control versus 24-mo control, and 9-mo L-NAME versus 24-mo L-NAME.
In the 9-mo group, we observed that the lymphatic tone was diminished 32% during the increases in transmural pressure from 1 to 7 cm H2O, and the contraction amplitude changed in a bell-shaped fashion being highest at a transmural pressure of 3 cm H2O with decline at higher pressures. An increase in vessel stretch induced a positive chronotropic effect leading to a 96% increase in contraction frequency of MLV segments as the transmural pressure was raised from 1 to 7 cm H2O. Cumulatively, maximum lymphatic pumping was observed at 3 cm H2O. In general, MLV segments obtained from 9-mo (adult) animals demonstrated a classic inotropic response to increases in wall stretch as previously described for these (17–19) and other lymphatic vessels (13,27,32).
Recent studies performed in vivo (4) demonstrated the presence of phasic NO release in rat mesenteric lymphatic vessels. While such phasic contraction-generated shear-dependent release of NO was predicted and pharmacologically confirmed in isolated TD experiments (22), their role in the regulation of isolated MLV contractions has never been evaluated. In our present experiments utilizing MLV segments obtained from 9-mo rats, we found that NOS blockade induced remarkable increases in lymphatic tone in MLV quite similar to the observations made in isolated TD segments (22). After L-NAME administration, lymphatic tone increased significantly at all levels of transmural pressure (157% and 83% compared to control at 1 cm H2O and 7 cm H2O, respectively) (Figure 1 A). At the same time, treatment of isolated MLV segments with L-NAME did not alter contraction amplitude (Figure 1 B) but did increase contraction frequency (Figure 1 C). After L-NAME application, contraction frequency was significantly higher at pressures 1, 3 and 5 cm H2O (99, 57 and 57%, respectively). As a result of the observed changes in vessel chronotropy, their minute productivity (indicated by FPF) was significantly greater after L-NAME application at transmural pressures of 1, 3 and 5 cm H2O (52, 63 and 74%, respectively) (Figure 1 D).
In the 24-mo group, segments of MLV under control conditions also demonstrated stretch-dependency of their contractile behavior. Directions of changes in lymphatic contractile parameters in the aged group were similar to those observed in the adult group including decreased lymphatic tone, bell-shaped changes in contraction amplitude, and positive chronotropy during increases in transmural pressure from 1 to 7 cm H2O with maximums of lymphatic pumping observed between 3 and 5 cm H2O. However, when comparing lymphatic contractions between 9-mo and 24-old MLV, we noted consistent evidence of aging-associated contractile decay. While lymphatic tone was unchanged (Figure 1 A), both the amplitude and frequency of lymphatic contractions were significantly reduced in aged MLV. Aging-associated alterations in lymphatic contractility resulted in a 28% –54% lowering of the contraction amplitude in aged MLV compared to adult at different levels of transmural pressure (Figure 1 B). The negative chronotropic effects of aging on MLV contractility varied from 81% depletion at 1 cm H2O to 53% depletion at 7 cm H2O compared to adult MLV (Figure 1 C). Because of the aging-associated changes in MLV contractility described above, the minute productivity of aged vessels was significantly depleted 6.6-, 3.4-, 2.7- and 3.2-fold at the transmural pressures of 1, 3, 5 and 7 cm H2O, compared to corresponding levels of transmural pressure in adult MLV respectively (Figure 1 D).
The results of the experiments utilizing L-NAME application in aged MLV segments demonstrated additional differences between adult and aged groups during the increases in transmural pressure. Surprisingly, we found that NOS blockade was unable to increase lymphatic tone in the aged group. No differences in tone were observed between control and L-NAME treatment conditions in 24-mo MLV while 9-mo MLV displayed significantly higher tone following L-NAME treatment compared to control conditions and to conditions after L-NAME treatment in 24-mo MLV (Figure 1 A).
Contraction amplitude was increased in aged vessels after NOS blockade with a significant 100% increase observed at 1 cm H2O transmural pressure. With further elevations of transmural pressure, this post-L-NAME positive inotropy progressively declined to insignificant levels (33% increase at 3 cm H2O transmural pressure to 9% increase at 7 cm H2O transmural pressure). Interestingly, L-NAME application in aged MLV restored the contraction amplitude to the values in adult control vessels at all levels of transmural pressure except 7 cm H2O at which the contraction amplitude in aged L-NAME-treated MLV was significantly lower than in adult non-treated vessels (Figure 1 B). Frequency of lymphatic contractions in aged vessels was only slightly increased after L-NAME administration compared to control conditions at all levels of transmural pressure and these differences did not reach statistical significance (Figure 1 C).
As a result of the above post-L-NAME changes, the minute productivity of aged MLV was partially restored during NOS blockade. FPF of L-NAME-treated, aged MLV segments at 1 cm H2O was significantly increased (3.4 fold) compared to aged MLV under control conditions but was still significantly less (48%) than FPF in adult MLV during the control conditions (Figure 1 D). L-NAME treatment tended to increase FPF in aged MLV at all other levels of transmural pressure, but this trend increase became less dramatic and non-significant statistically at higher levels of transmural pressure (81% at pressure 3 cm H2O, 24% at pressure 5 cm H2O and 47% at 7 cm H2O). FPF in L-NAME-treated aged MLV was always significantly lower than FPF in adult vessels during the control part of the tests at these pressure levels.
Table 1 presents the raw data of active lymph pump parameters in MLV segments obtained in experiments to determine the effects of aging on pressure/stretch-dependent regulation of contractility in these vessels. All illustrative calculations of the percentage of change of contractile parameters between adult and aged MLV described in text section above are based on the data presented in this table.
Table 1.
Influence of transmural pressure on parameters of active lymph pump in segments of rat mesenteric lymphatic vessels (control and after administration of L-NAME) in 9-mo and 24-mo old animals.
| Transmural Pressure (cm H2O) |
Treatment | LTI (%) |
AMP (%) |
FREQ (min−1) |
FPF (min−1) |
|---|---|---|---|---|---|
| 1 | 9 mo Control | 7.7 ± 1.5 | 24 ± 3 | 6.9 ± 1.6 | 3.3 ± 0.6 |
| 9 mo L-NAME 100 µM | 19.8 ± 3.3 | 22 ± 4 | 13.7 ± 4.1 | 5.0 ± 0.7 | |
| 24 mo Control | 6.9 ± 0.9 | 11 ± 3 | 1.3 ± 0.6 | 0.5 ± 0.2 | |
| 24 mo L-NAME 100 µM | 8.8 ± 2.0 | 22 ± 5 | 3.0 ± 0.8 | 1.7 ± 0.5 | |
| 3 | 9 mo Control | 6.5 ± 1.1 | 27 ± 2 | 12.1 ± 2.1 | 5.4 ± 0.7 |
| 9 mo L-NAME 100 µM | 12.4 ± 3.8 | 28 ± 4 | 19.0 ± 4.1 | 8.8 ± 0.7 | |
| 24 mo Control | 6.3 ± 0.5 | 18 ± 2 | 4.2 ± 1.1 | 1.6 ± 0.4 | |
| 24 mo L-NAME 100 µM | 5.5 ± 0.7 | 24 ± 4 | 6.5 ± 1.3 | 2.9 ± 0.7 | |
| 5 | 9 mo Control | 5.6 ± 1.0 | 21 ± 2 | 12.5 ± 2.0 | 4.6 ± 0.7 |
| 9 mo L-NAME 100 µM | 10.3 ± 3.2 | 24 ± 3 | 19.7 ± 4.5 | 8.0 ± 0.8 | |
| 24 mo Control | 5.5 ± 0.4 | 15 ± 2 | 5.5 ± 1.6 | 1.7 ± 0.3 | |
| 24 mo L-NAME 100 µM | 4.8 ± 0.7 | 17 ± 3 | 6.6 ± 1.3 | 2.1 ± 0.4 | |
| 7 | 9 mo Control | 5.3 ± 1.7 | 20 ± 2 | 13.5 ± 3.2 | 4.8 ± 1.2 |
| 9 mo L-NAME 100 µM | 9.7 ± 3.1 | 19 ± 3 | 20.3 ± 4.5 | 6.8 ± 0.8 | |
| 24 mo Control | 5.3 ± 0.8 | 11 ± 2 | 6.4 ± 1.3 | 1.5 ± 0.3 | |
| 24 mo L-NAME 100 µM | 5.0 ± 0.9 | 12 ± 2 | 8.8 ± 2.0 | 2.2 ± 0.6 |
Values are means ± SE; n=17 (9-mo), n=17 (24-mo); LTI, lymphatic tone index; AMP, contraction amplitude; FREQ, contraction frequency; FPF, fractional pump flow.
Effects of aging on imposed flow gradient-generated shear-dependent regulation of contractility in mesenteric lymphatic vessels
In the second part of this study we compared the imposed flow gradient-generated shear-dependent changes in contractility of isolated segments of MLV obtained from 9-mo (n=14) and 24-mo (n=14) old F-344 rats. In addition, we used NOS blockade by L-NAME (100 µM) to evaluate the importance of NO release by MLV in mediating these contractile responses in vessels obtained from both age groups. Figure 2 demonstrates the results of these studies.
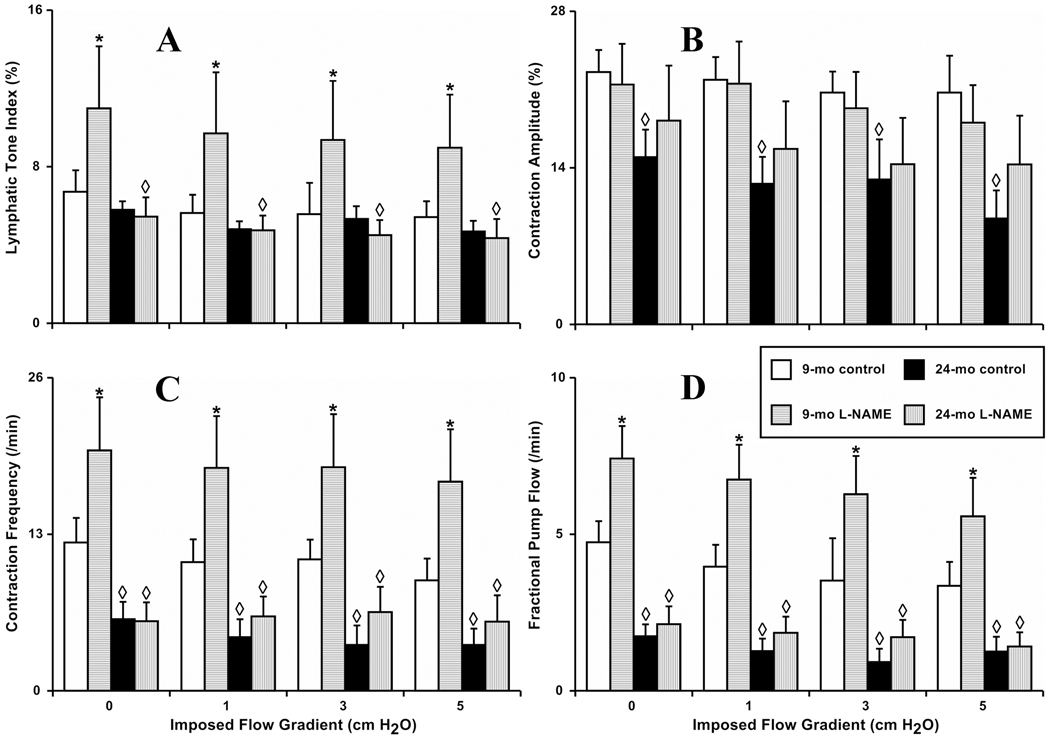
Figure 2. Aging-associated alterations in the contractile response of isolated segments of rat mesenteric lymphatic vessels to increases in imposed flow gradient.
A, B, C and D: 9-mo control and 24-mo control – segments isolated from 9 and 24 months old rats, respectively; 9-mo L-NAME and 24-mo L-NAME – L-NAME (100 µM) administration to the segments from 9 and 24 months old rats. Significant differences (P ≤ 0.05) between active lymph pump parameters indicated by * - 9-mo control versus 9-mo L-NAME conditions; by ◊ - 9-mo control versus 24-mo control, and 9-mo L-NAME versus 24-mo L-NAME.
In the 9-mo group, we observed that during the increases in imposed flow gradient from 0 to 5 cm H2O, the lymphatic tone, contraction amplitude, and frequency diminished 19%, 18%, and 25%, respectively, over this range of imposed flow gradients. Because of the observed changes, lymphatic pumping (indexed by FPF) declined (30%) in the presence of the highest selected imposed flow gradient of 5 cm H2O (compared to 0 cm H2O of imposed flow gradient). In general, MLV segments from the 9-mo (adult) animals under control conditions demonstrated the same type of the well-described (16–19) contractile responses to increases in steady wall shear stress, induced by raise of the imposed flow gradient applied to vessels.
In our current study on isolated MLV segments obtained from 9-mo rats, we found that NOS blockade induced a remarkable increase in lymphatic tone in MLV quite similar to the results observed in MLV segments obtained from SD rats (18). After L-NAME administration in adult MLV, lymphatic tone significantly increased 64–73% at the different levels of imposed flow gradient compared to control (no L-NAME treatment) conditions (Figure 2 A). Interestingly, after L-NAME treatment of 9-mo old MLV, we still observed an 18% decrease in lymphatic tone at 5 cm H2O imposed flow gradient compared to no imposed flow gradient.
The L-NAME treatment of isolated adult MLV segments has no effect on the contraction amplitude (Figure 2 B). We coincidentally noticed that after NOS inhibition, the pattern of the imposed flow gradient-generated shear-induced weakening of the lymphatic pump was similar to that observed in absence of such treatment. After L-NAME treatment during the increases in imposed flow gradient from 0 to 5 cm H2O, the contraction amplitude diminished 16%. We also found that NOS blockade significantly increased contraction frequency in the 9-mo MLV segments 63–88% at the various levels of imposed flow gradient compared to control (no L-NAME treatment) conditions. Interestingly, after L-NAME treatment of 9-mo old MLV, we still observed a 14% decrease in contraction frequency at 5 cm H2O imposed flow gradient compared to no imposed flow gradient (Figure 2 C). Because of the observed changes in the 9-mo MLV contractile characteristics after L-NAME application, the minute productivity of these vessels (indexed by FPF) was significantly greater (57–80%) at all levels of imposed flow gradient (Figure 2 D). NOS blockade was not able to eliminate the 25% reduction in lymphatic pumping (FPF) induced by the highest selected imposed flow gradient of 5 cm H2O (compared to the absence of the imposed flow gradient) (Figure 2 D).
In the 24-mo old segments of MLV under control conditions, we found that during the increases in imposed flow gradient from 0 to 5 cm H2O, the lymphatic tone, contraction amplitude, and contraction frequency diminished 19%, 40%, and 36%, respectively. Because of these observed changes, lymphatic pumping declined 30% in the presence of the highest selected imposed flow gradient of 5 cm H2O (compared to no imposed flow gradient). In general, MLV segments from the 24-mo (aged) animals under control conditions demonstrated contractile responses similar in magnitude and polarity to the changes observed in 9-mo group under control conditions during the same increases in imposed flow gradients.
Following L-NAME application, we found differences between adult and aged MLV segments in response to increasing imposed flow gradients. In these experiments as well as in the pressure/stretch-related experiments described earlier, we discovered that NOS blockade was unable to increase significantly lymphatic tone in the aged group (Figure 2 A). Over the range of imposed flow gradients (from 0 to 5 cm H2O), lymphatic tone decreased 19% in the aged L-NAME-treated MLV. Contraction amplitude of aged vessels after NOS blockade was increased slightly but non-significantly. At 5 cm H2O of imposed flow gradient, contraction amplitude in aged post-L-NAME MLV was still 22% lower than that measured under no imposed flow gradient (Figure 2 B). Compared to no imposed flow conditions, the frequency of lymphatic contractions in aged MLV was insignificantly decreased; however, this subtle evidence of imposed flow gradient-dependency of the contraction frequency in aged MLV was eliminated after NOS blockade (Figure 2 C). Finally, aged MLV minute productivity only tended to increase in the absence of NO at all levels of imposed flow gradients; however, FPF in L-NAME-treated aged MLV was always significantly lower than FPF in adult vessels during the control part of the tests at these levels of the imposed flow gradients (Figure 2 D).
Table 2 presents the raw data of active lymph pump parameters in MLV segments obtained in experiments to determine the effects of aging on the imposed flow gradient-generated shear-dependent regulation of contractility in these vessels. All illustrative calculations of the percentage of change of contractile parameters between adult and aged MLV described in text section above are based on the data presented in this table.
Table 2.
Influence of imposed flow gradients on parameters of active lymph pump in segments of rat mesenteric lymphatic vessels (control and after administration of L-NAME) in 9-mo and 24-mo old animals.
| Imposed Flow Gradient (cm H2O) |
Treatment | LTI (%) |
AMP (%) |
FREQ (min−1) |
FPF (min−1) |
|---|---|---|---|---|---|
| 0 | 9 mo Control | 6.7 ± 1.1 | 23 ± 2 | 12.3 ± 2.1 | 4.7 ± 0.7 |
| 9 mo L-NAME 100 µM | 11.0 ± 3.2 | 21 ± 4 | 20.0 ± 4.4 | 7.4 ± 1.3 | |
| 24 mo Control | 5.8 ± 0.4 | 15 ± 2 | 5.9 ± 1.5 | 1.7 ± 0.4 | |
| 24 mo L-NAME 100 µM | 5.4 ± 1.0 | 18 ± 5 | 5.8 ± 1.6 | 2.1 ± 0.6 | |
| 1 | 9 mo Control | 5.6 ± 0.9 | 22 ± 2 | 10.7 ± 1.9 | 4.0 ± 0.7 |
| 9 mo L-NAME 100 µM | 9.7 ± 3.1 | 22 ± 4 | 18.5 ± 4.3 | 6.7 ± 1.1 | |
| 24 mo Control | 4.8 ± 0.4 | 13 ± 2 | 5.5 ± 1.5 | 1.3 ± 0.4 | |
| 24 mo L-NAME 100 µM | 4.8 ± 0.8 | 16 ± 4 | 6.1 ± 1.7 | 1.9 ± 0.5 | |
| 3 | 9 mo Control | 5.5 ± 1.6 | 21 ± 2 | 10.9 ± 1.7 | 3.5 ± 1.4 |
| 9 mo L-NAME 100 µM | 9.4 ± 3.0 | 19 ± 3 | 18.6 ± 4.4 | 6.3 ± 1.2 | |
| 24 mo Control | 5.3 ± 0.7 | 13 ± 4 | 3.8 ± 1.6 | 0.9 ± 0.4 | |
| 24 mo L-NAME 100 µM | 4.5 ± 0.8 | 14 ± 4 | 6.5 ± 2.1 | 1.7 ± 0.6 | |
| 5 | 9 mo Control | 5.4 ± 0.8 | 21 ± 3 | 9.2 ± 1.8 | 3.3 ± 0.8 |
| 9 mo L-NAME 100 µM | 9.0 ± 2.7 | 18 ± 3 | 17.3 ± 4.3 | 5.6 ± 1.2 | |
| 24 mo Control | 4.7 ± 0.5 | 9 ± 3 | 3.8 ± 1.4 | 1.2 ± 0.5 | |
| 24 mo L-NAME 100 µM | 4.4 ± 1.0 | 14 ± 4 | 5.7 ± 2.2 | 1.4 ± 0.5 |
Values are means ± SE; n=14 (9-mo), n=14 (24-mo); LTI, lymphatic tone index; AMP, contraction amplitude; FREQ, contraction frequency; FPF, fractional pump flow.
DISCUSSION
In this study, we completed the first comprehensive evaluation of aging-associated alterations in the contractile responses of mesenteric lymphatic vessels. Our new data allow us to make several important conclusions that widen our basic knowledge of the regulatory mechanisms of mesenteric lymph flow in adulthood and senility, demonstrating therefore the complex nature of lymphatic system. Our previous studies have demonstrated regional heterogeneity in the contractile behavior of lymphatic vessels (17,19,29). Our current studies provided the first evidence of regionally different influence of aging on different lymphatic networks. To illustrate such conclusions, we will compare later in the discussion our current data with published data (21) obtained from experiments utilizing isolated rat thoracic duct segments obtained from similar groups of adult (9-mo old) and aged (24-mo old) F-344 rats and treated with similar experimental conditions.
Sensitivity of mesenteric lymphatic vessels to wall stretch, phasic contractions-generated and steady flow-generated wall shear stress in adulthood
We performed a series of tests to determine the contractile responses of MLV isolated from 9-mo old (adult) animals to increases in wall stretch generated by different levels of transmural pressure. These tests provided adult control data for comparison to data obtained with aged MLV, and expanded our knowledge on the basic contractile characteristics of the adult mesenteric lymphatic vessels highlighting their important differences with the lymphatic contractions in the thoracic duct. In the first set of the experiments, we blocked the NOS in conditions without steady flow through the vessel segment, induced by the imposed flow. In this set of experiments, the endothelial cells in the MLV therefore only experienced shear stress due to the flow generated by the spontaneous phasic contractions of the lymphatic muscle cells. No exogenous pressure gradient favorable to flow was present in the lymphatic specimen. This contractions-generated “active” flow in MLV is responsible for phasic fluctuations in wall shear stress (12) and leads to the appearance of spikes of NO release by the lymphatic endothelium that are synchronous with lymphatic contractions (4). By analyzing the physiological importance of such phasic contractions-generated shear-dependent regulation in thoracic duct (22), we previously made following important conclusions. This large lymphatic duct, with lower resistance to flow, does not need strong, long-distance lymph active pumping and presumably because this, and due to the low variability in lymph flow patterns during the different periods of day, the thoracic duct has a somewhat simple shear-dependent regulatory mechanism to support an energy-sufficient contractile pattern solely through NO-dependent self regulation.
In the current study of adult mesenteric lymphatic vessels, we found that opposite to thoracic duct the NOS blockade by L-NAME cannot induce negative inotropy. The amplitude of lymphatic contractions remained the same after NOS blockade in adult MLV even though their contraction frequency increased over all selected levels of transmural pressure under such conditions (Figure 1, B & C). As a result, the minute productivity of the adult MLV increased after NOS blockade (Figure 1 D). It seems unlikely that, in natural in situ conditions, the NO molecules will serve a role to limit the productivity of the active lymph pump, and thus, the increase in FPF in isolated adult mesenteric vessels after L-NAME administration looks somewhat artificial. On the other hand, the fact that NOS blockade induced a significant increase in MLV tone and frequency (therefore limiting diastolic time and diastolic filling) allows us to expect by default that the contraction amplitude in the absence of NO should go down as it does in TD at the same conditions (22). Therefore, our data supports the assumption that the phasic shear-dependent regulation of MLV contraction interplays with additional important mechanism(s) to control the lymphatic contractile cycle in these vessels seems to be more complex compared to TD.
Our current data and data previously published by us and others allow us to conclude that there is a complex interplay between the influences of phasic and tonic components of the stretch-dependent myogenic responses and the influences of phasic contractions-generated NO release in MLV. In the mesenteric lymphatic network, the lymph formation and lymph flow may increase very fast with digestion. During such periods, the intralymphatic pressure may rise dramatically fast resulting in the development of myogenic constriction (10), the tonic component of which, i.e. prolonged vessel constriction, by itself may lead to a local increase in vessel resistance to flow. The latter is unnecessary for lymphatic vessels during periods of high lymph formation. Stronger phasic contractions during the myogenic response (“compensatory increase in amplitude” (10)) will support stronger fluid propulsion, higher fluctuations in wall shear, and therefore higher phasic release of NO. The latter of which will enhance the diastolic relaxation effect through easing the lymphangion filling and diminishing subsequently local resistance to flow. In other words, after pressure elevation, the positive phasic inotropy during the development of the myogenic phasic response would counter balance by the release of additional NO. In the rat thoracic duct where there is lower total resistance to flow, lesser necessity for strong active pumping, and generally smaller tone-induced changes in lymphatic diameter relative to maximal diameter, the tonic and phasic components of the myogenic response may play a less functional role. Conversely, the data indicates a dominant functional importance for nitric oxide release in the control of the contractility in this largest lymphatic vessel of the body (15,17,20,22).
In our experiments with L-NAME, we artificially misbalanced the cascade of contractile regulatory reactions by eliminating the phasic contraction-generated shear-dependent NO release. At the same time, we did not eliminate the phasic contraction-generated shear itself so the potential existence of an additional as of yet unidentified shear-dependent, but NO-independent, mechanism for the regulation of lymphatic contractile strength in the MLV cannot be excluded. Further support of this idea can be seen in results of our experiments with NOS blockade in MLV during periods of increased imposed flow. Opposite to adult thoracic duct, L-NAME cannot completely abolish the influences of the imposed flow gradient on the MLV contractile pump (Figure 2 and Table 2), which are similar to effects of LNMMA observed by us earlier in these lymphatic vessels (18).
In conclusion, our present findings in adult MLV under control and L-NAME-treated conditions provide new support for the complexity and regional variability in the mechanisms controlling lymph flow in the body, which still requires additional investigations.
Aging of the mesenteric lymphatic pump
When comparing the contractile activity of adult and aged MLV, we found that aging significantly diminishes their contraction amplitude (negative inotropic effect) (Figure 1 B), and contraction frequency (negative chronotropic effect) (Figure 1 C). Due to these aging-associated alterations in contractility, the minute productivity of aged MLV decreased 6.6-fold at lower levels of transmural pressure and ~3-fold at moderate and high levels of wall stretch in old vessels (Figure 1 D). Overall, we conclude that aging induces weakening of the mesenteric lymph pump potentially by altering both pacemaking and contractile mechanisms; the specific causes of such events still need to be discovered. We believe that the weakened aged MLV may not provide enough contractile force to facilitate the transport of newly formed, nutrient rich lymph from the gut thus elongating the duration of natural digestion-related events in whole body. Additionally, the aging-induced weakening of the mesenteric lymph pump may lead to a delay in the clearance of excessive fluid as well as potentially hazardous substances and foreign particles from the aged gut and mesentery. This negative impact of aging on the fluid/macromolecule balance in the gut occurs constantly in the elderly including the periods of functional rest when levels of lymph formation and lymphatic filling are relatively low. During such periods, aged MLV are experiencing lower levels of wall stretch, a point at which the aging-associated depletion of their pumping is most remarkable (Figure 1 D).
While analyzing the contractile behavior of aged MLV during transmural pressure elevations, we noted a greater negative chronotropic effect of aging in these vessels compared to the aged TD (21). As a result, the aging-associated depletion of MLV minute productivity at lower levels of transmural pressure is relatively greater than that observed in aged TD. Fractional pump flow in aged TD segments was 1.9- and 1.9-fold down compared to their adult (9-month-old) counterparts at 1- and 3-cm H2O transmural pressures, respectively (21). In our current investigation of aged MLV segments, FPF was 6.6- and 3.4-fold down at the same levels of transmural pressure, respectively (Table 1). The causes and functional importance of these differences in the ability of these two aged lymphatic vessels to work against increases in preloads (diastolic intraluminal pressure) are yet to be evaluated.
Experiments with the treatment of aged MLV by L-NAME in the absence of imposed flow demonstrated that NOS blockade influenced contraction amplitude in aged MLV rather than in did not affect inotropy in adult vessels (Figure 1 B, data presented in Table 1). The contraction amplitude is lower in aged MLV (Figure 1 B) so the phasic contractions-generated shear-dependent release of NO in aged MLV presumably is also weaker than in adult MLV (smaller fluctuations in wall shear due to the weaker contractions). The fact that L-NAME application in the weakened aged vessels has a greater effect on inotropy than in adult vessels may indicate the existence of additional aging-associated shear-independent NO release in aged MLV. Future direct measurements of NO concentrations in aged MLV in situ will help provide clear understanding of these changes.
In conclusion, we found additional confirmation of earlier conclusions that mesenteric lymphatic vessels have a greater pumping component to their contractile activity compared to the thoracic duct where the relaxatory changes in its tone play a much greater functional role. In mesenteric lymphatic vessels, a constant interplay between the tonic and phasic components of the myogenic response and the shear-dependent release of the nitric oxide determines the level of contractile activity while the existence of another shear-dependent but NO-independent regulatory mechanism is likely present. Aging remarkably weakens MLV contractility; latter would predispose this lymphatic network to lower values of total lymph flow in resting conditions and would limit the ability of these vessels to respond to an edemagenic challenge in the elderly.
ACKNOWLEDGMENTS
This work was supported in parts by the National Institutes of Health (NIH RO1 AG-030578 and HL-094269) and by Texas A&M Health Science Center College of Medicine and Department of Systems Biology and Translational Medicine.
REFERENCES
- 1.Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by tnf-{alpha} antagonism. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H1259–H1263. doi: 10.1152/ajpheart.00990.2005. [DOI] [PubMed] [Google Scholar]
- 2.Benoit JN, Zawieja DC. Gastrointestinal lymphatics. In: Johnson L, editor. Physiology of the gastrointestinal tract. Third Ed. New York: Raven Press; 1994. pp. 1669–1692. [Google Scholar]
- 3.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am. J. Physiol. 1989;257:H2059–H2069. doi: 10.1152/ajpheart.1989.257.6.H2059. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1319–H1328. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandon Dixon J, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 6.Bulekbaeva LE. [the volume rate of lymph flow in dogs in postnatal ontogeny] Zh Evol Biokhim Fiziol. 1988;24:599–600. [PubMed] [Google Scholar]
- 7.Chevalier S, Ferland G, Tuchweber B. Lymphatic absorption of retinol in young, mature, and old rats: Influence of dietary restriction. FASEB J. 1996;10:1085–1090. doi: 10.1096/fasebj.10.9.8801171. [DOI] [PubMed] [Google Scholar]
- 8.Datte JY, Yapo PA, Offoumou MA. Nitric oxide effect on 5-hydroxytryptamine-induced vasoconstrictions of isolated smooth muscle. Pharmacol Rep. 2005;57:113–120. [PubMed] [Google Scholar]
- 9.Davis MJ. An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation. 2005;12:361–372. doi: 10.1080/10739680590934772. [DOI] [PubMed] [Google Scholar]
- 10.Davis MJ, Davis AM, Ku CW, Gashev AA. Myogenic constriction and dilation of isolated lymphatic vessels. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H293–H302. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol. 2006;4:3–10. doi: 10.1089/lrb.2006.4.3. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- 13.Gashev AA. [the pump function of the lymphangion and the effect on it of different hydrostatic conditions] Fiziol. Zh. SSSR Im .I. M .Sechenova. 1989;75:1737–1743. [PubMed] [Google Scholar]
- 14.Gashev AA. Physiologic aspects of lymphatic contractile function: Current perspectives. Ann. N .Y. Acad. Sci. 2002;979:178–187. doi: 10.1111/j.1749-6632.2002.tb04878.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- 15.Gashev AA. Lymphatic vessels: Pressure- and flow-dependent regulatory reactions. Ann. N .Y. Acad. Sci. 2008;1131:100–109. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- 16.Gashev AA. Basic mechanisms controlling lymph transport in the mesenteric lymphatic net. Ann. N .Y. Acad. Sci. 2010;1207 Suppl 1:E16–E20. doi: 10.1111/j.1749-6632.2010.05710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 18.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2295–H2308. doi: 10.1152/ajpheart.00260.2005. [DOI] [PubMed] [Google Scholar]
- 20.Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology. 2010;17:277–287. doi: 10.1016/j.pathophys.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation. 2007;14:827–839. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- 22.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated no-dependent lymphatic relaxation: A self-regulatory mechanism in rat thoracic duct. J. Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granger HJ, Kovalcheck S, Zweifach BW, Barnes GE. Quantitative analysis of active lymphatic pumping; Proceedings of the VII Summer Computer Simulation Conference; Simulation Council, La Jolla, CA. 1977. pp. 562–565. [Google Scholar]
- 24.Hollander D, Dadufalza V. Influence of aging on vitamin a transport into the lymphatic circulation. Exp Gerontol. 1990;25:61–65. doi: 10.1016/0531-5565(90)90010-y. [DOI] [PubMed] [Google Scholar]
- 25.Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: Role of endothelial prostanoids. Am. J. Physiol. 1999;277:R1683–R1689. doi: 10.1152/ajpregu.1999.277.6.R1683. [DOI] [PubMed] [Google Scholar]
- 26.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am. J. Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 27.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J. Physiol. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am. J. Physiol. 1998;274:R790–R796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 29.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje. [DOI] [PubMed] [Google Scholar]
- 30.Nadon NL. Maintaining aged rodents for biogerontology research. Lab Anim (NY) 2004;33:36–41. doi: 10.1038/laban0904-36. [DOI] [PubMed] [Google Scholar]
- 31.Nakaike R, Shimokawa H, Yasutake H, Sumimoto H, Ito A, Numaguchi K, Egashira K, Takeshige K, Takeshita A. Effects of l-arginine analogues on vasomotion of isolated porcine coronary arteries. Am. J. Physiol. 1995;268:H1966–H1972. doi: 10.1152/ajpheart.1995.268.5.H1966. [DOI] [PubMed] [Google Scholar]
- 32.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am. J. Physiol. 1980;239:H88–H95. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- 33.Orlov RS, Borisov AV, Borisova RP. Lymphatic vessels. Structure and mechanisms of contractile activity. (in russian) Leningrad, USSR: Nauka; 1983. [Google Scholar]
- 34.Rabinovitz AJ, Saphir O. The thoracic duct; significance of age-related changes and of lipid in the wall. Circulation. 1965;31:899–905. doi: 10.1161/01.cir.31.6.899. [DOI] [PubMed] [Google Scholar]
- 35.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 37.Wang A, Nishihashi T, Trandafir CC, Murakami S, Ji X, Shimizu Y, Kurahashi K. Involvement of endothelial cyclo-oxygenase metabolites in noradrenaline-induced contraction of rat coronary artery. Clin Exp Pharmacol Physiol. 2005;32:628–632. doi: 10.1111/j.0305-1870.2005.04242.x. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of no and edhf in flow-induced vasodilation in isolated hamster cremasteric arterioles. J. Vasc. Res. 2005;42:137–147. doi: 10.1159/000083652. [DOI] [PubMed] [Google Scholar]
- 39.Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am. J. Physiol. 1991;260:H1935–H1943. doi: 10.1152/ajpheart.1991.260.6.H1935. [DOI] [PubMed] [Google Scholar]