Abstract
The inflammatory response, and its subsequent resolution, are the result of a very complex cascade of events originating at the site of injury or infection. When the response is severe and persistent, Systemic Inflammatory Response Syndrome can set in, which is associated with a severely debilitating systemic hypercatabolic state. This complex behavior, mediated by cytokines and chemokines, needs to be further explored to better understand its systems properties and potentially identify multiple targets that could be addressed simultaneously. In this context, short term responses of serum cytokines and chemokines were analyzed in two types of insults: rats receiving a “sterile” cutaneous dorsal burn on 20% of the total body surface area (TBSA); rats receiving a cecum ligation and puncture treatment (CLP) to induce infection. Considering the temporal variability observed in the baseline corresponding to the control group, the concept of area under the curve (AUC) was explored to assess the dynamic responses of cytokines and chemokines. MCP-1, GROK/KC, IL-12, IL-18 and IL-10 were observed in both burn and CLP groups. While IL-10 concentration was only increased in the burn group, Eotaxin was only elevated in CLP group. It was also observed that Leptin and IP-1 concentrations were decreased in both CLP and sham-CLP groups. The link between the circulating protein mediators and putative transcription factors regulating the cytokine/chemokine gene expression was explored by searching the promoter regions of cytokine/chemokine genes in order to characterize and differentiate the inflammatory responses based on the dynamic data. Integrating multiple sources together with the bioinformatics tools identified mediators sensitive to type and extent of injury, and provided putative regulatory mechanisms. This is essential to gain a better understanding for the important regulatory points that can be used to modulate the inflammatory state at molecular level.
Keywords: Cytokines, Chemokines, Burn injury, Cecum ligation and puncture
1. Introduction
Inflammation is a complex biological response to injuries and infections, involving different inflammatory mediators, such as cytokines and chemokines, as well as nervous system reflexes, and hormones. Precise regulation and coordination of the processes linking them is necessary to mount an appropriate response enabling an eventual return to homeostasis [1]. It is generally accepted that the severity of the inflammatory response and the involvement of the various components can vary depending on type and severity of the injury, infectious agent, as well as genetic and physiological factors (sex, body weight, preexisting conditions including immune system deficiencies).
Burns and infections lead to one or several of the following “primary” events in the inflammatory cascade: denaturation of proteins, loss of plasma membrane integrity, activation of complement proteins, and secretion of local mediators, such as histamine, nitric oxide, and reactive oxygen species [2–4]. This is quickly followed by the production of pro-inflammatory cytokines (such as IL-1, TNF-α, IL-18, IL-6, and IL-12), and chemokines (such as Eotaxin, G-CSF, GM-CSF, GRO/KC, MCP-1, MIP-1, Rantes). The initial pro-inflammatory response also activates a modulating anti-inflammatory reaction consisting of anti-inflammatory cytokines (such as IL-4, IL-10, IL-13). Inflammatory cytokines also impact afferent signals to the nervous system which in turn increase the secretion of anti-inflammatory stress hormones [5] and activate the cholinergic anti-inflammatory pathway [1]. Although these different signals may result in distinct cellular responses depending on the type and severity of the injury, there is a large degree of overlap in the underlying signaling pathways, including MAPK, Jac/STAT, and Ik-B/NF-kβ activated during the inflammation.
Animal models of burn injury, cecal ligation and puncture, and endotoxin injection have previously been used to profile circulating mediator concentrations after an insult [6–13]. Gauglitz et al. [6] used male rats receiving a full thickness burn over 60% of the total body surface area (TBSA), where they observed that serum concentrations of several pro- and anti-inflammatory mediators (IL-1 beta, IL-6, IL-10, MCP-1, and CINC) were significantly elevated after burn. Barber et al. [9] studied the relation between burn size (20%, 30%, 40%, and 60% TBSA) and cytokine concentrations (TNA-alpha, IL-1 beta, and IL-6) at one time point (24 h) after burn injury, and observed burn size-dependent increases. Ertel et al. [11] induced sepsis by CLP in male rats and analyzed the TNF, IL-1 and IL-6 cytokines profiles in serum within 20 hour time course after CLP. They observed a persistent elevation of plasma TNF until 10 hours, steadily increasing IL-1 plasma concentrations, and enhanced IL-6 plasma concentrations at all time points compared to the sham group.
It is noteworthy that these studies are limited to one type of insult or a limited number of cytokines. Chemokines and other inflammatory mediators including Leptin and IL18 are gaining more attention in the attempts to characterize the inflammatory responses. For example, elevated concentrations of IL-18, which is a proinflammatory cytokine involved in the regulation of cell-mediated and innate immune responses, have been reported to correlate with organ dysfunction after injury [14]. Peter et abserved that GCSF, a chemokine mainly produced by monocytes and macrophages, modified the immune system by increasing the white blood cells and decreasing the TNF-α and IFN-γ concentrations in a rat animal model receiving 30 % TBSA burn injury [15]. Leptin is also playing an important role in regulating energy metabolism. It was observed that Leptin reduced elevated tissue associated myeloperoxidase activity and microscopic damage scores in various tissues including liver, stomach, colon and kidney in rats receiving 30 % TBSA burn [16].
In this study, we used nonlethal rat models of burn injury and cecal ligation and puncture (CLP) to profile the early temporal inflammatory response by measuring 23 different cytokines and chemokines. Moreover, the binding sites of promoter regions of the cytokines and chemokines whose concentrations were significantly altered in the treatment groups were explored to identify putative transcription factors. Comparison of the timing of cytokine/chemokine concentration changes among the groups revealed mediators sensitive to type and extent of injury. Connecting the cytokine/chemokine dynamic patterns to transcription factor activation further provided insights on the regulatory mechanism of inflammation process.
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats (Charles River Labs, Wilmington, MA) weighing between 150 and 200 g were used. The animals were housed in a temperature-controlled environment (25°C) with a 12-hour light-dark cycle and provided water and standard chow ad libitum. All experimental procedures were carried out in accordance with National Research Council guidelines and approved by the Rutgers University Animal Care and Facilities Committee.
2.2 Experimental Plan
Animals were randomly divided into four groups. Two different insults were investigated: a dorsal cutaneous burn (B) to mimic trauma with no infection, and cecal ligation and puncture (CLP) to mimic infection and sepsis. Control treatments included sham burn and sham CLP. In total four different groups of animals were investigated: sham-burn, burn, sham-CLP and CLP (labeled S, B, SCLP, and CLP, respectively).
2.3 Burn Injury
A full-thickness burn on the dorsal skin corresponding to 20% of the total body surface area (TBSA) was performed as described previously [17]. This model was chosen because it has nearly 100% long-term survival, no evidence of systemic hypoperfusion, and no significant effect on the feeding pattern [18]. Rats were anesthetized by intraperitoneal injection of 80 to 100 mg/kg ketamine + 12 to 10 mg/kg xylazine. All hair removed from the dorsal abdominal area using electric clippers. The animal’s back was immersed in water at 100°C for 10 s to produce a full-thickness scald injury over 20% TBSA. Immediately after burns, the animals were resuscitated with 50 mL/kg of saline injected intraperitoneally. Sham burn controls consisted of animals treated identically but immersed in warm water (37°C). Rats were single caged after burn or sham burn and given standard rat chow and water ad libitum until sacrifice. No post-burn analgesics were administered, consistent with other studies with this full thickness burn model since the nerve endings in the skin are destroyed and the skin becomes insensate [19]. Furthermore, after the animals woke up, they ate, drank, moved freely about the cage, responded to external stimuli, and did not show clinical signs of pain or distress.
2.4 Cecum Ligation and Puncture
CLP was used as an infection model because it is thought to closely mimic the physiological changes in human sepsis [20]. Rats were anesthetized and given the analgesic buprenorphrine subcutaneously at 0.01 to 0.05 mg/kg. Animals were then placed in supine position and hair was shaved on the abdomen. Bupivicaine (0.125% to 0.25%) was applied subcutaneously around the incision site for additional perioperative and postoperative analgesia. The abdominal cavity was cut open by a 2 cm midline incision. The cecum of the rat was exposed and ligated just below the ileocecal valve so as to avoid intestinal obstruction. Suture was passed between the body of the cecum and the main artery, so that the latter was not ligated. The cecum was punctured 4 times (not through and through) with a 20 gauge needle and replaced in the peritoneum. The abdominal incision was then sutured in layers using interrupted monofilament sutures. The animal received 10 mL/kg saline intraperitoneally for resuscitation. Sham-CLP controls consisted of animals treated identically without receiving CLP, i.e. they were anesthetized, underwent laparotomy as described above, but no surgical manipulation of the cecum was performed. Rats were single caged after the treatments and given standard rat chow and water ad libitum until sacrifice.
2.5 Cytokine Analysis
Animals were anesthetized at different time points (2, 4, 8, 12, 16, 20, and 24) (n=3 per time point per group) post burn or CLP. Blood samples were immediately collected from the vena cava by heparinized catheters, kepted on ice, and then centrifuged at 4500 rpm for 3 min at 4 °C. The serum supernatant was collected and stored at −80 °C until analysis. A MILLIPLEX MAP Rat Cytokine/Chemokine Panel (Millipore) was used for the simultaneous quantification of 23 different cytokines (Eotaxin, G-CSF, GM-CSF, GRO/KC, IFN-γ, IL-10, IL-12 (p70), IL-13, IL-17, IL-18, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IP-10, Leptin, MCP-1, MIP-1α, RANTES, TNF-α, VEGF) according to the manufacturer’s guidelines.
2.6 Data Analysis
We recently developed an algorithm to estimate the area under the curve (AUC) of a variable baseline and compare it with the AUC of the response curve [21]. This method was used to discover significant net responses such as transcriptional regulatory effects in gene expression data. Given the fact that cytokine production may be altered throughout the observation period, the concept of area under the curve can be used to assess the dynamic responses and the deviation from their baseline values [22–23]. This method was selected here since it is proven to be more informative for experiments having high sampling frequencies after the experimental perturbations [21]. In this study, for each cytokine, the overall AUC (area under the cytokine concentration–time curve in treatment group, i.e. B and CLP groups) and baseline AUC (area under the cytokine concentration–time curve in control group, i.e. S group) were calculated numerically [22]. These values were then compared to determine if the overall AUC significantly deviates from the baseline AUC by identifying p-values using a t-distribution and Sattertwaite’s approximation for degrees of freedom [23]. Finally, the algorithm proposed by Benjamini and Hochberg [24] was used to determine a data-based p-value threshold controlling the false discovery rate at the level α (α=0.05 in this study).
Briefly, AUC was calculated by the trapezoidal rule using the means of replicates, X̄jk, at each time point tj [22] as follows:
| (1) |
where m and f are the total number of time points and number of groups, respectively, and njk is the total number of replicates or animals at time points tj and group k. The variance of linear AUC estimator is obtained as:
| (2) |
where σjk is the standard deviation of the concentration values at time j in group k. A test of null hypothesis of no difference between two AUCs has been presented by Heinzl [23]. The null hypothesis of equality among two AUC’s (k=1, 2) is given as:
| (3) |
Assuming t-distribution and Sattertwaite’s approximation, t value and degree of freedom, v, are calculated as follows in order to obtain p-value (tobs, v):
| (4) |
| (5) |
Then, the calculated p-values are ordered, p(1) ≤....≤ p(z), where z is total number of cytokines. Using Benjamini and Hochberg approach [24], l̂ is calculated as:
| (6) |
If l̂ exists, the first l̂ null hypotheses are rejected, implying that the cytokines corresponding to p(1) ≤....≤ p(l̂)are significantly changed.
Heat maps were generated by the “clustergram” function in MATLAB, which was used to cluster the differentially produced cytokines and chemokines (hierarchical clustering). This further enabled to compare and analyze the dynamic patterns of the inflammatory mediators.
2.7 Predicting Putative Transcription Factors
In order to identify potential transcription factors (TFs) that regulate cytokine gene expression, we explored the basic underlying assumption of comparative genomics which states that functional regions evolve in a constrained fashion and therefore at a lower rate than non-functional regions [25–26]. It implies that conserved regions in a set of orthologous sequences survive due to their special functional implications i.e. TF families associated with binding sites identified on these conserved regions are more likely to function as transcriptional regulators. Promoters of cytokine genes of rats were extracted from the Genomatix database of promoter information with a default length (500bp upstream and 100bp downstream of the transcription start site) unless an experimentally defined length has been reported [27]. Each promoter is characterized by a set of orthologous promoters from the same gene of other vertebrate species, if available (e.g. Homo sapiens, Macaca mulatta, Pan troglodytes, Mus musculus, Equus caballus, Canis lupus familiaris, and Bos Taurus). DiAlign TF [27] with default parameters (core similarity: 0.75, matrix similarity: optimal threshold for each position weight matrix suggested from MatBase [27]) was subsequently applied to identify conserved regions on promoter P and then transcription factor binding sites (TFBSs) that are enriched on corresponding conserved regions from the set of orthologous promoters with a common threshold (70% in this study). In addition, in order to increase the confidence of the predicted binding sites ModelInspector [27] was used to search for a list of cis-regulatory modules (L) from a library of functional, experimentally-verified, modules (MatBase[27]) that match on promoter P. Identified TFBSs on P are then scanned over L and only those that are present on modules in L are selected. Associated TF families with those selected TFBSs are inferred and considered as transcriptional regulators for corresponding cytokines.
3. Results and Discussion
3.1 Animal Weight Changes and Mortality
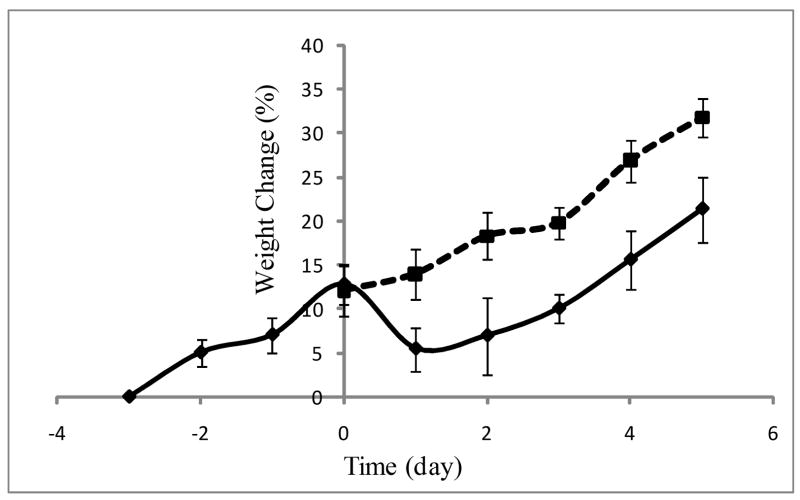
All animal groups had 100% survival for at least one week following treatment (n≥3 for each treatment). The time course of whole body weight changes was also monitored. CLP caused a ~10% weight loss, although after 24 hours, the animals started to regain weight at a rate similar to that observed pre-CLP (Figure 1). Sham-CLP did not cause any change in body weight gain rate. These observations are consistent with prior literature using a similar model [17]. Burn injury and sham-burn did not result in any significant change in the rate of body weight gain (data not shown).
Figure 1.
Body weight change (%) in CLP (solid line) and SCLP (dotted line) groups. n≥10.
CLP in rodents results in a hypermetabolic and catabolic state [17] and has been extensively used as a model of sepsis. While it is generally viewed as more realistic than endotoxin injection models, it is important to note that the response and mortality rate to CLP can vary depending on the specific procedural details (e.g. length of the cecum ligated, size of the needle, and the number of punctures) of the technique used, as well as rat strain [20, 28]. In the procedure used herein, we took care to not ligate the cecal branch of the ileocecal artery, thus preserving viability of the cecum itself, which explains our high survival rate, consistent with observations published by Banta and co workers [17]. We did not want high mortality since our purpose was to compare injury vs. infection induced systemic inflammatory responses in nonlethal and reversible conditions.
3.2 Cytokine Profiles
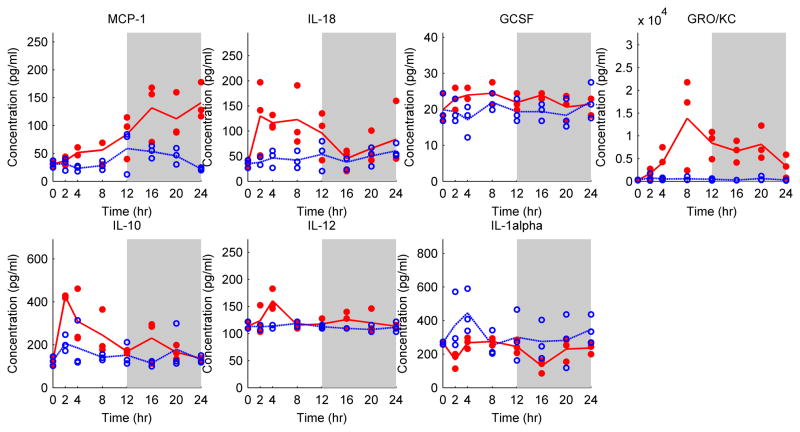
Cytokine profiles in the Sham group (which did not receive any injury or surgical manipulation) were used as reference to which the profiles obtained for all other groups were compared. Table 1 shows the cytokines which have been filtered out by the AUC method incorporated with Benjamin and Hochberg false discovery rate correction. MCP-1 (Monocyte chemotactic protein), IL-18 (Interleukin-18), GCSF (granulocyte colony-stimulating factor), GRO/KC (growth related oncogene), IL-10 (Interleukin-10), IL-12P70 (Interleukin-12 or IL-12) and IL-1α (Interleukin-1α) were significantly altered in the animals receiving a 20% TBSA burn injury. Concentrations of the chemokines GRO/KC (P=0.003), MCP-1 (P=0.0002) and GCSF (P=0.0017) - which are chemotactic for leukocytes to injured areas- were increased in the burn group. GRO/KC showed a peak around t=8 h after the burn injury whereas MCP-1 steadily increased within 24 hours postburn (Figure 2). Similarly, IL-12 (P=0.004), which can be regarded as both a pro- and an anti-inflammatory cytokine, IL-10, an anti inflammatory cytokine (P=0.004), and IL-18 (P=0.0008), a pro inflammatory cytokine, reached a maximum at t=4h. On the other hand, IL-1α (P=0.007), a pro-inflammatory cytokine, seems to be down-regulated after burn.
Table 1.
Differentially produced cytokines and chemokines. Observed P values of cytokines obtained from AUC method which are filtered out by Benjamin & Hochberg criteria are only given.
| S vs B | S vs SCLP | S vs CLP | SCLP vs CLP | Benjamin & Hochberg | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokines & Chemokines | P value (AUC) | Cytokines & Chemokines | P value (AUC) | Cytokines & Chemokines | P value (AUC) | Cytokines & Chemokines | P value (AUC) | l | pl=α.(l/z) |
| MCP-1 | 0.00023 (P ≤ pl=1) | IP-10 | 0.00012 (P ≤ pl=1) | MCP-1 | 1.00E-05 (P ≤ pl=1) | IL-12P70 | 0.0005 (P ≤ pl=1) | l=1 | 0.0022 |
| IL-18 | 0.0008 (P ≤ pl=1) | GROKC | 0.00036 (P ≤ pl=1) | GMCSF | 0.00037 (P ≤ pl=1) | IL-18 | 0.001 (P ≤ pl=1) | l=2 | 0.0044 |
| GCSF | 0.0017 (P ≤ pl=3) | MCP-1 | 0.0004 (P ≤ pl=3) | IP-10 | 0.0004 (P ≤ pl=3) | Eotaxin | 0.0014 (P ≤ pl=3) | l=3 | 0.0065 |
| GROKC | 0.003 (P ≤ pl=4) | IL-1beta | 0.0024 (P ≤ pl=4) | IL-18 | 0.0008 (P ≤ pl=4) | IL-4 | 0.009 (P ≤ pl=4) | l=4 | 0.009 |
| IL-10 | 0.004 (P ≤ pl=5) | Leptin | 0.004 (P ≤ pl=5) | IL-12P70 | 0.001 (P ≤ pl=5) | l=5 | 0.011 | ||
| IL-12P70 | 0.004 (P ≤ pl=6) | IL-17 | 0.01 (P ≤ pl=6) | Leptin | 0.0022 (P ≤ pl=6) | l=6 | 0.013 | ||
| IL-1alpha | 0.007 (P ≤ pl=7) | IFN | 0.011 (P ≤ pl=7) | GROKC | 0.012 (P ≤ pl=7) | l=7 | 0.015 | ||
| Eotaxin | 0.012 (P ≤pl=8) | l=8 | 0.017 | ||||||
Figure 2.
Cytokine and chemokine profiles in Burn (red dot) and Sham (blue circle) groups. Each circle or dot represents an independent sample, and lines pass through average cytokine profiles at each time point. The white and grey colors represent light and dark cycles respectively.
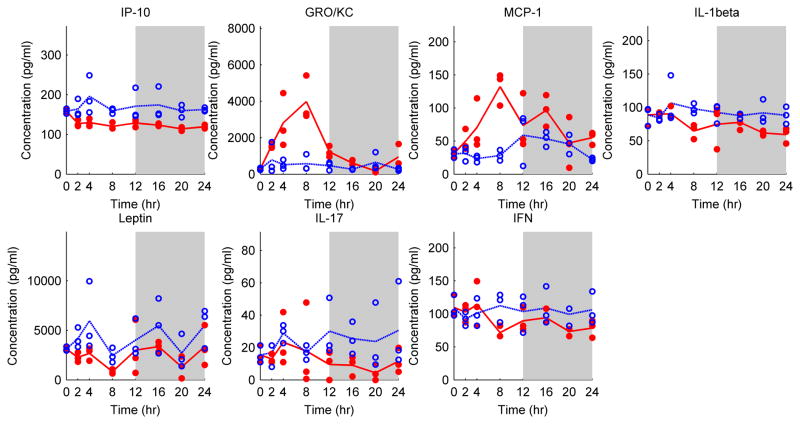
SCLP, which involves laparotomy without ligation or puncturing of the cecum, caused changes in several markers. It was observed that some of the chemokines, including IP-10 (interferon gamma induced protein) (P=0.0001), GRO/KC (P=0.0004), and MCP-1 (P=0.0004) were significantly different from the Sham group (Table 1). GRO/KC and MCP-1 peaked around t=8 h after SCLP treatment (Figure 3). On the other hand, IP-10 concentrations decreased (Figure 3). Similarly, some pro-inflammatory cytokines, including IL-1β, IL-17, and IFN-γ (P = 0.002, 0.01 and 0.01, respectively) and Leptin (P= 0.004), a hormone regulating energy intake and energy expenditure, were decreased after SCLP treatment.
Figure 3.
Cytokine and chemokine profiles in SCLP (red dot) and Sham (blue circle) groups. Each circle or dot represents an independent sample, and lines pass through average cytokine profiles at each time point. The white and grey colors represent light and dark cycles respectively.
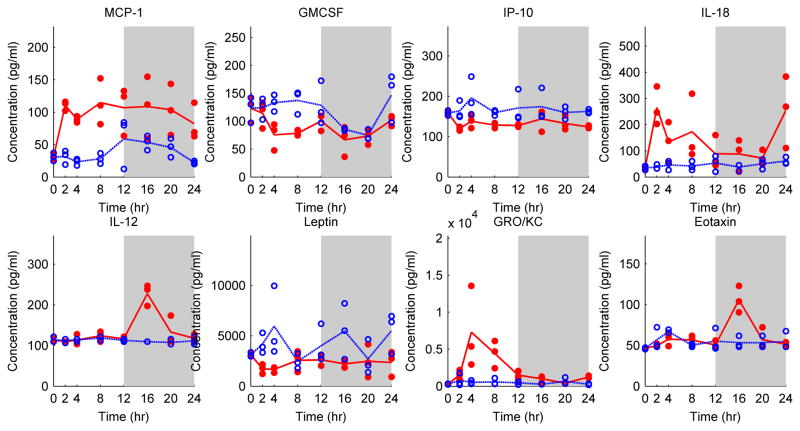
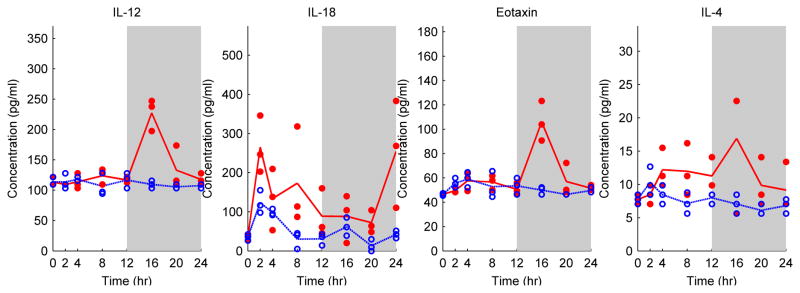
MCP-1, GMCSF, IP-10, IL-18, IL-12, Leptin, GRO/KC and Eotaxin were significantly altered by CLP treatment compared to the Sham group (Table 1 and Figure 4). IL-18, a pro inflammatory cytokine, was significantly elevated (P=0.0008) and demonstrated two peaks at around t=2 h and t=24 h. Similar to previous observations, GRO/KC peaked at 8 h post-CLP. On the other hand, IL-12 peaked later, at t= 16 h, which is also later than the observed IL-12 profile in the burn group (Figure 2). Eotaxin, a chemoattractant protein, also showed significant elevation at t=16 h (P=0.01). MCP-1 (P=0.00002) significant increased within the 24 h post-burn period. Other chemokines, namely GMCSF, IP-10, and Leptin, decreased after the CLP treatment.
Figure 4.
Cytokine and chemokine profiles in CLP (red dot) and Sham (blue circle) groups. Each circle or dot represents an independent sample, and lines pass through average cytokine profiles at each time point. The white and grey colors represent light and dark cycles respectively.
Finally we compared the CLP group to the SCLP group to identify infection-specific effects. IL-12, IL-18, Eotaxin, and IL-4 were significantly different (Table 1). IL-4, an anti-inflammatory cytokine, was significantly decreased compared to the SCLP group (P= 0.01) (Figure 5). On the other hand, IL-12, IL-18, and Eotaxin were increased in the CLP group compared to the SCLP group (as well as the Sham group).
Figure 5.
Cytokine and chemokine profiles in CLP (red dot) and SCLP (blue circle) groups. Each circle or dot represents an independent sample, and lines pass through average cytokine profiles at each time point. The white and grey colors represent light and dark cycles respectively.
When baseline values of the control group (S) were used for comparison to the injured animals (B, SCLP and CLP), we observed that GRO/KC and MCP-1 chemokine concentrations were significantly elevated in all treatment groups. Moreover, significant alterations in IP-10, Leptin and Eotaxin profiles in CLP and/or SCLP group (Figures 3 and 4) were also detected. Leptin is a hormone regulating food intake and metabolic functions, and its concentration was decreased in CLP and SCLP groups in this study. Although IL-1β and TNF-α contribute to the up-regulation of leptin production in animals, results from studies conducted in septic patients are contradictory since both up or down regulation in leptin production could be observed in the patients [29]. MCP-1 and Eotaxin are CC chemokines where the first two cysteines are adjacent to each other. They attract mononuclear cells to sites of chronic inflammation [30]. GRO/KC and IP-10 are chemokines in CXC group (the first two cysteines are separated by an amino acid) which are neutrophil and lymphocytes chemo-attractants and induce granule exocytose, fibroblast differentiation and restrain angiogenesis [30].
IL-18, a pro-inflammatory cytokine, was increased in the early phase of inflammation in burn and CLP groups in this study. It has structural similarities with IL-1 family proteins [31]. It can be expressed by different cell types including macrophages, Kupffer cells, keratinocytes and adrenal cortical cells [32]. Similar to IL-1 and TNF-α pro inflammatory cytokines, IL-18 activates similar signaling molecules [33]; thus, one can speculate that IL-18 might be functionally similar to TNF-α and IL-1β. In fact, Bohn and co-workers demonstrated that IL-18 is involved in the regulation of cytokine production during the early phase of bacterial infections and in the clearance of bacteria in mice [34]. IL-12 was another cytokine demonstrating increased concentrations in both the burn and CLP groups in this study. IL-12 plays an important role in the development of T helper (Th) 1 autoimmune responses [32]. It is produced by monocytes, macrophages, dendritic cells, neutrophils and B cells [35]. Administration of IL-12 in chimpanzees by intravenous injection induced increases in plasma concentrations of IL-15, IL-18, and IFN-γ, and a marked anti-inflammatory cytokine response (IL-10, IL-1 receptor antagonist) and secretion of different chemokines [36]. IL-10 is one of the most important anti-inflammatory cytokines which has been studied extensively to characterize the immune responses in different animal models. However, only in the burn group an increase in IL-10 concentration was detected. IL-10 is mainly synthesized by CD4+ Th2 cells, monocytes and B cells [37]. IL- 10 is regarded as modulator of the pro- inflammatory response by inhibiting production of TNF-α and IL-1β. IL-10 is also capable of decreasing the IL-18 mRNA expression in monocytes [38].
3.3 Comparison of Injury Models
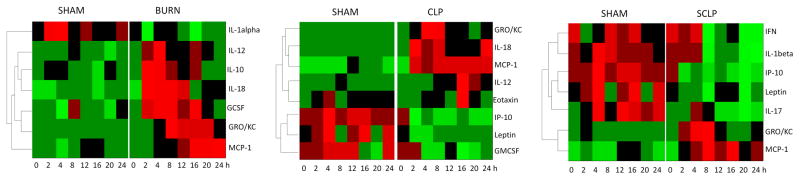
In this study three different injury models were analyzed. When these are compared, certain mediators exhibit insult-specific behavior whereas others exhibit similar response across all animal groups (Table 2). GCSF, IL-10 and IL-1alpha were only identified in the burn group, whereas GMCSF and Eotaxin were specific to CLP. IL-17, IFN and IL-1beta were only observed in Sham-CLP group which received a sterile surgical treatment. A significant number of overlapping cytokines/chemokines between CLP and other groups were identified, including IL-18, IL-12, IP-10, Leptin, MCP-1 and GROKC (Table 2). Among them, MCP-1 and GROKC are the only mediators observed in all groups.
Table 2.
Grouping the cytokines and chemokines based on their concentration changes in three injury models. Some mediators (MCP-1 and GRO/KC) are observed in more than one group whereas some are specific to an injury type.
| Cytokines/Chemokines | Sham vs Burn | Sham vs CLP | Sham vs SCLP |
|---|---|---|---|
| GCSF | √ | ||
| IL-10 | √ | ||
| IL-1A | √ | ||
| GMCSF | √ | ||
| EOTAXIN | √ | ||
| IL-17 | √ | ||
| IFN | √ | ||
| IL-1beta | √ | ||
| IL-18 | √ | √ | |
| IL-12 | √ | √ | |
| IP-10 | √ | √ | |
| LEPTIN | √ | √ | |
| MCP-1 | √ | √ | √ |
| GRO/KC | √ | √ | √ |
We further clustered the temporal profiles of the differentially produced cytokines in all three groups and represented them as heat maps (Figure 6) to elucidate and compare the dynamic patterns. IL-1alpha was down regulated in the burn group whereas GMCSF, Leptin and IP-10 concentrations decreased following the CLP treatment in a similar fashion. In SCLP group, additional cytokines and chemokines were down-regulated including IFN, IL-1beta, IP-10, Leptin and IL-17. In the burn group, IL-12, IL-10, IL-18 and GCSF were up-regulated at the early stage while GRO/KC and MCP-1 at the late stage around t=8–12 h. Similarly, early up-regulation of IL-10 concentration was also reported by Gauglitz et al. [6] whereas they showed MCP-1 concentration was increased at the early stage in the rat model receiving 60 % TBSA burn. Finnerty et al. [7] also elucidated an early up-regulation in GRO/KC concentration in mice following the 35 % TBSA burn. The chemokine/cytokine dynamic patterns in the burn group (Figure 6) were not observed in SCLP or CLP groups in this study. MCP-1 and GRO/KC concentrations were increased at the early stage and moreover MCP-1concentration showed a persistent elevation in both groups. It is noteworthy that IL-12 profile peaked early around 4 hour postburn, while it peaked much later, at 16 hours following CLP (Figure 6). Similarly, Eotaxin concentration was only increased in the CLP group and also exhibited a peak around t=16 h. However concentrations of both Eotaxin and IL-12 were up-regulated for a short period of time in CLP group.
Figure 6.
Heat maps comparing the treatment groups (Burn, CLP and SCLP) with the control group (Sham). Green indicates the lowest level while red indicates the highest and black average level.
In this study, we did not observe any significant change in the concentration of TNF-α, IL-1 β and IL-6, in either burn or CLP groups. Similarly, Murphy et al. [39] observed no significant differences between sham and burn (25 % TBSA) mice in the plasma concentrations of TNF-α, IL-6 and IL-10 after the injury. In fact, conflicting observations regarding the cytokine expressions in animal models have been extensively reported in literature. It is obvious that variations in experimental procedures, and size and severity of injuries as well as utilizing different species might result in different consequences in cytokine profiles. Barber and co-workers [9] observed that cytokine concentrations significantly increased when burn size increased in rats. Walley et al. [10] used mice and three needle sizes (18 GA, 21 GA and 26 GA) for CLP treatments. They observed that as the diameter of the CLP needle decreased, the TNF-α and IL-6 concentrations decreased and IL-10 concentration increased. Klein and co-workers [12] used rats for 30% TBSA burn, and observed that IL-1 beta and TNFalpha increased after burn. On the other hand, Gauglitz and co-workers [6] utilized rats receiving full thickness burn of 60% TBSA. They observed that serum concentrations of TNF-α were not found to be significantly different although other cytokines or chemokines including IL-10, MCP-1 and GRO (CINC-1) were significantly elevated after burn. We also observed significant elevations in IL-10, MCP-1 and GRO concentrations in rats receiving 20 % TBSA burn injury. Conflicting observations were also reported in the studies conducted in burn or septic patients or human subjects. Interestingly in some studies, a transient increase in TNF-α concentration was observed in burn patients [40]. It has been also shown that IL-1β was not detected in the plasma of burn patients [41] although its plasma concentration generally increases after burn injury with septic shocks in human or it is positively correlated with burn size [42].
3.4 Putative Transcription Factors
By searching the conserved regions of promoters, we identified putative transcription factors (TFs) of the significantly altered cytokines that might participate in the regulation of their corresponding genes (Table 3). It is well known that many signaling pathways including MAPK, Jac/STAT, and Ik-B/NF-kβ cascades where NF-kβ, Stat, and C/EBP-β transcription factors play key roles are activated in various tissues in the early phase of burn and septic shocks [12, 43–45]. NF-kβ, Stat, and C/EBP-β are well known regulators involved in cytokine production. These were also identified in our analysis (Table 3) and were found to be associated with GRO/KC, MCP-1, Eotaxin and IL-10. It is also remarkable to say that cytokines or chemokines produced by these TFs eventually trigger the signaling pathways activating the similar TFs. IL-18, IL-12 and most of the chemokines activate JAK/STAT and MAPK signaling pathways [46]. IL-10 (which was significantly elevated in the burn group in our study) induces STAT activation which promotes the transcription of Suppressor of Cytokine Signaling 3 (SOCS3), a negative feedback regulator inhibiting many inflammatory cytokines such as TNF-α, IL-6, and IL-1. There are also other putative TFs identified (Table 3) such as ETS, SP1, GATA and VTBP which have been known to regulate the function of immune system and play important role in the inflammation. ETS transcription factors activate immunity-related genes including promoters of IL-12 and IL-18 [47].
Table 3.
Putative transcription factors of some cytokines and chemokines observed in burn and CLP groups.
| Transcription factors | GRO/KC | MCP-1 | Eotaxin | Leptin | IL-18 | lL-12 | IL-10 |
|---|---|---|---|---|---|---|---|
| ETSF (Human and murine ETS1 factor- E-twenty six family) | √ | √ | √ | √ | |||
| MYOD (Myoblast determining factors) | √ | √ | |||||
| NFKB (Nuclear factor kappa B/c-rel) | √ | √ | |||||
| PBXC (PBX1 - MEIS1 complexes) | √ | ||||||
| SORY (SOX/SRY-sex/testis determining and related HMG box factors) | √ | ||||||
| STAT (Signal Transducer and Activator of Transcription) | √ | √ | √ | ||||
| PARF (PAR/bZIP family) | √ | ||||||
| CEBP (CCAAT-enhancer-binding proteins) | √ | √ | |||||
| SP1F (GC-Box factors SP1/GC) | √ | √ | √ | ||||
| EREF (Estrogen Response Element family) | |||||||
| VTBP (TATA binding protein factor) | √ | √ | √ | ||||
| BRNF (Brn POU domain factors) | √ | ||||||
| CAAT (CCAAT binding factors) | √ | ||||||
| CREB (cAMP response element-binding) | √ | ||||||
| FKHD (Fork head domain factors) | √ | ||||||
| HNF1 (Hepatocyte nuclear factor 1) | √ | ||||||
| NF1F (Nuclear Factor 1) | √ | ||||||
| OCT1(Octamer-binding transcription factor 1) | √ | ||||||
| GATA (GATA binding factors) | √ | √ | |||||
| HAND (Twist subfamily of class B bHLH transcription factors) | √ | ||||||
| KLFS (Krupple like factor family) | √ |
Note: These are transcription factor families, each of which includes several TFs that have similar binding sites.
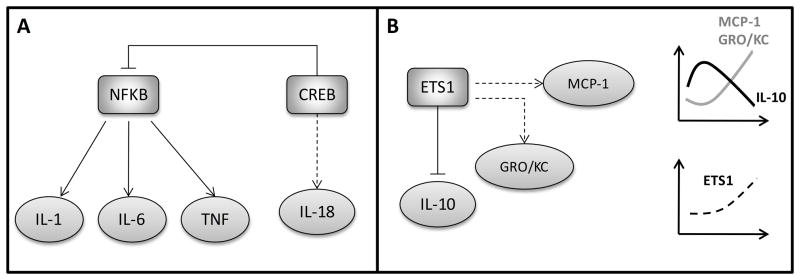
Although more complicated experimental analysis is required to identify the functional transcription factors regulating cytokine expression levels, bioinformatics analysis like the one presented in this work provides insights on the signaling system and identifies possible targets for the experimental researches. It is well known that NF-kβ regulates the expression of a number of cytokines and chemokines including IL-1, TNF and IL-6 [48]. It is also known that the transcription factor CREB might show an anti-inflammatory behavior by inhibiting NF-kβ signaling [49]. We identified that CREB is a putative TF of IL-18 whose concentration was elevated in both burn and CLP groups. On the other hand, IL-1, TNF and IL-6, mainly regulated by NF- kβ, were not identified in burn and CLP groups in this study. Another interesting finding is that ETS1 factors might have potential to regulate the MCP-1 and GRO/KC chemokines which had similar temporal profiles. It has also been reported in literature that inhibition of ETS1 up-regulates IL-10 concentration [50]. IL-10 was significantly elevated at the early phase of the inflammatory response in the burn group. However, MCP-1 and GRO/KC concentrations were elevated when IL-10 concentration decreased in the burn group (see Figure 6). On the other hand, in CLP and SCLP groups, we did not observe significant changes in IL-10 concentration, but GRO/KC and MCP-1 were increased at the early stage and more persistent elevated MCP-1 was observed in CLP and SCLP groups when compared to the burn group (Figure 6). Due to the high interdependency of physiological processes, integrating multiple sources together with the bioinformatics tools in attempt to answer the question to what extend transcription factor activation affects the circulatory inflammatory mediators provide an overview of the global regulation. This is also crucial to elucidate important regulatory points that can be used to modulate the inflammatory state at molecular level.
It is interesting that there is a certain number of putative TFs that might regulate the same cytokine (Table 3). Similarly, a TF might also regulate more than one cytokines. It should be noted that the tissue specificity was not considered in this study to determine the binding sites in the promoters. The sequence of the promoter regions of the cytokine genes was only analyzed to determine the putative TF binding sites. Although a set of TFs has potential to regulate the same cytokine, the question is whether, and how, this affects cytokine expression patterns in different injury models. It should be kept in mind that peripherally circulating cytokines are secreted by various tissues or cells including leukocytes, glial cells and liver, etc. It is well known that inflammatory response of a tissue might be different than that of another tissue, and similarly the host response depends on type of the injury [51]. Temporal and quantitative differences in the activation of TFs in various injury models explain the diversity of cytokines’ patterns.
Complex interactions of cytokines or chemokines through the signaling network is a big challenge for studies aiming at determining new therapeutic targets to eliminate deleterious effects of chemokine or cytokine related disease states. Inhibition of certain mediator receptor interactions might be required in order to eliminate the negative effects of disorders caused by inappropriate mediator receptor regulations. However, this might lead to activation or deactivation of undesired upstream signaling pathways. For example, using inhibitors or animal models deficient in certain chemokines-receptors including CXCR2 or CCR1 demonstrated improvement in sepsis formation and sepsis related lethality in animals [52–53]. On the other hand, inhibiting chemokine receptors might also weaken antibacterial resistance of host by up-regulating the IL-10 [54]. Consequently, a comprehensive understanding of underlying mechanisms of the inflammatory response is essential to have a more control over the proposed therapeutic approaches.
The mammalian regulatory landscape is highly complex and redundant conferring highly robust characteristics to the host [55–56]. Although very preliminary, the results of our TF analysis begin to point towards the direction of elucidating the structure and networks of transcription factors as these emerge as major contributors to regulation [57]. The idea of inferring functional interactions by observing the dynamics of regulated signals has been previously explored in the context of liver-specific responses to soluble signals [58], whereas we recently demonstrated how the regulated dynamics can begin to elucidate implicit upstream interactions among transcription factors [59–60]. In this context the information generated through our preliminary analyses enables the initial formulation of putative injury-specific modules of regulatory interactions (Figure 7). Undoubtedly, a lot of work still remains to be accomplished, but we wish to demonstrate that it is indeed plausible to integrate in vivo responses and bioinformatics tools towards the ultimate goal of describing testable hypotheses related to critical regulatory interactions.
Figure 7.
A. The regulatory mechanism between NF-kβ and CREB. In burn and CLP groups, only IL-18 of which CREB is a putative TF was elevated, however no changes in IL-1, IL-6 and TNF concentrations were observed in both treatment groups. B. The regulatory mechanism of ETS1. ETS1 was identified as a putative TF of MCP-1 and GRO/KC. In burn group, these two cytokines’ concentrations were increased while IL-10 concentration was decreased. Note that straight lines indicate known regulatory mechanisms (based on literature) and dashed lines represent putative mechanisms estimated from this study based on experimental observations and TF analysis.
4. Concluding Remarks
In this study, we characterized the circulating cytokine profiles in the first 24 hours following 20% TBSA burn injury, SCLP, and CLP. Burn injury alone was used to investigate the effect of a systemic but “sterile” pro-inflammatory insult. CLP was used to investigate the effect of a bacterial infection, also a pro-inflammatory, but different, stimulus. Since CLP involves laparotomy, a surgical procedure, it was also necessary to include a SCLP group to ascertain the effect of the surgical procedure vs. that of the infection itself. While none of the insults caused mortality, CLP caused a transient decrease in body weight. We found that a certain number of cytokines and chemokines, belonging to the pro- and anti- inflammatory types, were altered by these insults. Furthermore, each insult was associated with a different cytokine signature profile.
Experimental observations can be very noisy. Problems can always arise in multiplex assays because of potential interactions between different antibodies and cytokines, and the presence of varying dynamic mutual-effects between rare and abundant cytokines in the samples [61]. The limited number of replicates in animal experiments can be also an additional complicating factor. In serial sacrifice designs where only one sample is taken from each animal, significant differences in the physiologic concentrations of circulatory proteins between the samples can be observed. Moreover, cyclic fluctuations (or circadian rhythm) in cytokine expressions, which affects the inflammatory response of the body [62], can display 24 h period and even persist in the absence of external timing information [63]. Therefore, due to the dynamic behaviors and fluctuations observed in physiological processes, it was important to utilize an appropriate statistical approach to identify differentially expressed proteins over a time course. Although existing statistical methods such as ANOVA test are more appropriate for static sampling designs, these might not be applicable for time course experiments where detecting differences in the physiological behaviors of two or more groups over time is essential [64]. The AUC is considered as an important indicator for drug availability and assessing the net pharmacological response of a given dose of drug [22–23]. We recently explored this method to analyze gene expression time course data [21]. Similarly, the same concept can be applied for temporal experiments aiming at identifying differentially expressed proteins. This provides a quantitative estimate of overall exposure to cytokines which can be obtained by integrating the concentration curve over time. This also considers the temporal variability in the cytokine concentrations of control groups.
In conclusion, we analyzed the early stage (first 24 hours) serum cytokine and chemokine profiles in moderately injured rats receiving 20 % TBSA burn injury and CLP treatment. We identified cytokines and chemokines which were significantly elevated in the burn and CLP groups. Determining cytokine expression patterns in different animal models receiving different injuries would provide critical insights regarding the complex interactions of those inflammatory mediators at the cellular level enabling a better characterization of the inflammatory response.
Acknowledgments
The authors gratefully acknowledge the financial support from NIH grant GM082974.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 2.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19:777–83. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 3.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. A Study of Cytokines in Burn Blister Fluid Related to Wound-Healing. Burns. 1995;21:352–5. doi: 10.1016/0305-4179(95)00005-4. [DOI] [PubMed] [Google Scholar]
- 5.Correa SG, Maccioni M, Rivero VE, Iribarren P, Sotomayor CE, Riera CM. Cytokines and the immune-neuroendocrine network: What did we learn from infection and autoimmunity? Cytokine Growth Factor Rev. 2007;18:125–34. doi: 10.1016/j.cytogfr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning D, Barral JM, et al. Characterization of the Inflammatory Response During Acute and Post-Acute Phases after Severe Burn. Shock. 2008;30:503–7. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finnerty CC, Przkora R, Herndon DN, Jeschke MG. Cytokine expression profile over time in burned mice. Cytokine. 2009;45:20–5. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kataranovski M, Magic Z, Pejnovic N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res. 1999;48:473–82. [PubMed] [Google Scholar]
- 9.Barber RC, Maass DL, White DJ, Horton JW. Increasing percent burn is correlated with increasing inflammation in an adult rodent model. Shock. 2008;30:388–93. doi: 10.1097/SHK.0b013e318164f1cd. [DOI] [PubMed] [Google Scholar]
- 10.Walley ER, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–8. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The Complex Pattern of Cytokines in Sepsis - Association between Prostaglandins, Cachectin, and Interleukins. Ann Surg. 1991;214:141–8. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein D, Einspanier R, Bolder U, Jeschke MG. Differences in the hepatic signal transcription pathway and cytokine expression between thermal injury and sepsis. Shock. 2003;20:536–43. doi: 10.1097/01.shk.0000093345.68755.98. [DOI] [PubMed] [Google Scholar]
- 13.Shelley O, Murphy T, Paterson H, Mannick JA, Lederer JA. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–9. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 14.Stassen NA, Breit CM, Norfleet LA, Polk HC. IL-18 promoter polymorphisms correlate with the development of post-injury sepsis. Surgery. 2003;134:351–6. doi: 10.1067/msy.2003.248. [DOI] [PubMed] [Google Scholar]
- 15.Peter FW, Schuschke DA, Barker JH, Fleishcher-Peter B, Pierangeli S, Vogt PM, et al. The effect of severe burn injury on proinflammatory cytokines and leukocyte behavior: its modulation with granulocyte colony-stimulating factor. Burns. 1999;25:477–86. doi: 10.1016/s0305-4179(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 16.Çakir B, Çevik H, Contuk G, Ercan F, Eksioglu-Demiralp E, Yegen BÇ. Leptin ameliorates burn-induced multiple organ damage and modulates postburn immune response in rats. Regulatory Peptides. 2005;125:135–44. doi: 10.1016/j.regpep.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Banta S, Vemula M, Yokoyama T, Jayaraman A, Berthiaume F, Yarmush ML. Contribution of gene expression to metabolic fluxes in hypermetabolic livers induced through burn injury and cecal ligation and puncture in rats. Biotechnol Bioeng. 2007;97:118–37. doi: 10.1002/bit.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herndon DN, Wilmore DW, Mason JAD. Development and analysis of a small animal model simulating the human postburn hypermetabolic response. Journal of Surgical Research. 1978;25:394–403. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 19.Valenti LM, Mathieu J, Chancerelle Y, De Sousa M, Levacher M, Tuan Dinh-Xuan A, et al. High levels of endogenous nitric oxide produced after burn injury in rats arrest activated T lymphocytes in the first G1 phase of the cell cycle and then induce their apoptosis. Experimental Cell Research. 2005;306:150–67. doi: 10.1016/j.yexcr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4:31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheff J, Almon R, DuBois D, Jusko W, Androulakis I. Assessment of Pharmacologic Area Under the Curve When Baselines are Variable. Pharmaceutical Research. 2011:1–9. doi: 10.1007/s11095-010-0363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfsegger M. Establishing Bioequivalence in Serial Sacrifice Designs. Journal of Pharmacokinetics and Pharmacodynamics. 2007;34:103–13. doi: 10.1007/s10928-006-9037-x. [DOI] [PubMed] [Google Scholar]
- 23.Heinzl H. A note on testing areas under the curve when using destructive measurement techniques. Journal of Pharmacokinetics and Pharmacodynamics. 1996;24:651–5. doi: 10.1007/BF02353485. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate- a practical and powerful approach to multiple testing. J R Stat Soc Ser B-Methodol. 1995;57:289–300. [Google Scholar]
- 25.Doniger SW, Huh J, Fay JC. Identification of functional transcription factor binding sites using closely related Saccharomyces species. Genome Res. 2005;15:701–9. doi: 10.1101/gr.3578205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardison RC. Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet. 2000;16:369–72. doi: 10.1016/s0168-9525(00)02081-3. [DOI] [PubMed] [Google Scholar]
- 27.Genomatix. http://www.genomatix.de.
- 28.Otero-Anton E, Gonzalez-Quintela A, Lopez-Soto A, Lopez-Ben S, Llovo J, Perez LF. Cecal ligation and puncture as a model of sepsis in the rat: Influence of the puncture size on mortality, bacteremia, endotoxemia and tumor necrosis factor alpha levels. Eur Surg Res. 2001;33:77–9. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 29.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 30.Charo IF, Ransohoff RM. Mechanisms of disease - The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 31.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127:117–26. doi: 10.1067/msy.2000.101584. [DOI] [PubMed] [Google Scholar]
- 33.Thomassen E, Bird TA, Renshaw BR, Kennedy MK, Sims JE. Binding of interleukin-18 to the interleukin-1 receptor homologous receptor IL-1Rrp1 leads to activation of signaling pathways similar to those used by interleukin-1. J Interferon Cytokine Res. 1998;18:1077–88. doi: 10.1089/jir.1998.18.1077. [DOI] [PubMed] [Google Scholar]
- 34.Bohn E, Sing A, Zumbihl R, Bielfeldt C, Okamura H, Kurimoto M, et al. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 35.Paunovic V, Carroll HP, Vandenbroeck K, Gadina M. Signalling, inflammation and arthritis - Crossed signals: the role of interleukin (IL)-12,-17,-23 and-27 in autoimmunity. Rheumatology. 2008;47:771–6. doi: 10.1093/rheumatology/kem352. [DOI] [PubMed] [Google Scholar]
- 36.Lauw FN, Dekkers PEP, te Velde AA, Speelman P, Levi M, Kurimoto M, et al. Interleukin-12 induces sustained activation of multiple host inflammatory mediator systems in chimpanzees. J Infect Dis. 1999;179:646–52. doi: 10.1086/314636. [DOI] [PubMed] [Google Scholar]
- 37.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 38.Marshall JD, Aste-Amezaga M, Chehimi SS, Murphy M, Olsen H, Trinchieri G. Regulation of human IL-18 mRNA expression. Clin Immunol. 1999;90:15–21. doi: 10.1006/clim.1998.4633. [DOI] [PubMed] [Google Scholar]
- 39.Murphy TJ, Paterson HM, Kriynovich S, Zang Y, Kurt-Jones EA, Mannick JA, et al. Linking the “two-hit” response following injury to enhanced TLR4 reactivity. J Leukoc Biol. 2005;77:16–23. doi: 10.1189/jlb.0704382. [DOI] [PubMed] [Google Scholar]
- 40.Vindenes HA, Ulvestad E, Bjerknes R. Concentrations of cytokines in plasma of patients with large burns: Their relation to time after injury, burn size, inflammatory variables, infection, and outcome. Eur J Surg. 1998;164:647–56. doi: 10.1080/110241598750005525. [DOI] [PubMed] [Google Scholar]
- 41.Debandt JP, Cholletmartin S, Hernvann A, Lioret N, Duroure LD, Lim SK, et al. Cytokine Response to Burn Injury - Relationship with Protein-Metabolism. J Trauma-Injury Infect Crit Care. 1994;36:624–8. doi: 10.1097/00005373-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Drost AC, Burleson DG, Cioffi WG, Mason AD, Pruitt BA. Plasma Cytokines after Thermal-Injury and Their Relationship to Infection. Ann Surg. 1993;218:74–8. doi: 10.1097/00000658-199307000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho K, Adamson LK, Jeong J, Crivello SD, Vanhook TG, Palmieri T, et al. CD14-dependent alterations in c-Jun expression in the liver after burn injury. J Surg Res. 2004;122:36–42. doi: 10.1016/j.jss.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Ogle CK, Kong FS, Guo XL, Wells DA, Aosasa S, Noel G, et al. The effect of burn injury on suppressors of cytokine signalling. Shock. 2000;14:392–8. [PubMed] [Google Scholar]
- 45.Andrejko KM, Chen JD, Deutschman CS. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am J Physiol-Gastroint Liver Physiol. 1998;275:G1423–G9. doi: 10.1152/ajpgi.1998.275.6.G1423. [DOI] [PubMed] [Google Scholar]
- 46.Soriano SF, Serrano A, Hernanz-Falcon P, de Ana AM, Monterrubio M, Martinez C, et al. Chemokines integrate JAK/STAT and G-protein pathways during chemotaxis and calcium flux responses. European Journal of Immunology. 2003;33:1328–33. doi: 10.1002/eji.200323897. [DOI] [PubMed] [Google Scholar]
- 47.Gallant S, Gilkeson G. ETS transcription factors and regulation of immunity. Archivum Immunologiae et Therapiae Experimentalis. 2006;54:149–63. doi: 10.1007/s00005-006-0017-z. [DOI] [PubMed] [Google Scholar]
- 48.Pahl HL. Activators and target genes of Rel/NF-kappa B transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 49.Wen AY, Sakamoto KM, Miller LS. The Role of the Transcription Factor CREB in Immune Function. J Immunol. 2010;185:6413–9. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell L, Garrett-Sinha LA. Transcription factor Ets-1 in cytokine and chemokine gene regulation. Cytokine. 2010;51:217–26. doi: 10.1016/j.cyto.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Feezor RJ, Cheng A, Paddock HN, Baker HV, Moldawer LL. Functional Genomics and Gene Expression Profiling in Sepsis: Beyond Class Prediction. Clinical Infectious Diseases. 2005;41:S427–S35. doi: 10.1086/431993. [DOI] [PubMed] [Google Scholar]
- 52.Ness TL, Carpenter KJ, Ewing JL, Gerard CJ, Hogaboam CM, Kunkel SL. CCR1 and CC chemokine ligand 5 interactions exacerbate innate immune responses during sepsis. J Immunol. 2004;173:6938–48. doi: 10.4049/jimmunol.173.11.6938. [DOI] [PubMed] [Google Scholar]
- 53.Ness TL, Hogaboam CM, Strieter RM, Kunkel SL. Immunomodulatory role of CXCR2 during experimental septic peritonitis. J Immunol. 2003;171:3775–84. doi: 10.4049/jimmunol.171.7.3775. [DOI] [PubMed] [Google Scholar]
- 54.Feterowski C, Mack M, Weighardt H, Bartsch B, Kaiser-Moore S, Holzmann B. CC chemokine receptor 2 regulates leukocyte recruitment and IL-10 production during acute polymicrobial sepsis. Eur J Immunol. 2004;34:3664–73. doi: 10.1002/eji.200425294. [DOI] [PubMed] [Google Scholar]
- 55.Tan K, Tegner J, Ravasi T. Integrated approaches to uncovering transcription regulatory networks in mammalian cells. Genomics. 2008;91:219–31. doi: 10.1016/j.ygeno.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 57.Kyrmizi I, Hatzis P, Katrakili N, Tronche F, Gonzalez FJ, Talianidis I. Plasticity and expanding complexity of the hepatic transcription factor network during liver development. Genes Dev. 2006;20:2293–305. doi: 10.1101/gad.390906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King KR, Wang S, Irimia D, Jayaraman A, Toner M, Yarmush ML. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang E, Foteinou PT, King KR, Yarmush ML, Androulakis IP. A novel non-overlapping bi-clustering algorithm for network generation using living cell array data. Bioinformatics. 2007;23:2306–13. doi: 10.1093/bioinformatics/btm335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang E, Yarmush ML, Androulakis IP. Transcription factor network reconstruction using the living cell array. J Theor Biol. 2009;256:393–407. doi: 10.1016/j.jtbi.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou X, Fragala MS, McElhaney JE, Kuchel GA. Conceptual and methodological issues relevant to cytokine and inflammatory marker measurements in clinical research. Curr Opin Clin Nutr Metab Care. 2010;13:541–7. doi: 10.1097/MCO.0b013e32833cf3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holzheimer RG, Curley P, Saporoschetz IB, Doherty JM, Mannick JA, Rodrick ML. Circadian Rhythm of Cytokine Secretion Following Thermal Injury in Mice: Implications for Burn and Trauma Research. Shock. 2002;17:527–9. doi: 10.1097/00024382-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–12. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Storey JD, Xiao WZ, Leek JT, Tompkins RG, Davis RW. Significance analysis of time course microarray experiments. Proc Natl Acad Sci U S A. 2005;102:12837–42. doi: 10.1073/pnas.0504609102. [DOI] [PMC free article] [PubMed] [Google Scholar]