Abstract
Objective
Cytomegalovirus (CMV) has been implicated in cardiovascular disease, possibly through the induction of inflammatory processes. P-selectin and L-selectin are adhesion molecules that mediate early microvascular responses to inflammatory stimuli. This study examined the role of these selectins in the microvascular dysfunction that occurs during persistent CMV infection.
Methods
C57Bl/6, P- or L-selectin-deficient mice were mock-inoculated or infected with murine CMV (mCMV), and 5 wk later placed on normal diet (ND) or high cholesterol diet (HC) for 6 wk. P-selectin expression was measured, or intravital microscopy was performed to determine arteriolar vasodilation, and venular blood cell recruitment.
Results
P-selectin expression was significantly increased in the heart, lung and spleen of mCMV-ND, but not mCMV-HC C57Bl/6. mCMV-ND and mCMV-HC exhibited impaired arteriolar function, which was reversed by treatment with an anti-P-selectin antibody, but not L-selectin deficiency. mCMV-HC also showed elevated leukocyte and platelet recruitment. P-selectin inhibition abrogated, whereas L-selectin deficiency partially reduced, these responses.
Conclusions
We provide the first evidence for P-selectin upregulation by persistent mCMV infection, and implicate this adhesion molecule in the associated arteriolar dysfunction. P-selectin, and to a lesser extent L-selectin, mediates the leukocyte and platelet recruitment induced by CMV infection combined with hypercholesterolemia.
Keywords: Cytomegalovirus infection, cardiovascular disease, P-selectin, microvasculature, arteriolar vasodilation
Introduction
In recent years pathogens have been implicated in the pathogenesis of several diseases, including cardiovascular disease (CVD) [14,16], inflammatory bowel disease, and cancer [34]. One such pathogen is cytomegalovirus (CMV), which is a β-herpesvirus that infects a majority of the world’s population, mostly during childhood. This virus activates inflammatory pathways as part of its survival strategy therefore it has been proposed that this feature may be how it contributes to other diseases, for example CVD [3,6,17,21,34]. In particular, it has been shown that primary CMV infection of isolated vascular endothelial cells can increase oxidative stress [36,41,44], upregulate endothelial adhesion molecules [2,5,11,31,33,35], and promote leukocyte [2,22,35] and platelet adhesion [30]. Such responses are characteristic of the impaired vasodilation responses and inflammatory and thrombogenic phenotype observed in microvascular and macrovascular beds exposed to cardiovascular risk factors such as hypercholesterolemia [7,19,23,27,38-40,42]. However, CVD develops over decades, primarily during the persistent phase of CMV infection and less is known about whether persistent CMV infection activates similar mechanisms in vivo. In healthy individuals and diabetics, CMV seropositivity has been associated with impaired arterial vasodilation [20], and we have recently described a murine model of CMV-induced arteriolar dysfunction [26]. Furthermore, there were mild transient venular inflammatory and platelet recruitment responses that were exacerbated by the presence of another cardiovascular risk factor, hypercholesterolemia. To date, the mechanisms underlying this CMV-induced microvascular dysfunction remain unclear.
One of the key adhesion molecules implicated in CVD as well as in the early microvascular responses to different cardiovascular risk factors is P-selectin. It is primarily located in the Wiebel-Palade bodies of vascular endothelium and in α-granules in platelets, and moves to the respective cell surfaces upon cell activation [25]. P-selectin on endothelial cells supports leukocyte tethering and rolling via interaction with PSGL-1 on the leukocytes [45]. In addition, L-selectin, which is expressed on the surface of leukocytes can support secondary capture and therefore subsequent rolling in inflamed venules [37]. These capture and rolling steps are critical in the leukocyte recruitment cascade. Platelet P-selectin is known to also bind PSGL-1 on leukocytes and the resulting signaling may lead to leukocyte activation, and ultimately recruitment of both cell types to the vessel wall. In terms of cardiovascular disease, both endothelial and platelet P-selectin have been implicated in atherosclerotic plaque development [4,24], and its expression is upregulated in aortas early before lesion development [32]. In addition, secondary capture, an L-selectin-dependent event, mediates approximately one quarter of leukocyte rolling over atherosclerotic plaques [15] and L-selectin has been implicated in monocyte and lymphocyte recruitment to atherosclerosis-prone aortas [1,18]. P-selectin has also been shown to play a role in the microvascular responses to hypercholesterolemia long before clinical evidence of disease is observed in large arteries, in that P-selectin mediates leukocyte and platelet recruitment in postcapillary venules, as well as the impaired endothelium-dependent vasodilation in arterioles [39,42]. In fact in closely paired arterioles and venules, the P-selectin-mediated venular events contribute to arteriolar dysfunction [27]. Less is known about the role of L-selectin in microvascular responses to hypercholesterolemia, although it has been shown to support leukocyte recruitment to postcapillary venules in response to oxidized low density lipoprotein [28]. Whether P-selectin or L-selectin can mediate CMV-induced arteriolar dysfunction in the absence of concurrent venular blood cell recruitment, or a role for these selectins is only present when hypercholesterolemia accompanies CMV infection remains to be elucidated.
Little is known about selectins during CMV infection. During primary infection of human pulmonary artery endothelial cells, human CMV (HCMV) promotes platelet adhesion [30] and the supernatant from infected endothelial cells led to platelet P-selectin expression and shedding, although a role for P-selectin in the platelet-endothelial interactions was not tested. Endothelial P-selectin appeared to be downregulated but not solubilized. These findings are of particular importance in the context of primary infection or perhaps reactivation of the virus, however it is unclear whether persistent CMV infection can upregulate P-selectin in different organs in vivo, or whether this adhesion molecule actively participates in the associated microvascular dysfunction. Information about L-selectin in CMV infection is primarily focused on its use as a marker for the immune response to the virus [10], and its role in the vascular responses to CMV has yet to be elucidated.
Cardiovascular patients typically present with “traditional” risk factors such as hypercholesterolemia, and a majority of these are CMV-positive. In our previous studies, we used a high cholesterol diet containing cholate to aid with reabsorption of cholesterol, and were able to show that CMV synergized with hypercholesterolemia in the generation of microvascular dysfunction, in particular blood cell recruitment [26]. In this study we first redefined our model to exclude cholate from our high cholesterol diet in order to avoid any potential direct effect of this dietary component on microvascular responses. Then we assessed the impact of CMV alone, or in combination with hypercholesterolemia on P-selectin expression in organs of mice infected with murine CMV (mCMV). Next, using a blocking antibody against P-selectin, we tested the functional role of P-selectin in the microvascular responses to mCMV only or mCMV plus elevated cholesterol levels. Lastly, we addressed the role of L-selectin in these responses by monitoring the microvasculature in infected L-selectin-deficient mice.
Materials and Methods
Animals
Wild-type C57BL/6J (WT) and B6.129S7-Selptm1Bay/J (P-sel−/−) mice (on a C57BL/6J background) were obtained from Jackson Laboratories, Bar Harbor, ME. L-selectin-deficient (L-sel−/−) mice were obtained from Dr. Thomas Tedder (Duke University) [43] and bred in-house. Mice (3-5 wk old) were injected with mock inoculum or 3×104 plaque forming units (PFU) of mCMV (Smith strain; prepared as previously described [26]). 5 wks later, mice were divided into those maintained on normal diet (ND) or mice placed on HC (Teklad 94059 containing 1.25% cholesterol, 15.8% fat, Harlan Teklad, Madison, WI). Dual-radiolabelled antibody or intravital microscopy experiments were performed on WT mice at 11 wks post-infection (p.i.). Separate groups of mCMV-infected WT mice received anti-mouse P-selectin antibody (clone RB40.34) (P-sel Ab) or isotype-matched (IgG1λ) control antibody (P-sel iso Ab) IP, 24 h prior to observation of the microvasculature. n=4-11 for all groups.
Surgical Protocol
At the time of experimentation, mice were anesthetized with ketamine hydrochloride (150 mg/kg body weight, IP) and xylazine (7.5 mg/kg body weight, IP). Core body temperature was maintained at 35±0.5°C. Animal handling procedures were approved by the LSU Health Sciences Center Institutional Animal Care and Use Committee and were in accordance with the guidelines of the American Physiological Society.
Dual-Radiolabelled Antibody Technique (DRL)
This method was used to measure the relative expression of P-selectin in vivo in WT Mock-ND, Mock-HC, mCMV-ND and mCMV-HC groups. Calculations of P-selectin expression were based on the relative accumulation (ng mAb/g tissue), in any regional vascular bed, of a binding monoclonal antibody (mAb) to P-selectin (clone RB40.34) and an isotype-matched non-binding Ab (which compensates for non-specific accumulation of the binding Ab) (BD Pharmingen, San Diego, CA), as previously described [13]. Organs were harvested, weighed and radioactivity was measured on a gamma counter. Receptor levels in the various vascular beds were expressed as ng mAb/g tissue.
Intravital Microscopy
Intravital microscopy was used to measure leukocyte recruitment and adhesion of exogenous platelets in postcapillary venules, as well as arteriolar vasodilation responses to acetylcholine (endothelium-dependent) and papaverine (endothelium-independent), as previously described [26,39]. Rolling leukocytes were defined as leukocytes moving at a velocity lower than the red blood cell velocity. These were identified as visible moving leukocytes interacting with the vessel wall. Rolling flux was calculated as the # leukocytes rolling past a specific point of the venular segment over the 1 min observation period. Leukocyte rolling velocity was derived from the average velocity of up to 10 leukocytes visibly rolling along the entire 100 μm segment under observation, and was expressed as μm/sec. A leukocyte was considered adherent if it remained stationary for ≥30 s (#/mm2 vessel wall) and was measured throughout the observation period. Leukocyte emigration was measured online at the end of each 1 min observation period. Emigrated leukocytes were expressed as the number of interstitial leukocytes per mm2 of interstitium (#/mm2) based on the count per field of view adjacent to the segment under observation (=0.028mm2).
All platelet donors matched the source of the recipients in terms of infection, diet and, in all but one group, strain. In the unmatched group, platelets from P-sel−/− mice were observed in WT recipients (both mCMV+HC). Platelets were considered saltating if they paused transiently (for ≥2 s but <30s) on the vessel wall, and firmly adherent if they arrested for ≥30 s. Total adhesion was calculated as the sum of saltation and firm adhesion, and all platelet parameters were expressed as # platelets/mm2 vessel wall.
Once the venular data had been collected, arteriolar vasodilation responses to ACh were recorded. Arteriolar vasorelaxation responses were expressed as the percentage diameter change versus baseline. Arteriolar diameters were then allowed to return to baseline with BBS superfusion, before papaverine was applied to test for maximal endothelium-independent vasodilation. Arterioles that were not responsive to papaverine were excluded from the study.
Serum Cholesterol Levels
Serum was frozen for subsequent measurement of cholesterol levels using a spectrophotometric assay (Sigma Chemicals Co., St. Louis, MO).
Statistical Analysis
All values are reported as mean±SEM. ANOVAs with Fisher’s (Dual-radiolabelled antibody data) or Scheffe’s (intravital microscopy data) post-hoc tests were used for statistical comparison of experimental groups, with statistical significance set at P<0.05.
Results
P-Selectin Expression
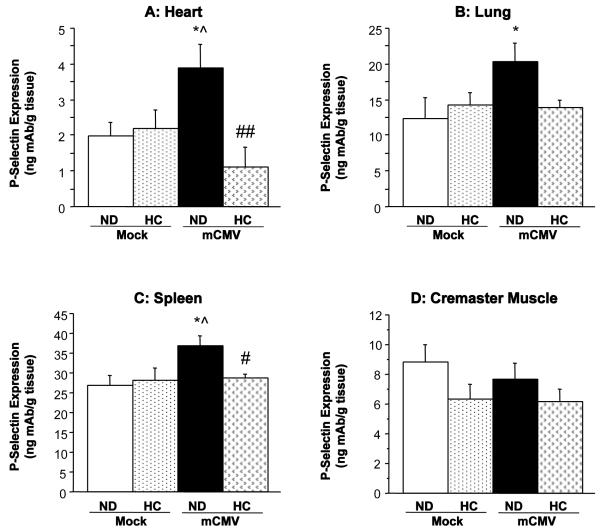
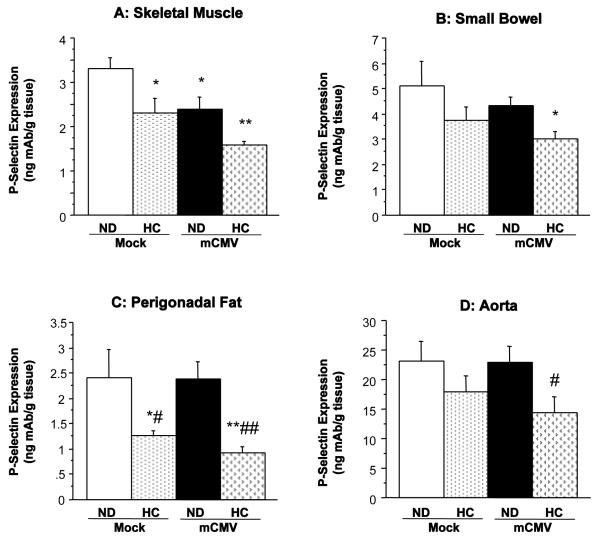
mCMV infection in WT mice led to a significant upregulation of P-selectin in the vascular beds of the heart, lung, and spleen, when compared to Mock-ND mice (Figure 1). There was also a trend towards increased P-selectin expression in the eyes (more than 2-fold; not shown), although this was variable and did not reach significance. The cremaster muscle did not exhibit any alteration in P-selectin expression (Figure 1D), whereas there was a reduction in P-selectin in skeletal muscle (Figure 2A). Interestingly, the mCMV-induced elevations in P-selectin expression shown in Figure 1 were not observed in hypercholesterolemic mCMV-infected WT mice. In fact exposure of WT mice to 6 wks of HC led to a significant reduction in P-selectin expression in the small bowel and skeletal muscle as well as in fat deposits (Figure 2), versus Mock-ND counterparts. In the adipose tissues, this decrease appeared to be primarily due to the diet. P-selectin remained unchanged from control in other organs from these mice. However, in the aorta (Figure 2D), eyes, and large bowel (data not shown), P-selectin expression in the WT CMV-HC group was significantly lower than in their normocholesterolemic infected counterparts (CMV-ND group).
Figure 1.
P-selectin expression, as measured by DRL, in (A) heart, (B) lung, (C) spleen and (D) cremaster muscle of WT mice 11 wks after exposure to mock inoculum or mCMV. Mice were either maintained on ND, or placed on HC at 5 wks p.i. for 6 wks. * P<0.05 vs. Mock-ND; ^ P<0.05 vs. Mock-HC; # P<0.05 vs. mCMV-ND; ## P<0.01 vs. mCMV-ND.
Figure 2.
P-selectin expression in (A) skeletal muscle, (B) small bowel, (C) perigonadal fat, and (D) aorta, of WT mice 11 wks after injection with mock inoculum or mCMV. Mice were maintained on ND or changed to HC at 5 wks p.i. until P-selectin assessment. * P<0.05 vs. Mock-ND; ** P<0.01 vs. Mock-ND; # P<0.05 vs. mCMV-ND; ## P<0.01 vs. mCMV-ND.
Arteriolar Vasodilation Responses
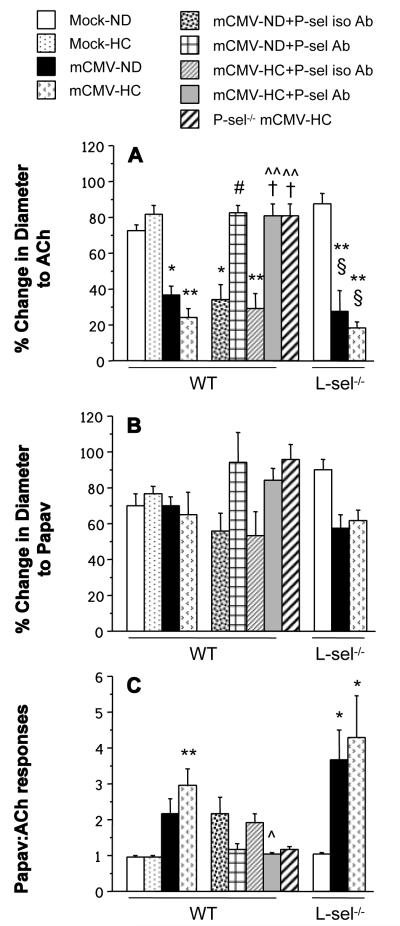
Arteriolar vasodilation in response to acetylcholine was significantly impaired in mCMV-infected WT mice, when compared to mock-inoculated controls (Figure 3A). Vasodilation was unchanged by HC alone, and the cholesterol-enriched diet did not render the arteriolar dysfunction any worse in the mCMV-HC WT mice, when compared to normocholesterolemic counterparts. Administration of isotype-matched control Ab 24 h prior to observation did not elicit any changes in the vasodilation responses, however anti-P-selectin restored normal levels of function in both mCMV-ND and mCMV-HC WT groups.Arteriolar vasodilation in P-sel−/− mCMV-HC mice was comparable to anti-P-sel Ab-treated WT counterparts. In contrast, deficiency of L-selectin did not confer any protection against the arteriolar dysfunction in either mCMV group. Papaverine induced vasodilation (endothelium-independent) was comparable in all groups (Figure 3B). While the changes in diameter in response to acetylcholine or papaverine were approximately equal in the Mock groups and the P-selectin Ab-treated mice (Figure 3C), there was a tendency for the endothelium-independent responses to be at least twofold higher than the endothelium-dependent vasodilation in the untreated and isotype-matched control Ab-treated WT mCMV groups, as well as the L-sel−/− mCMV mice. No blood cells were observed to be interacting with arteriolar walls in any of the groups examined.
Figure 3.
% change in diameter from baseline in arterioles in responses to the endothelium-dependent vasodilator, acetylcholine (ACh) (A) or the endothelium-independent vasodilator, papaverine (papav) (B). The ratio between papav and ACh responses is depicted in panel C. WT, P-sel−/− or L-sel−/− mice received mock inoculum or mCMV, and were either kept on ND, or switched to HC at 5 wks p.i., until observation at 11 wks p.i. Separate WT mCMV groups were treated with anti-P-selectin antibody (+P-sel Ab) or an isotype-matched control antibody (+P-sel iso Ab) 24 h prior to the experiment. * P<0.05 vs. both WT Mock groups; ** P<0.005 vs. both WT Mock groups; # P<0.005 vs. WT mCMV-ND and WT mCMV-ND+P-sel iso Ab; ^ P<0.005 vs. WT mCMV-HC; ^^ P<0.0005 vs. WT mCMV-HC; † P<0.01 vs. WT mCMV-HC+P-sel iso Ab; § P<0.005 vs. L-sel−/− Mock-ND.
Blood Cell Recruitment in Postcapillary Venules
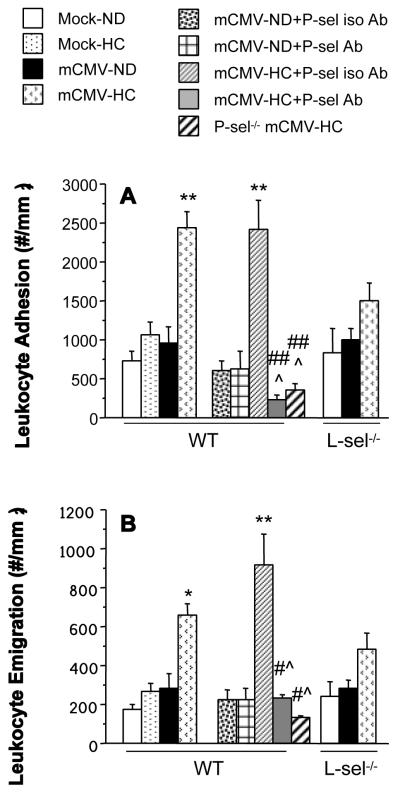
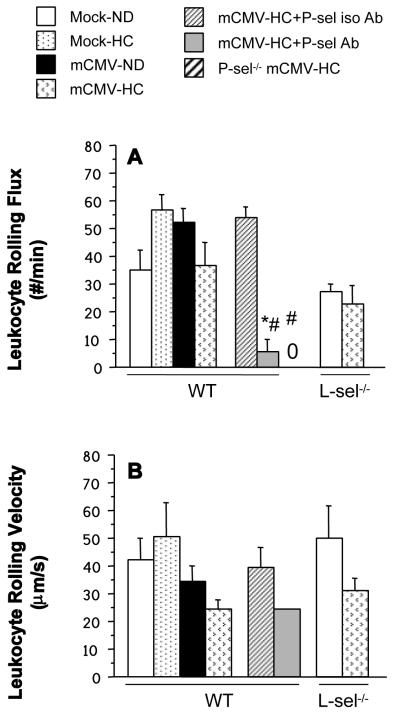
Neither HC nor mCMV alone led to significant leukocyte recruitment in postcapillary venules of WT mice. However the combination of mCMV+HC led to significant elevations in leukocyte adhesion and emigration (Figure 4). This was not due to significant decreases in wall shear rate (data not shown). Pre-treatment of WT mCMV-HC mice with anti-P-selectin Ab 24 h prior to observation led to a significant attenuation of leukocyte adhesion to below Mock-ND levels. Leukocyte emigration in these mice was also reduced to Mock-ND levels by treatment with anti-P-sel Ab. As expected, the isotype control Ab did not alter leukocyte adhesion or emigration when compared with untreated counterparts.Findings in P-sel−/−-mCMV-HC mice confirmed those from the anti-P-sel Ab experiments. L-selectin deficiency led to a partial reduction of leukocyte adhesion and emigration in mCMV-HC mice when compared with WT counterparts. Because both P-selectin and L-selectin support the rolling interactions of leukocytes, we measured rolling flux and rolling velocity to determine whether exposure to the combination of mCMV and HC altered these parameters in WT mice, thereby leading to enhanced adhesion (Figure 5). Rolling flux was unaltered by exposure to mCMV or/and HC (Figure 5A), however these mice exhibited a 42% decrease in leukocyte rolling velocity when compared with Mock-ND controls, although this did not reach significance (Figure 5B). When all rolling leukocytes were monitored it was found that more rolling leukocytes rolled for the entire 100 μm section of the venule in mCMV-HC mice (71%) when compared with mock-ND controls (53%; not significant; data not shown). Treatment of theseWT mice with the control Ab did not affect rolling flux. In contrast, anti-P-selectin Ab treatment completely inhibited rolling flux in eight out of ten mice. In one of the remaining two mice, rolling flux was low (15.5 cells/min) but none of these leukocytes rolled for the entire 100 μm section being analyzed, whereas in the other mouse, rolling behavior was comparable to that observed in untreated mCMV-HC mice (Figure 5). Similar to 80% of the anti-P-selectin Ab-treated mice, P-sel−/−-mCMV-HC mice exhibited a complete abrogation of leukocyte rolling, confirming a role for P-selectin in this process. Leukocyte rolling flux in L-sel−/− Mock-ND and mCMV-HC mice was approximately 77% and 63% of that seen in WT counterparts, with velocities that were comparable to the WT mice.
Figure 4.
The number of adherent leukocytes in postcapillary venules (A) and leukocytes emigrated into the surrounding interstitium (B) in the cremaster muscle of WT, P-sel−/− or L-sel−/− mice exposed to mock inoculum or mCMV. Mice remained on ND or were changed to HC at 5 wks p.i. until observation at 11 wks p.i. Separate WT mCMV groups received anti-P-selectin antibody (+P-sel Ab) or an isotype-matched control antibody (+P-sel iso Ab) 24 h prior to observation. * P<0.05 vs. both WT Mock groups and mCMV-ND; ** P<0.005 vs. both WT Mock groups and mCMV-ND; # P<0.01 vs. WT mCMV-HC; ## P<0.0001 vs. WT mCMV-HC; ^ P<0.0005 vs. WT mCMV-HC+P-sel iso Ab.
Figure 5.
Leukocyte rolling flux (# of leukocytes rolling past a specific point per minute) (A), and leukocyte rolling velocity (B) in postcapillary venules of WT, P-sel−/− or L-sel−/− mice injected with mock inoculum and maintained on ND or infected with mCMV and placed on HC at 5 wk p.i. Intravital microscopy observation of the venules was performed at 11 wks p.i. Only one out of ten mice had leukocytes that rolled for 100 μm in the P-sel Ab group, and could be considered for measurement of velocity. No leukocyte rolling was detected in the P-sel−/− mice (denoted by “0”). * P<0.05 vs. WT mCMV-HC; # P<0.005 vs. WT mCMV-HC+P-sel iso Ab.
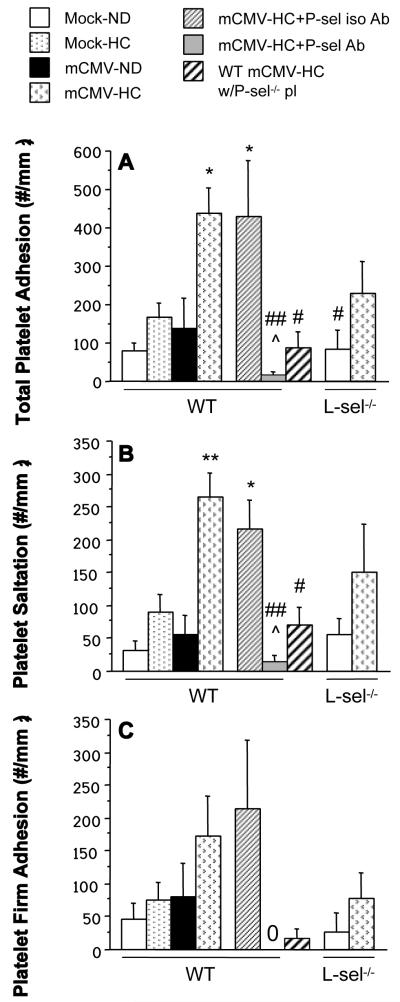
Platelet adhesion was not increased by either HC or mCMV alone, however, like leukocyte recruitment, total platelet adhesion was significantly elevated in venules of WT mice exposed to both mCMV and HC (Figure 6A). This was primarily made up of transient (saltation) interactions, (Figure 6B), with firm adhesion increasing 3.8-fold (comparable to the 3.4-fold increase observed in leukocyte adhesion) (Figure 6C). The isotype-matched control Ab did not affect platelet interactions, however anti-P-selectin Ab almost completely inhibited platelet saltation and completely obliterated firm adhesion in the mCMV-HC mice. When we observed platelets derived from P-selectin-deficient donors (P-sel−/−) in WT recipients, both exposed to mCMV-HC, platelet saltation and firm adhesion were significantly reduced, when compared to adhesion of WT mCMV-HC platelets in WT mCMV-HC recipients, although some residual platelet recruitment remained. L-sel deficiency led to a modest decrease in mCMV-HC-induced platelet recruitment (both saltation and firm adhesion), although this did not reach significance versus WT mCMV-HC mice.
Figure 6.
Total adhesion (A), saltation (B) and firm adhesion (C) of exogenous platelets in postcapillary venules of WT, P-sel−/− or L-sel−/− mice receiving mock inoculum or mCMV, and maintained on ND or placed on HC at 5 wks p.i. All platelet donors matched recipients in terms of WT mouse strain, infection and diet, with the exception of the 7th bar, which represents platelets from P-sel−/− mCMV-HC donors observed in WT mCMV-HC recipients (WT mCMV-HC w/P-sel−/− pl). “0” denotes zero adhesion as measured in the mCMV-HC+P-sel Ab group. * P<0.05 vs. WT Mock-ND; ** P<0.005 vs. both WT Mock groups and WT mCMV-ND; # P<0.05 vs. mCMV-HC; ## P<0.005 vs. mCMV-HC; ^ P<0.01 vs. mCMV-HC+P-sel iso Ab.
Cholesterol levels
Plasma cholesterol levels were elevated approximately 2-fold by 6 wks HC, and this was unaltered by mCMV infection, antibody administration or mouse strain (data not shown).
Discussion
CMV infection is a lifelong persistent infection that affects a majority of individuals. It results in many pro-inflammatory consequences, including upregulation of endothelial adhesion molecule expression [2,5,11,31,33,35], leukocyte adhesion [2,22,35] and increased generation of cytokines [8,9,11], as well as platelet adhesion [30]. These types of responses have been implicated in diseases such as CVD. However, much of this information about CMV is derived from primary infection of isolated endothelial cells, and less is known about the impact of persistent infection on the intact vasculature. We have recently shown that CMV infection leads to arteriolar dysfunction, and transient low-grade inflammation in venules [26]. Exposure of CMV-infected mice to hypercholesterolemia (important because a majority of the hypercholesterolemic patient population are infected with CMV), led to a synergism between these two risk factors. Here we tested the roles of P-selectin and L-selectin, adhesion molecules that have been implicated in CVD [4,24] in these microvascular responses to CMV infection. Our findings indicate that persistent CMV infection leads to the upregulation of P-selectin in lungs, heart, and spleen that is not observed in their hypercholesterolemic counterparts. Nonetheless we show a role for P-selectin in both the arteriolar dysfunction induced by mCMV infection, and in the venular blood cell recruitment observed in mice exposed to both mCMV and hypercholesterolemia. L-selectin played a minor role in the events in the venules of mCMV-HC mice. These results indicate CMV may predispose tissues to a pro-disease state characterized by upregulated P-selectin expression in select organs and P-selectin-dependent vasodilation impairment, and that this virus can synergize with another cardiovascular risk factor to induce P-selectin-, and to a lesser extent L-selectin-, dependent inflammatory and thrombogenic responses.
Using 4 wks of a cholate-containing high cholesterol diet, we previously demonstrated that persistent mCMV infection synergized with hypercholesterolemia to exaggerate and prolong leukocyte and platelet adhesion responses in post-capillary venules [26]. In order to eliminate the possibility that dietary cholate, rather than cholesterol, was the cause of this synergism, we redefined our model for the current study using a cholate-free HC. It should be noted that the cholate content and diet duration differ from that previously used to show that P-selectin expression is upregulated [29], arteriolar function is impaired, and leukocytes and platelets are recruited to postcapillary venules during hypercholesterolemia [39], responses we did not observe here with HC alone. As before, we waited for the primary infection period to subside before switching the mice to HC. It was not surprising (because of the role of cholate in cholesterol absorption) that we had to extend our dietary regime from 4 to 6 weeks (and therefore our infection period to 11 weeks), in order to observe levels of leukocyte and platelet adhesion that were comparable to the 9 wk time-point in the original model [26]. Nonetheless, this suggests that it is the hypercholesterolemia, not the cholate, which synergizes with mCMV to produce the exacerbated responses we observe. mCMV-induced arteriolar dysfunction was not impacted by HC at this time-point.
P-selectin is an adhesion molecule expressed primarily by endothelial cells and platelets, and which plays a major role in CVD [4,7,12,24]. Unlike many other adhesion molecules (e.g. E-selectin, ICAM-1, VCAM-1) [2,5,11,31,33,35], it was shown not to be upregulated on isolated endothelial cells during primary CMV infection [30]. However, platelets exposed to the supernatant of primary infected pulmonary artery endothelial cells did exhibit enhanced P-selectin expression and increased shedding of this adhesion molecule. Using an in vivo model of persistent mCMV infection we demonstrated significantly increased levels of P-selectin expression in a primary target organ of CMV, the spleen, and organs important for CVD, the heart and lungs. The eyes, which are affected by CMV in immunocompromised patients also showed a tendency for P-selectin upregulation. This could be relevant not only for CVD but also for other diseases to which CMV has been linked, such as cancer [34]. When we tested the role for P-selectin in mCMV-induced arteriolar dysfunction, we found that, despite the lack of blood cell recruitment in these vessels, or local elevation of P-selectin, this adhesion molecule contributed to the impaired vasodilation observed during mCMV infection. Whether this is due to platelet P-selectin mediated interactions between circulating leukocytes and platelets, that in turn causes the release of vasoactive factors e.g. thromboxane A2, that impair the vasodilation responses remains unclear. Alternatively, P-selectin-dependent responses in select organs where P-selectin is upregulated may lead to the liberation of soluble factors into the systemic circulation that result in arteriolar dysfunction at distal sites, but are insufficient to create a prolonged inflammatory or thrombogenic phenotype throughout the organism. If the latter is the case, it appears that L-selectin is not a key regulator of these responses, for example through secondary tethering leading to P-selectin-dependent leukocyte rolling, because L-selectin-deficient mice exhibited no such protection against mCMV-induced arteriolar dysfunction. This P-selectin-mediated impairment of endothelium-dependent vasodilation responses during persistent CMV infection could have important implications for the recovery of a tissue from ischemic events where arteriolar control of blood flow during reperfusion is a key factor.
Our findings that the mCMV-induced P-selectin expression in key organs was not sustained or even exaggerated in the hypercholesterolemic mCMV-infected group, but instead decreased in some tissues, including the aorta, was somewhat surprising. A potential explanation is that the P-selectin upregulation in the mCMV-infected mice is transient, and the addition of HC accelerated the timecourse of induction such that it was no longer expressed at 11wks. However, the arteriolar dysfunction, and venular leukocyte and platelet recruitment in mCMV-HC mice were inhibited by blocking P-selectin for 24 hours, suggesting some cells are expressing this adhesion molecule at the 11 wk time-point. There are several possibilities as to why this apparent discrepancy occurred. First, the addition of HC may lead to shedding or more rapid re-internalization of P-selectin such that levels expressed on the vascular wall remain relatively constant or reduced, despite the receptor being continually mobilized to the cell surface. Second, given the enhanced leukocyte adhesion observed in postcapillary venules of mCMV+HC mice, P-selectin that may have been upregulated on endothelial cells could have been shielded from the detection antibody, thereby resulting in an apparent lack of change in expression. Third, P-selectin upregulated on circulating platelets or that shed into the blood may act to “mop” up the antibody, giving a false negative result. The latter two are potential limitations of our technique that is designed to look at cell surface expression of a molecule. It is also plausible that upregulation of P-selectin on the vessel wall is not necessary for the events we observed, rather basal levels of P-selectin are sufficient to support leukocyte rolling, allowing the leukocytes to interact with other upregulated adhesion molecule(s) that mediate adhesion. Alternatively, P-selectin on circulating platelets, via interactions with circulating leukocytes, may have initiated the release of soluble factors from either/both cell types that led to the responses on both sides of the microvasculature, therefore vascular P-selectin was not upregulated and thus not detected. In fact, our finding that platelets were the primary cellular source of the P-selectin that mediated their adhesion supports the third or last possibilities in that it shows P-selectin is mobilized to the surface of these cells. There is a growing appreciation for the link between platelets and inflammatory responses, and whether platelet P-selectin mediates the other responses in our model is the subject of ongoing studies. In contrast to P-selectin, L-selectin was involved in approximately 50% of the leukocyte and platelet recruitment observed in mCMV-HC mice. Although we did not specifically assess primary and secondary tethering in our mice the different levels of protection against leukocyte recruitment achieved by blocking either selectin are in agreement with the current literature showing an overlap between these two selectins, where P-selectin mediates primary tethering and rolling along the endothelium, and L-selectin is primarily responsible for mediating secondary tethering between circulating leukocytes and other rolling or adherent leukocytes that subsequently transitions to interactions (potentially P-selectin-dependent) with the endothelium [37]. While our attempts to identify whether platelets were interacting with the vessel wall directly or via leukocytes were inconclusive, the partial reduction of platelet recruitment by L-selectin deficiency suggests at least some of the recruited platelets were adhering to leukocytes.
In the case of the arteriolar dysfunction in mCMV+HC mice, it is possible that not only are soluble factors released from circulating cells via a platelet P-selectin-dependent pathway, leading to impaired vasodilation responses, but the leukocyte and platelet recruitment in nearby venules may also contribute. This is based on findings by Kim et al. [27] who demonstrated an association between P-selectin-dependent leukocyte and platelet recruitment in venules and the impaired vasodilation responses of arterioles during hypercholesterolemia. Although this relationship was stronger for paired arterioles, and we did not specifically assess this pairing relationship, one could argue that since our L-selectin deficiency conferred approximately 50% protection in the venules but no restoration of arteriolar function, the events in the venules did not influence those in arterioles. While this does appear to be the case specifically in relation to L-selectin-dependent blood cell recruitment, and there appears to be an overlap between L-selectin and P-selectin-dependent leukocyte and platelet accumulation, we cannot exclude the possibility that at least some of the P-selectin-mediated blood cell recruitment may influence arteriolar function.
Our findings that P-selectin is upregulated in several organs during persistent mCMV infection and mediates mCMV-induced arteriolar dysfunction offer new insight into how this virus may contribute to diseases such as CVD. The exaggerated inflammatory and thrombogenic phenotype we observed in the CMV+HC mice represents a prolongation of an early response to hypercholesterolemia, suggesting that CMV may both enhance the intensity and duration of P-selectin-dependent pathways of microvascular dysfunction, which could have important implications for a majority of hypercholesterolemic patients. These venular events appear to be supported, at least in part, by L-selectin. While in vitro work suggests that, unlike other cardiovascular risk factors, CMV may not directly alter platelet function or activation state [30], we were able to show that platelet P-selectin appears to be a major contributor to the thrombogenic phenotype induced by CMV+HC. With the growing appreciation of the interplay between platelets and inflammation, it is conceivable that platelets play an important role in mCMV and mCMV+HC induced inflammatory, and perhaps arteriolar, responses. Our findings provide the first step for future studies designed to understand how CMV alters or augments microvascular responses to other cardiovascular risk factors.
Acknowledgments
This work was supported by grants from the American Heart Association (0730294N) and NIH (P20RR018724)
References
- 1.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol. 2006;80:11539–11555. doi: 10.1128/JVI.01016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum A, Giladi M, Weinberg M, Kaplan G, Pasternack H, Laniado S, Miller H. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am J Cardiol. 1998;81:866–868. doi: 10.1016/s0002-9149(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 4.Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 5.Burns LJ, Pooley JC, Walsh DJ, Vercellotti GM, Weber ML, Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins. Transplantation. 1999;67:137–144. doi: 10.1097/00007890-199901150-00023. see comment. [DOI] [PubMed] [Google Scholar]
- 6.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 7.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craigen JL, Yong KL, Jordan NJ, MacCormac LP, Westwick J, Akbar AN, Grundy JE. Human cytomegalovirus infection up-regulates interleukin-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997;92:138–145. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crough T, Burrows JM, Fazou C, Walker S, Davenport MP, Khanna R. Contemporaneous fluctuations in T cell responses to persistent herpes virus infections. Eur J Immunol. 2005;35:139–149. doi: 10.1002/eji.200425548. [DOI] [PubMed] [Google Scholar]
- 11.Dengler TJ, Raftery MJ, Werle M, Zimmermann R, Schonrich G. Cytomegalovirus infection of vascular cells induces expression of pro-inflammatory adhesion molecules by paracrine action of secreted interleukin-1beta. Transplantation. 2000;69:1160–1168. doi: 10.1097/00007890-200003270-00022. [DOI] [PubMed] [Google Scholar]
- 12.Dong ZM, Brown AA, Wagner DD. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation. 2000;101:2290–2295. doi: 10.1161/01.cir.101.19.2290. [DOI] [PubMed] [Google Scholar]
- 13.Eppihimer MJ, Wolitzky B, Anderson DC, Labow MA, Granger DN. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 14.Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–3141. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–218. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W, Schlumberger W, Meyer J. Impact of infectious burden on progression of carotid atherosclerosis. Stroke. 2002;33:2581–2586. doi: 10.1161/01.str.0000034789.82859.a4. [DOI] [PubMed] [Google Scholar]
- 17.Freeman RB., Jr. The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant. 2009;9:2453–2458. doi: 10.1111/j.1600-6143.2009.02824.x. [DOI] [PubMed] [Google Scholar]
- 18.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier TW, Scalia R, Murohara T, Guo JP, Lefer AM. Nitric oxide protects against leukocyte-endothelium interactions in the early stages of hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:1652–1659. doi: 10.1161/01.atv.15.10.1652. [DOI] [PubMed] [Google Scholar]
- 20.Grahame-Clarke C, Chan NN, Andrew D, Ridgway GL, Betteridge DJ, Emery V, Colhoun HM, Vallance P. Human cytomegalovirus seropositivity is associated with impaired vascular function. Circulation. 2003;108:678–683. doi: 10.1161/01.CIR.0000084505.54603.C7. [DOI] [PubMed] [Google Scholar]
- 21.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 22.Grundy JE, Lawson KM, MacCormac LP, Fletcher JM, Yong KL. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J Infect Dis. 1998;177:1465–1474. doi: 10.1086/515300. [DOI] [PubMed] [Google Scholar]
- 23.Huo Y, Ley KF. Role of platelets in the development of atherosclerosis. Trends Cardiovasc Med. 2004;14:18–22. doi: 10.1016/j.tcm.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 25.Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–3287. [PubMed] [Google Scholar]
- 26.Khoretonenko MV, Leskov IL, Jennings SR, Yurochko AD, Stokes KY. Cytomegalovirus infection leads to microvascular dysfunction and exacerbates hypercholesterolemia-induced responses. Am J Pathol. 2010;177:2134–2144. doi: 10.2353/ajpath.2010.100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MH, Carter PR, Harris NR. P-selectin-mediated adhesion impairs endothelium-dependent arteriolar dilation in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2007;292:H632–638. doi: 10.1152/ajpheart.00780.2006. [DOI] [PubMed] [Google Scholar]
- 28.Liao L, Starzyk RM, Granger DN. Molecular determinants of oxidized low-density lipoprotein-induced leukocyte adhesion and microvascular dysfunction. Arterioscler Thromb Vasc Biol. 1997;17:437–444. doi: 10.1161/01.atv.17.3.437. [DOI] [PubMed] [Google Scholar]
- 29.Petnehazy T, Stokes KY, Russell JM, Granger DN. Angiotensin II type-1 receptor antagonism attenuates the inflammatory and thrombogenic responses to hypercholesterolemia in venules. Hypertension. 2005;45:209–215. doi: 10.1161/01.HYP.0000154085.27868.93. [DOI] [PubMed] [Google Scholar]
- 30.Rahbar A, Soderberg-Naucler C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J Virol. 2005;79:2211–2220. doi: 10.1128/JVI.79.4.2211-2220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricotta D, Alessandri G, Pollara C, Fiorentini S, Favilli F, Tosetti M, Mantovani A, Grassi M, Garrafa E, Dei Cas L, Muneretto C, Caruso A. Adult human heart microvascular endothelial cells are permissive for non-lytic infection by human cytomegalovirus. Cardiovasc Res. 2001;49:440–448. doi: 10.1016/s0008-6363(00)00258-3. [DOI] [PubMed] [Google Scholar]
- 32.Sakai A, Kume N, Nishi E, Tanoue K, Miyasaka M, Kita T. P-selectin and vascular cell adhesion molecule-1 are focally expressed in aortas of hypercholesterolemic rabbits before intimal accumulation of macrophages and T lymphocytes. Arterioscler Thromb Vasc Biol. 1997;17:310–316. doi: 10.1161/01.atv.17.2.310. [DOI] [PubMed] [Google Scholar]
- 33.Shahgasempour S, Woodroffe SB, Garnett HM. Alterations in the expression of ELAM-1, ICAM-1 and VCAM-1 after in vitro infection of endothelial cells with a clinical isolate of human cytomegalovirus. Microbiol Immunol. 1997;41:121–129. doi: 10.1111/j.1348-0421.1997.tb01177.x. [DOI] [PubMed] [Google Scholar]
- 34.Soderberg-Naucler C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41:218–223. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Span AH, Mullers W, Miltenburg AM, Bruggeman CA. Cytomegalovirus induced PMN adherence in relation to an ELAM-1 antigen present on infected endothelial cell monolayers. Immunology. 1991;72:355–360. [PMC free article] [PubMed] [Google Scholar]
- 36.Speir E. Cytomegalovirus gene regulation by reactive oxygen species. Agents in atherosclerosis. Ann N Y Acad Sci. 2000;899:363–374. doi: 10.1111/j.1749-6632.2000.tb06200.x. [DOI] [PubMed] [Google Scholar]
- 37.Sperandio M, Smith ML, Forlow SB, Olson TS, Xia L, McEver RP, Ley K. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197:1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokes KY, Granger DN. The microcirculation: a motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolemia? J Physiol. 2005;562:647–653. doi: 10.1113/jphysiol.2004.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stokes KY, Calahan L, Russell JM, Gurwara S, Granger DN. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation. 2006;13:377–388. doi: 10.1080/10739680600745877. [DOI] [PubMed] [Google Scholar]
- 40.Stokes KY, Russell JM, Jennings MH, Alexander JS, Granger DN. Platelet-associated NAD(P)H oxidase contributes to the thrombogenic phenotype induced by hypercholesterolemia. Free Radic Biol Med. 2007;43:22–30. doi: 10.1016/j.freeradbiomed.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki S, Kameoka M, Nakaya T, Kimura T, Nishi N, Hirai K, Ikuta K. Superoxide generation by monocytes following infection with human cytomegalovirus. Immunopharmacology. 1997;37:185–190. doi: 10.1016/s0162-3109(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 42.Tailor A, Granger DN. Hypercholesterolemia promotes P-selectin-dependent platelet-endothelial cell adhesion in postcapillary venules. Arterioscler Thromb Vasc Biol. 2003;23:675–680. doi: 10.1161/01.ATV.0000056742.97580.79. [DOI] [PubMed] [Google Scholar]
- 43.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weis M, Kledal TN, Lin KY, Panchal SN, Gao SZ, Valantine HA, Mocarski ES, Cooke JP. Cytomegalovirus infection impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine in transplant arteriosclerosis. Circulation. 2004;109:500–505. doi: 10.1161/01.CIR.0000109692.16004.AF. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb Haemost. 1999;81:1–7. [PubMed] [Google Scholar]