Abstract
Repeated cycles of ethanol intoxication and withdrawal associated with dependence induce neuroadaptations in a variety of brain systems. Withdrawal-induced negative emotional states can be ameliorated by ethanol consumption; a learned process termed negative reinforcement. Accordingly, a dependence-induced phenotype is escalated ethanol self-administration. Matrix metalloproteinases (MMPs) are proteolytic enzymes which degrade the extracellular matrix to allow for synaptic reorganization and plasticity. To test the hypothesis that an intact MMP system is required for animals to learn about the negative reinforcing effects of ethanol and display escalated self-administration during acute withdrawal when ethanol-dependent, male Wistar rats were trained to self-administer ethanol and then assigned to either acute or chronic MMP inhibition treatment groups. The chronic treatment group received intracerebroventricular (ICV) infusions of the broad spectrum MMP inhibitor FN-439 or artificial cerebrospinal fluid (aCSF) via osmotic minipumps during a one month ethanol dependence induction period and subsequent post-dependence induction self-administration sessions that occurred during acute withdrawal. The acute treatment group only received ICV FN-439 or aCSF on the day of self-administration sessions following dependence induction during acute withdrawal. The results showed that inhibition of MMPs attenuated escalated ethanol self-administration following chronic and acute exposure conditions. Furthermore, once learning (i.e., plasticity) had occurred, MMP inhibition had no impact on escalated response patterns and animals previously subjected to MMP inhibition that did not escalate evidenced normal escalations in operant ethanol self-administration once FN-439 treatments were terminated. Thus, the present data identified that an intact MMP system is required for the escalated responding that occurs during acute withdrawal in dependent animals and implicate such escalation as a learned response.
Keywords: Addiction, Alcohol, Dependence, Ethanol, Extracellular Matrix, Learning, Intermittent Ethanol Vapor Exposure, Matrix Metalloproteinases, Negative Reinforcement, Self-administration, Withdrawal
1. Introduction
Ethanol dependence produces profound neurochemical and morphological changes within the central nervous system that underlie altered motivational and affective behavior (e.g., Koob, 2009b). Behaviors indicative of dependence–like phenotypes include excessive ethanol self-administration (O'Dell, Roberts, Smith, & Koob, 2004; Rimondini, Sommer, & Heilig, 2003; Walker & Koob, 2007), and the development of anxiety- (Valdez et al., 2002) and depressive-like (Walker et al., 2010) states. The removal of these negative states via ethanol consumption is reinforcing (termed negative reinforcement; Koob, 2009a) and this ‘self-medication’ contributes to excessive ethanol consumption and relapse (Heilig & Egli, 2006; Heilig, Egli, Crabbe, & Becker, 2010; Markou, Kosten, & Koob, 1998). The pattern of acquisition concerning dependence-induced escalation of ethanol self-administration during acute withdrawal follows a standard learning curve, in that self-administration steadily increases with each acute withdrawal self-administration session until a plateau is reached and responding stabilizes (e.g., Walker & Koob, 2008).
Morphological and intracellular signaling changes in response to chronic ethanol and drugs of abuse have been identified (Nestler, 1993; e.g., Ortiz et al., 1995). Extracellular matrix (ECM) proteins provide structural support in the nervous system and in order for synaptic plasticity (e.g., Hebbian, homeostatic and metaplasticity) to occur, the extracellular matrix must be degraded (Dityatev & Fellin, 2008; Lee, Tsang, & Birch, 2008; Wright & Harding, 2004; Wright & Harding, 2009). Furthermore, the ECM is involved in the regulation of intracellular / extracellular signaling, receptor localization in a number of neurotransmitter systems and astrocytic functions (Dityatev et al., 2008). Matrix metalloproteinases (MMPs), a family of proteolytic enzymes, can cleave extracellular matrix proteins to allow for the reconfiguration of neural pathways (Ethell & Ethell, 2007; Lee et al., 2008; Wright et al., 2004). MMP secretion can be stimulated by growth factors and ECM-intracellular signaling pathways (Wright et al., 2009). MMP expression appears to be required for hippocampal-based learning and inhibition of MMPs interferes with induction and maintenance of long-term potentiation, as well as memory tasks (Meighan, Meighan, Davis, Wright, & Harding, 2007; Meighan et al., 2006; Nagy, Bozdagi, & Huntley, 2007; Wright, Brown, & Harding, 2007).
To test the hypothesis that MMPs are required for dependence-induced escalation of self-administration during acute withdrawal, two experiments were conducted in which a broad spectrum MMP inhibitor (FN-439) or artificial cerebrospinal fluid (aCSF) were infused intracerebroventricularly (ICV) under chronic or acute conditions. FN-439 is a well characterized compound that has repeatedly been shown to attenuate the effects of MMPs on the ECM and reduce various indices of (and the underlying process that contribute to) associative and non-associative learning (Brown et al., 2007; Brown, Wilson, Cocking, & Sorg, 2009; Meighan et al., 2006; Wiediger & Wright, 2009; Wright et al., 2007), without producing non-specific effects on behavior (e.g., see Brown et al., 2009).
Animals were trained to self-administer ethanol and dependence was induced using an intermittent ethanol vapor exposure paradigm. Following the induction of dependence, escalation of self-administration was evaluated during acute withdrawal. An initial cohort of animals demonstrated that, following dependence induction, escalation of self-administration occurs according to a typical learning curve. In the first experiment, once animals had learned to self-administer ethanol, ICV FN-439 was chronically infused during the dependence induction period and subsequent post-dependence self-administration sessions. In the second experiment, following the dependence induction period, FN-439 was infused ICV prior to and immediately after self-administration sessions. To protect against Type I error in the second experiment (i.e., rejecting the null hypothesis when it is actually true) that could be attributable to non-specific effects of FN-439, the specificity of FN-439 for learning-related processes was confirmed by evaluating FN-439 effects in animals that displayed escalated responding. Further, to ensure that FN-439 did not permanently impair the ability of animals to learn, those animals that had originally been treated with FN-439 were subsequently infused with aCSF and the presence or absence of escalated responding was evaluated.
2. Materials and Methods
2.1. Animals
Thirty-eight adult male Wistar rats (bred from Charles River Laboratory stock) approximately 70 days old were communally housed (2-3 per cage) in a temperature (21 ± 2oC) controlled vivarium on a 12-hr reverse light cycle (lights off at 6 am) with ad libitum food and water available. Prior to training, animals were handled daily for a one-week period. This work adheres to the National Research Council Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and was approved by the Washington State University Institutional Animal Care and Use Committee.
2.2. Acquisition of Operant Ethanol Self-Administration
During 30 minute sessions that occurred 5 days per week, animals were trained to self-administer (according to a continuous schedule of reinforcement) a 10% ethanol (w/v) solution (0.1 ml) in standard operant chambers (Med Associates, St. Albans, VT) with custom drinking wells (Behavioral Pharma, La Jolla, CA) using a sweetener-fade procedure (Samson, 1986) that has been described in detail elsewhere (Walker et al., 2007). Acquisition of the operant response occurred using a sweetened fluid (0.125% saccharin and 3% glucose) that is preferred to other sweeteners such as sucrose (Valenstein, Cox, & Kakolewski, 1967) without necessitating water or food deprivation. Next, 10% ethanol (w/v) was added to the solution and then over three weeks, the sweetener was removed from the solution with 10% ethanol as the final solution. Once responding stabilized (defined as three sessions with < 10% deviation), animals were assigned to one of the three different treatment conditions (example of dependence-induced escalation, chronic FN-439 treatment or acute FN-439 treatment) that were matched based on ethanol self-administration.
2.3. Treatment Conditions
2.3.1. Condition 1
Serving as escalation control animals, the first two groups did not receive any surgical procedures and were divided into groups receiving either one-month of chronic intermittent ethanol vapor (n=5) or air exposure (n=5).
2.3.2. Condition 2
The chronic treatment animals were implanted with osmotic minipumps (OMP; 42-day pumps; Alzet, Cupertino, CA) that were connected to unilateral cannulae (28 gauge; Alzet, Cupertino, CA) targeting one lateral ventricle. One group received chronic FN-439 infusion (n=7,1.5 μg/hr in 0.15μl/hr), whereas the other received chronic aCSF (n=7; 0.15μl/hr) during the dependence induction period and post-vapor self-administration sessions.
2.3.3. Condition 3
The acute treatment animals were implanted with bilateral guide cannulae (22 gauge; Plastics One, Roanoke, VA) targeting the lateral ventricles and, following dependence induction, were designated to receive 35μg FN-439 (17.5/side; n=7) or aCSF (n=7) 15 minutes prior to and 45 miinutes following the post-dependence ethanol self-administration sessions.
2.3.4. Controls for Condition 3
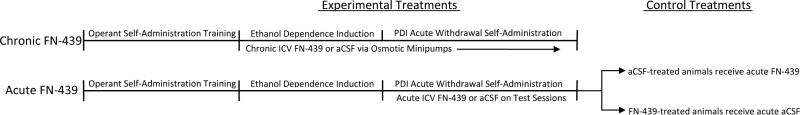
Two important control conditions were included in the acute treatment experiment. Because FN-439 was administered prior to and following the self-administration sessions, the possibility existed that FN-439 could have rate-decreasing effects that are independent of a MMP mechanism of action. Therefore, a failure to observe elevated self-administration during acute withdrawal could actually be a result of the FN-439 pretreatment and unrelated to the blockade of learning. Thus, the first control condition received an acute ICV FN-439 pretreatment 15 min prior to a final self-administration session following stable elevated ethanol self-administration in the aCSF-treated group. The second control condition was designed to address the possibility that FN-439 exposure might induce a permanent alteration in an organism's capacity to develop an escalated ethanol self-administration pattern during acute withdrawal when dependent. Once the FN-439 infusions were terminated for the FN-439 acute treatment group, the animals continued to receive aCSF infusions under the same timing conditions as the FN-439 treatments (i.e., 15 min prior to and 45 min. after) to evaluate whether the animals would show normal negative reinforcement learning. A diagram describing the treatment regimens for conditions 2 and 3 is presented in Figure 1.
Figure 1.
Diagram of the experimental design for the acute and chronic FN-439 treatment regimens following the acquisition of operant ethanol self-administration, dependence induction and post-dependence induction self-administration sessions during acute withdrawal. aCSF = artificial cerebrospinal fluid, ICV = intracerebroventricular and PDI = post-dependence induction.
2.4. Surgical Procedures
For surgical implantation of the OMPs and ICV guide cannulae, animals were anesthetized via isoflurane inhalation (5% for induction / 3% maintenance). OMPs were implanted subcutaneously approximately 3 cm below the right shoulder blade and attached via polyvinylchloride tubing to a unilateral guide cannula implanted via stereotaxic surgery. The cannula guides connected to the OMPs were counterbalanced for hemisphere using the coordinates AP -0.8, DV -4.7, ML±1.7 from bregma (Paxinos and Watson, 2005) and bilateral guide cannulae (22 gauge; Plastics One, Roanoke,) were implanted using the coordinates AP -0.8, DV -3.9, ML ±1.5 (from bregma according to Paxinos and Watson, 2005). Prior to the implantation of the OMPs, the pumps were filled with the appropriate solution (FN-439 or aCSF), attached to ICV cannulae and incubated in sterile 0.9% NaCl at 37°C for 72 hours. The coordinates for the acute condition were modified slightly based on the histological results from chronic treatment condition. For both conditions, stainless steel screws served as anchor points and cannulae were held in place using dental acrylic. The bilateral guide cannulae were sealed by inserting obdurators (31 gauge; PlasticsOne, Roanoke, VA) to maintain cannulae patency and reduce risk of infection. The obdurators were held in place with a stainless steel cap nut (Fastenal, Moscow, ID). Following surgery, post-operative analgesics (flunixin, 2.5 mg/kg; MWI Veterinary Supply, Meridian, ID) and antibiotics (Baytril, 5 mg/kg, MWI Veterinary Supply, Meridian, ID) were administered subcutaneously for 5 days. Following one week of recovery, the animals were transferred to the vapor chamber apparatus for dependence induction.
2.5. Induction of Ethanol Dependence
The present experiments utilized an intermittent ethanol vapor exposure regimen to induce dependence. Ethanol vapor exposure can induce ethanol dependence (Rogers, Wiener, & Bloom, 1979) and intermittent vapor exposure (14 hours on, 10 hours off) induces a wide range of behaviors indicative of a dependent-like phenotype such as excessive ethanol self-administration in both fixed- and progressive-ratio schedules of reinforcement (Walker and Koob 2007; O'Dell et al. 2004) and increased anxiety- (Valdez et al. 2002) and depressive-like (Walker et al. 2010b) behaviors. The vapor exposure apparatus (La Jolla Alcohol Research, Inc., La Jolla, CA) allows for the animal's blood alcohol levels (BALs) to beikated according to desired target BALs. Target BALs over the course of the experiment were 175 – 225 mg% and were determined twice weekly by collecting the blood from the tail (~0.25 ml). Blood samples were centrifuged at 15000 rpm for 15 min and the 5 μl of plasma was assayed using the Analox AM1 (Analox Instruments Ltd., Lunenburg, MA). Single point calibrations of the Analox AM1 were conducted daily prior to each set of BAL determinations.
2.6. Post-Dependence Operant Testing
Following the one-month dependence induction period, all animals were tested for operant ethanol self-administration twice weekly 6 hours into withdrawal (i.e. acute withdrawal). It has been previously shown that following 6 hrs of withdrawal, the BALs of ethanol-experienced animals that had achieved a BAL of approximately 225 mg% following ethanol vapor exposure have returned to baseline levels (Gilpin et al., 2009). BALs were collected immediately before the ethanol vapor terminated on test days to confirm that the animal's BALs were within the target range. Following each test, the rats were returned to ethanol vapor apparatus and were re-exposed to ethanol vapor between tests. Thus, each post-dependence self-administration session was conducted under identical conditions of acute withdrawal in dependent animals.
2.7. Drugs
FN-439 (EMD Biosciences, Gibbstown, NJ; Prod#444250) was soluble in aCSF (pH of 7.4 and compressed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2,1.2 mM CaCl2, 5.4 mM d-Glucose and 0.25 mM ascorbic acid; Alvarez-Jaimes, Stouffer and Parsons, 2009; Nealey, Smith, Davis, Smith and Walker, 2011). In Condition 2, FN-439 or aCSF were infused at a rate of 0.15 μl/hr, whereas the animals in Condition 3 received FN-439 or aCSF via internal cannulae (31 gauge that extended 1 mm past the guide cannula) at a volume and rate of 1 μl/side over 60 seconds.
2.8. Histology
Following the completion of each experiment, successful targeting of the lateral ventricles was confirmed by infusing 0.6% cresyl violet. Animal were injected with 100mg sodium pentobarbital (Sigma Aldrich, St. Louis, MO). The OMP animals had the tubing connecting the OMP to the cannula exposed and 2 μl of cresyl violet was infused over 60 s, whereas the animals in Condition 3 had 1 μl/side of cresyl violet infused over 60 s. Subsequently, the animal's brains were removed and the presence of cresyl violet in the ventricles was confirmed. Those animals that did not display ventricular cresyl violet were not included in the statistical analysis.
2.9. Statistical Analysis
To control for differences in animal weight that could influence operant ethanol self-administration levels, lever presses were converted to g/kg ethanol consumption for all analyses in the present manuscript. To confirm that intermittent ethanol vapor induced a dependence-like phenotype, a mixed model two-way analysis of variance (ANOVA) was conducted on ethanol consumption data from the four post-induction period sessions for air- and ethanol vapor-exposed animals. Although mixed-model ANOVAs are used in later analyses of MMP inhibition, those ANOVAS did not include a comparison of air- and vapor-exposed animal data as the initial demonstration of escalation did (Fig. 2), but instead focused exclusively on comparisons of vapor-exposed animals with or without acute or chronic MMP inhibition.
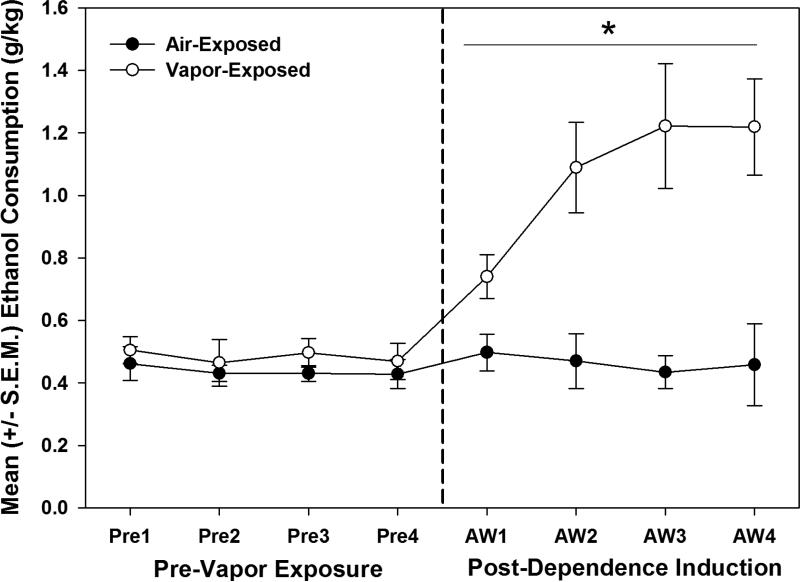
Figure 2.
Intermittent ethanol vapor induces escalated responding during acute withdrawal in dependent animals. Representative of the typical dependence-like phenotype during acute withdrawal, there is a significant Exposure × Session interaction (*= p≤0.05) for operant ethanol self-administration following one month of intermittent ethanol vapor exposure for testing that occurred 6 hrs into acute withdrawal. The dashed line indicates one month of chronic intermittent ethanol vapor exposure, with pre-vapor exposure sessions on the left and post-dependence induction sessions during acute withdrawal (AW) on the right (n=5 / grp for the air- and vapor-exposed animals).
In the present manuscript, escalation sessions were identified as such when one of the two groups of animals escalated as assessed by a one-tailed paired sample t-test comparing their performance on the most recent test session to the session immediately prior to the most recent session. To evaluate the effects of acute and chronic MMP inhibition on the development escalated ethanol self-administration, a mixed model two-way ANOVA was utilized with drug treatment and post-vapor session as the between-and within-subjects factors, respectively. In the acute and chronic FN-439 experiments, for the within-subject variable of session, the initial post-vapor exposure session(s) were compared to the session in which escalation of responding occurred. In the chronic FN-439 exposure experiment, only one initial test session took place prior to escalation. However, in the acute FN-439 treatment experiment, three sessions took place prior to escalation and in order to maintain consistent data analysis across experiments, the averages of those three sessions were used as the initial test session values in the two-way ANOVA. The acceptability of this action was determined by conducting one-way repeated-measures ANOVAs for the FN-439 and aCSF-treated animals to evaluate whether there were any differences between the three sessions and if not, the average of the three was used. This course of action was taken instead of “pooling” the data because the within-subject component of the analysis results in the exclusion of data that is not represented by equal sample sizes. In addition, to protect against the possibility of committing a Type I error, a two-way ANOVA was also conducted that utilized the data from the single session prior to escalation instead of the averaged values. Post-hoc independent-sample t-tests were conducted on the ethanol consumption data (g/kg) from the escalation sessions in order to compare the FN-439 and aCSF-treated animals for ethanol consumption.
Data from the acute FN-439 control condition was analyzed using a paired-sample t-test to compare the escalated and FN-439 sessions. The data from the second control condition consisting of aCSF infusions for those animals originally receiving acute FN-439 treatments was analyzed using a one-way repeated measure ANOVA with session as the within-subjects factor and followed by post-hoc Least Significant Difference (LSD) tests to compare ethanol intake (g/kg) over the three aCSF-treated sessions.
Results
As a result of the histological verification of cannula placement, two animals in the chronic aCSF treatment group were removed from the study. Additionally, three animals (one in the acute FN-439 and two from the acute aCSF group) removed their cannula guides during the one-month dependence induction phase and were eliminated from the study. Therefore, of the 38 animals that began the study, 33 were included in the data analysis.
When comparing the air- and vapor-exposed ethanol consumption (g/kg) for the four post-dependence induction period self-administration sessions that occurred during acute withdrawal (see Figure 2), the two-way ANOVA identified a main effect of Exposure condition (F (1, 8) = 20.445, p≤0.01) and an Exposure × Session interaction (F (3, 24) = 3.813, p≤0.05).Thus, the air- and vapor-exposed groups were different from each other and ethanol vapor-exposure resulted in significantly escalated ethanol self-administration. To provide an example of the rates of responding for the dependent and nondependent animals, the number of lever-presses necessary to achieve the mean levels of ethanol intake (g/kg) during the 4th post-dependence induction acute withdrawal self-administration session (see Figure 2) were 70 (± 3.84) for the vapor-exposed and 26 (± 3.04)for the air-exposed animals.
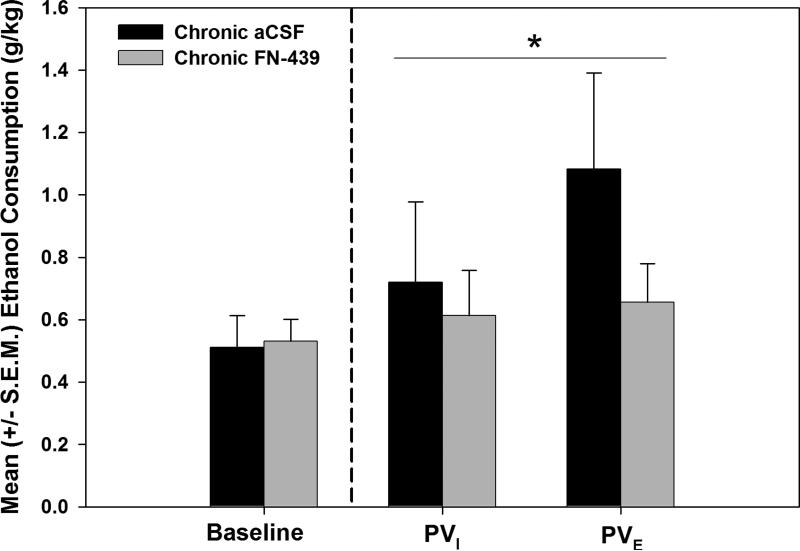
In the chronic FN-439 or aCSF condition, the aCSF-treated animals displayed escalated responding on the second post-dependence test session (t (4) = 2.23, p<0.05). The mixed model ANOVA conducted on the initial and escalated acute withdrawal self-administration sessions following chronic MMP inhibition or aCSF infusion via osmotic minipump (see Figure 3) during the dependence induction period and acute withdrawal test sessions indicated that there was a significant Drug Treatment × Session (F (1,14) = 6.833, p≤0.05). The Drug Treatment × Session interaction indicates that the animals acted differently depending on whether they were treated with chronic FN-439 or aCSF, although post-hoc independent samples t-tests comparing the performance of the chronic FN-439 vs. aCSF-treated rats were non-significant (t (10) = 1.446, p>0.05). The fact that the mixed model ANOVA shows a significant effect displays the importance of using the correct statistical tests (i.e., the use of the mixed-model ANOVA) to avoid making a Type II error (i.e., the null hypothesis is not rejected when it is in fact false).
Figure 3.
Chronic MMP inhibition prevents escalated responding for ethanol. Mean (+S.E.M) ethanol consumption during baseline and post-vapor self-administration sessions for animals that had chronic intracerebroventricular FN-439 (n = 7) or artificial cerebrospinal fluid (aCSF, n= 5) treatment via osmotic minipumps during the one month of intermittent vapor exposure and subsequent post-vapor testing. A significant Drug Treatment × Session interaction was found (*= p≤0.05) indicating that the escalation of ethanol self-administration differed over the sessions depending on whether the animals were aCSF-treated or had received FN-439. PVI = initial post-vapor sessions and PVE = escalated post-vapor session.
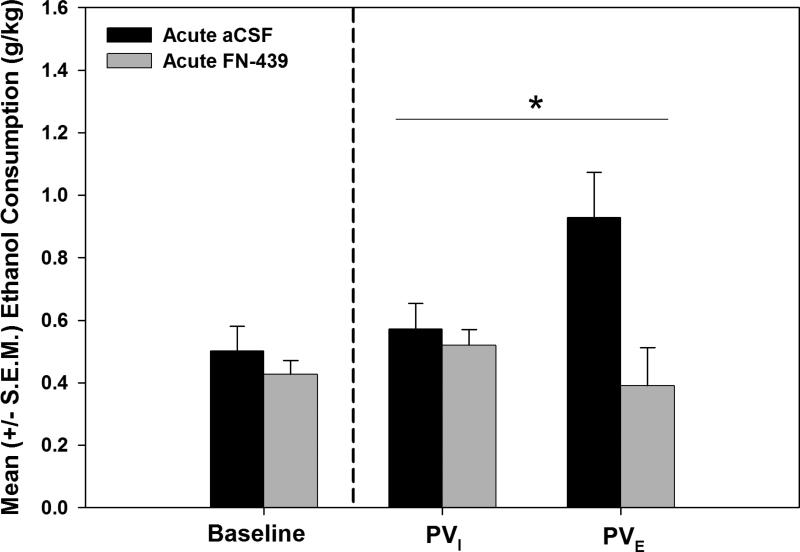
In the acute FN-439 or aCSF condition, the aCSF-treated animals displayed escalated responding on the fourth post-dependence test session (t (4) = 2.18, p<0.05). The mean (±S.E.M) ethanol consumption during the first three post-dependent self-administration sessions (data not graphically shown) for aCSF-treated animals (.61 ± .19, .64 ± .19 and .47 ± .18, respectively) and animals treated with FN-439 (.61 ± .14, .4 ± .11 and .54 ± .2, respectively) showed no significant differences over the sessions when analyzed with a one-way repeated measures ANOVA (F (2, 8) = 0.214, p > 0.05) anF (2, 10) = 0.383, p > 0.05, respectively). Although the three sessions are not significantly different from each other, averaging reduces variability, which could enhance the probability of rejecting the null hypothesis. Therefore, to protect against the commission of a Type I error, in addition to the two-way ANOVA that incorporated the averaged data from post-vapor sessions 1-3, a two-way ANOVA was conducted that only used the final session prior to escalation instead of the averaged data. When the two-way mixed model ANOVA was used to v analyze the post-vapor self-administration data, that included the averaged data, from animals challenged with acute FN-439 or aCSF infusions prior to and after self-administration sessions during acute withdrawal (see Figure 4), a significant main effect of Drug Treatment and a significant Drug Treatment × Session interaction were observed (F (1, 9) = 6.520, p≤0.05 and F (1, 9) = 6.980, p≤0.05, respectively). The two-way ANOVA that utilized the single post-vapor session data prior to escalation from animals challenged with acute FN-439 or aCSF infusions identified that the Drug Treatment × Session interaction (F (1, 9) = 12.347, p≤0.01) was maintained. Post-hoc independent samples t-tests comparing the performance of animals during the escalation session following the acute FN-439 vs. aCSF-treatments were significant (t (9) = 2.861, p < 0.05). These results showed that FN-439 and aCSF differentially affected the dependent animals and that the aCSF-treated group showed escalated self-administration when compared to the FN-439 treatment group.
Figure 4.
Acute MMP inhibition, restricted to post-dependence induction acute withdrawal test days, prevents escalated responding for ethanol. Mean (+S.E.M) ethanol consumption during baseline and post-vapor sessions for animals receiving acute intracerebroventricular FN-439 (n = 6) or artificial cerebrospinal fluid (n = 5) infusions following one-month of intermittent vapor exposure. A significant Drug Treatment × Session interaction was found (*= p≤0.05) indicating that acute exposure to FN-439 attenuated the negative reinforcement learning associated with the development of the dependence-like phenotype of excessive ethanol self-administration. PVI = initial post-vapor sessions and PVE = escalated post-vapor session.
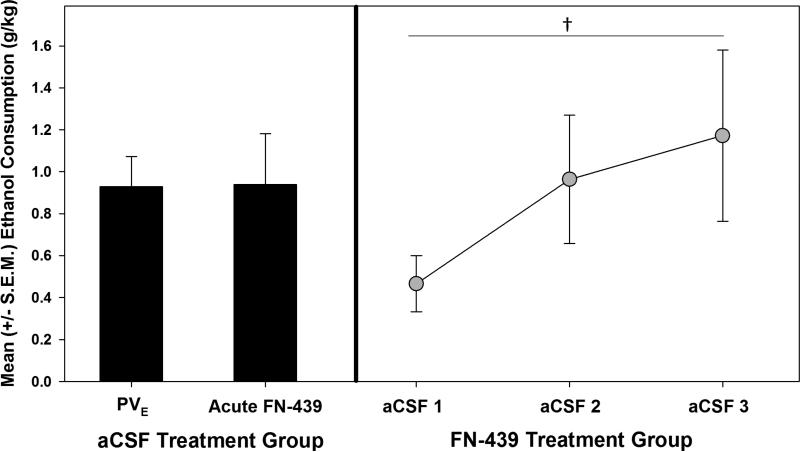
When analyzing the critical control conditions for interpretation of the present data from a learning perspective, a paired-sample t-test comparing the elevated self-administration and the acute FN-439 treatment session (see Figure 5) showed that there were no significant changes in behavior from the treatment (t (4) = -0.078, p > 0.05). This confirmed that once elevated responding was observed, that MMP inhibition did not have an impact, presumably because plasticity associated with learning had already occurred and MMPs were no longer activated. Conversely, when the originally treated FN-439 group was administered aCSF infusions prior to following self-administration session during acute withdrawal, a main effect of session (F (2, 10) = 4.049, p≤0.05) was observed, indicating an elevated ethanol self-administration pattern. Post-hoc LSD tests confirmed that the animals significantly (p < 0.05) consumed more alcohol during the final aCSF-treated session when compared to the first aCSF-treated session. This result confirms that prior FN-439 treatment did not permanently impair the ability of the animals to learn and show an escalated response pattern.
Figure 5.
Control conditions required for a learning-based explanation of dependence-induced escalation. Left panel: Mean (+S.E.M) ethanol consumption during the session in which the artificial cerebrospinal fluid (aCSF) – treated animals escalated (PVE) compared to those same animals receiving subsequent acute intracerebroventricular FN-439 infusion. The lack of an acute FN-439 effect shows that once negative reinforcement learning has occurred and the animals show escalated response patterns, inhibition of matrix metalloproteinases has no impact. Right Panel: Mean (+S.E.M) ethanol consumption following acute aCSF infusions in animals initially treated with FN-439 that did not display negative reinforcement learning (i.e., did not show escalated responding)–demonstrates that those animals are capable of learning and display a normal dependence-like phenotype in the absence of FN-439. A significant main effect of session (†= p≤0.05) was observed for the three aCSF-treated sessions.
4. Discussion
The current study prevented synaptic plasticity from occurring in the brain by disabling the molecular mechanisms which allow for ECM degradation and evaluated the consequences of such actions on escalated operant self-administration patterns that are observed during acute withdrawal in dependent rats. MMPs are required for the degradation of the ECM which accompanies plasticity, and so by inhibiting MMPs via FN-439, the ECM is essentially locked in place and plasticity may not occur (Wright and Harding, 2009). In the chronic exposure experiment, the MMP inhibitor FN-439, or aCSF, were chronically administered during the entire dependence induction period and during acute withdrawal operant self-administration sessions. This experiment was designed to broadly block synaptic plasticity associated with both dependence induction and escalated response patterns during acute withdrawal in dependent animals. The results showed that the animals chronically treated with FN-439 did not escalate as the aCSF-treated animals did. These initial findings confirmed that MMPs do have a role in dependence-like phenotypes. However, because the chronic FN-439 treatment spanned both the dependence induction period and post-dependence testing, these initial results did not distinguish between the blockade of plasticity (i.e., morphological changes) during dependence formation or acute withdrawal in dependent animals as contributors to the lack of escalation.
As a result, the second experiment was conducted in which the animals were allowed to develop dependence and then received acute administration of FN-439 immediately before and after the acute withdrawal operant self-administration sessions. This assessed whether MMP inhibition, at the time point at which negative reinforcement learning would be hypothesized to occur, would be sufficient to attenuate escalated operant self-administration even though any neuroadaptations associated with dependence-induction had been allowed to occur. Rats which received acute ICV infusions of FN-439 before and after self-administering ethanol did not develop the escalated response pattern that typifies acute withdrawal and is hypothesized to reflect negative reinforcement learning (Walker and Koob, 2008; Koob and Le Moal, 1997; 2008; Walker et al., 2011; Nealey et al., 2011). Thus, MMP inhibition during acute withdrawal in dependent animals was sufficient to attenuate escalations in operant self-administration even though the animals had an intact dependence induction phase. These results are consistent with those established previously showing that MMP expression is necessary for the occurrence of plasticity associated with learning (Meighan et al., 2007; Meighan et al., 2006; Wright et al., 2007; Wright et al., 2009). Furthermore, the acquisition and reconsolidation of cocaine-induced conditioned place preferences have been blocked by MMP inhibition via FN-439 (Brown et al., 2007).
However, the possibility existed that FN-439 has rate decreasing effects that were independent of FN-439 MMP inhibition. To rule this out, those animals that received aCSF during the post-dependence self-administration sessions and had shown normal escalation were treated with an acute infusion of FN-439 prior to a final self-administration session. The results showed that FN-439 did not suppress responding and confirmed that once escalation had occurred, MMP inhibition had no impact on operant self-administration rates. Another question that arose from the results of the second experiment was the possibility that MMP inhibition could cause a permanent suppression of learning. To confirm that this was not the case, those animals that were originally treated with FN-439 that did not escalate were treated with aCSF under the same conditions. The results showed that over the course of three sessions, ethanol self-administration escalated and therefore, normal negative reinforcement learning was observed.
Although the present experiments evaluated chronic FN-439 during dependence induction and acute withdrawal testing or during acute withdrawal test sessions only, we did not directly focus on inhibition of MMPs exclusively during the dependence induction period and the fact that the neuroadaptations that occur during that time period potentially serve as necessary prerequisites for typical escalated responding observed during acute withdrawal in dependent animals. Future studies will be needed to systematically investigate the precise changes produced by ethanol dependence and determine the role of MMPs in the dependence-induced neuroadaptations and plasticity.
Although FN-439 has been extensively characterized and repeatedly been shown to attenuate the effects of MMPs on the ECM and reduce various indices of associative and non-associative learning (including the underlying processes necessary for such learning to occur; Brown et al., 2007; Brown et al., 2009; Meighan et al., 2006; Wiediger et al., 2009; Wright et al., 2007), it is considered a general MMP inhibitor and does not allow for the precise specification of which MMPs are relevant to particular behaviors. Although the MMP family consists of over 25 enzymes, MMP-9 and MMP-3 have been heavily implicated in systems that could contribute to escalated responding and negative reinforcement learning. MMP-9 is required for the synaptic plasticity related to hippocampal-based long-term potentiation and memory (Nagy et al., 2006) and is involved in dendritic spine enlargement through a β1-integrin pathway (Wang et al., 2008). Furthermore, MMP-9 levels are increased following cocaine-primes in a reinstatement paradigm (Brown, Forquer, Harding, Wright, & Sorg, 2008), have been shown to be altered in the hippocampus by chronic cocaine exposure (Mash et al., 2007) and are increased in mice displaying behavioral sensitization to methamphetamine (Mizoguchi et al., 2007). Interestingly, a functional polymorphism in the MMP-9 gene is associated with alcohol dependence in humans (Samochowiec et al., 2010) and, although it relates to peripheral rather than brain MMPs, serum MMP-9 concentrations are increased alcoholics compared to controls (Sillanaukee, Kalela, Seppa, Hoyhtya, & Nikkari, 2002). In addition, MMP-3 is required for spatial learning (Meighan et al., 2006), as well as passive avoidance conditioning (Olson et al., 2008) and habituation (Wright et al., 2009). Future studies will need to be conducted in order to identify the precise involvement of the different MMPs, as well as specific brain nuclei in which synaptic remodeling is occurring as the basis of escalated operant self-administration.
Considerable evidence suggests that during dependence, allostatic dysregulation of motivational systems and negative reinforcement learning may promote the development of excessive alcohol consumption (Heilig et al., 2006; Heilig et al., 2010; Koob & Le Moal, 2008; Walker et al., 2008; Walker et al., 2010). This theory of allostatic dysregulation builds upon earlier theories of homeostasis (Solomon & Corbit, 1974) that describe the role of opponent processes in motivational and affective behaviors. In essence, when a drug induces a positive hedonic state, a compensatory negative hedonic state will follow. In cases of chronic alcohol or drug exposure, the positive hedonic state decreases and the negative hedonic state increases due to both within- and between-system changes that involve the recruitment of brain stress and negative affect circuits (Heilig & Koob, 2007; Koob2009a; Koob & Le Moal, 1997). Thus, when alcohol use is terminated, the neuroadaptations underlying these negative emotional states remain and the organism learns that continued ingestion of drugs self-medicates the symptoms associated with withdrawal (Heilig et al., 2006; Heilig et al., 2010; Markou et al., 1998). The combined results of the current experiments support this hypothesis by showing that preventing neural plasticity during acute withdrawal in dependent animals attenuates escalated operant self-administration of ethanol.
In summary, the present experiment confirmed that chronic intermittent ethanol vapor exposure induces the dependence-like phenotype of escalated ethanol self-administration that is hypothesized to represent negative reinforcement learning. Importantly, it was shown for the first time that inhibition of MMPs by FN-439 exclusively during acute withdrawal can disrupt the development of the dependence-like phenotype. This confirmed that escalated responding for ethanol during acute withdrawal in dependent animals is a learned response and that an intact MMP system is required for the plasticity that underlies such escalations in operant ethanol self-administration produced by dependence.
Acknowledgements
Support for this research was provided by R01AA020394 awarded to BMW by the National Institute on Alcohol Abuse and Alcoholism and WSU Alcohol and Drug Abuse Research Program grants awarded to AWS and BMW according to the State of Washington Initiative Measure No. 171. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington. The authors would like to thank Dr. Barbara Sorg for her assistance with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
None of the authors have any financial, personal or organizational conflicts of interest to report in relation to this manuscript.
Reference List
- Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J.Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA. Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn.Mem. 2007;14:214–223. doi: 10.1101/lm.476207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA. Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse. 2008;62:886–889. doi: 10.1002/syn.20562. [DOI] [PubMed] [Google Scholar]
- Brown TE, Wilson AR, Cocking DL, Sorg BA. Inhibition of matrix metalloproteinase activity disrupts reconsolidation but not consolidation of a fear memory. Neurobiol.Learn.Mem. 2009;91:66–72. doi: 10.1016/j.nlm.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol. 2008;4:235–247. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J.Neurosci.Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant Behavior and Alcohol Levels in Blood and Brain of Alcohol-Dependent Rats. Alcoholism: Clinical and Experimental Research. 2009 doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacology and Therapeutics. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict.Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009a;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009b;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos.Trans.R.Soc.Lond B Biol.Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Tsang VW, Birch NP. Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biol. 2008;4:223–234. doi: 10.1017/S1740925X09990172. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J. Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS.One. 2007;2:e1187. doi: 10.1371/journal.pone.0001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J.Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J.Neurochem. 2006;96:1227–1241. doi: 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T. Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J.Neurochem. 2007;100:1579–1588. doi: 10.1111/j.1471-4159.2006.04288.x. [DOI] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn.Mem. 2007;14:655–664. doi: 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. Journal of Neuroscience. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.012. doi:10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Critical Reviews in Neurobiology. 1993;7:23–39. [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism: Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olson ML, Meighan PC, Brown TE, Asay AL, Benoist CC, Harding JW, Wright JW. Hippocampal MMP-3 elevation is associated with passive avoidance conditioning. Regul.Pept. 2008;146:19–25. doi: 10.1016/j.regpep.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed ed. Elsevier, Academic Press; San Diego: 2005. [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J.Stud.Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behavioral and Neural Biology. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Samochowiec A, Grzywacz A, Kaczmarek L, Bienkowski P, Samochowiec J, Mierzejewski P, Preuss UW, Grochans E, Ciechanowicz A. Functional polymorphism of matrix metalloproteinase-9 (MMP-9) gene in alcohol dependence: family and case control study. Brain Research. 2010;1327:103–106. doi: 10.1016/j.brainres.2010.02.072. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcoholism: Clinical and Experimental Research. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P, Kalela A, Seppa K, Hoyhtya M, Nikkari ST. Matrix metalloproteinase-9 is elevated in serum of alcohol abusers. Eur.J.Clin.Invest. 2002;32:225–229. doi: 10.1046/j.1365-2362.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychological Review. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcoholism: Clinical and Experimental Research. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcoholism: Clinical and Experimental Research. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19520–19525. doi: 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiediger RV, Wright JW. Influence of dorsal hippocampal lesions and MMP inhibitors on spontaneous recovery following a habituation/classical conditioning head-shake task. Neurobiol.Learn.Mem. 2009;92:504–511. doi: 10.1016/j.nlm.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wright JW, Brown TE, Harding JW. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007;2007:73813. doi: 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Progress in Neurobiology. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Wright JW, Harding JW. Contributions of matrix metalloproteinases to neural plasticity, habituation, associative learning and drug addiction. Neural Plast. 2009;2009:57938. doi: 10.1155/2009/579382. [DOI] [PMC free article] [PubMed] [Google Scholar]