Abstract
The parathyroid hormone 1 receptor (PTH1R), a primary regulator of mineral ion homeostasis, is expressed on both the apical and basolateral membranes of kidney proximal tubules and in the LLC-PK1 kidney cell line. In LLC-PK1 cells, apical PTH1R subpopulations are far more effective at signaling via phospholipase (PLC) than basolateral counterparts, revealing the presence of compartmental signaling. Apical PTH1R localization is dependent upon direct interactions with ezrin, an actin-membrane cross-linking scaffold protein. Ezrin undergoes an activation process that is dependent upon phosphorylation and binding to phosphatidylinositol-4,5-bisphosphate (PIP2), a lipid that is selectively concentrated to apical surfaces of polarized epithelia. Consistently, the intracellular probe for PIP2, GFP-PLCδ1-PH, localizes to the apical membranes of LLC-PK1 cells, directly overlapping ezrin and PTH1R expression. Activation of the apical PTH1R shifts the GFP-PLCδ1-PH probe from the apical membrane to the cytosol and basolateral membranes, reflecting domain-specific activation of PLC and hydrolysis of PIP2. This compartmental signaling is likely due to the polarized localization of PIP2, the substrate for PLC. PIP2 degradation using a membrane-directed phosphatase shifts ezrin localization to the cytosol and induces ezrin de-phosphorylation, processes consistent with inactivation. PIP2 degradation also shifts PTH1R expression from brush border microvilli to basolateral membranes and markedly blunts PTH-elicited activation of the MAPK pathway. Transient expression of ezrin in HEK293 cells shifts PTH1R expression from the plasma membrane to microvilli-like surface projections that also contain PIP2. As a result, ezrin enhances PTH mediated activation of the PLC pathway in this cell model with increasing total receptor surface expression. Collectively, these findings demonstrate that the apical segregation of PIP2 to the apical domains not only promotes the activation of ezrin and the subsequent formation of the PTH1R containing scaffold, but also ensures the presence of ample substrate for propagating the PLC pathway.
Keywords: ezrin; parathyroid hormone; parathyroid hormone receptor; phosphatidylinositol-4,5-bisphosphate; phospholipase; LLC-PK1
1. Introduction
The parathyroid hormone 1 receptor2 (PTH1R) is a class b G protein coupled receptor (GPCR) that is activated by two prominent hormones; parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP) [1]. Through direct actions on bone and kidney, PTH, an endocrine factor, is a primary regulator of mineral ion homeostasis acutely controlling blood calcium levels and phosphate reabsorption in the proximal tubules of the kidney [2]. Conversely, PTHrP is a paracrine/autocrine factor that directs the development of many tissues [3]. Although coupling to Gs and signaling through the cAMP/PKA is the primary pathway [4], the PTH1R promiscuously activates the other major G protein sub-families, including Gq/11 [5], Gi [6, 7] and G12/13 [8], raising the question as to how these diverse signaling pathways are effectively coordinated to mediate desired cellular responses. With respect to other GPCR sub-families, one ligand typically activates several receptor isoforms, thus providing a level of regulation based on cell- and tissue-specific expression patterns. However, one receptor mediating physiological responses from two ligands appears to be unique to the GPCR superfamily, revealing an interesting signaling paradigm for the PTH1R. This attribute raises the question as to how one receptor can mediate such a diverse array of physiological processes.
As previously mentioned, the proximal tubule of the kidney is a primary target tissue for PTH actions, including the regulation of the type II sodium-phosphate co-transporters (NPT2a/c) [9] and the synthesis of vitamin D [10]. The PTH1R is expressed on both apical and basolateral membranes of this polarized epithelia [11, 12], thus providing an ideal model for the analysis of compartmental signal transduction. In both opossum kidney (OK) [6] and LLC-PK1 [13] cells, two model proximal tubule cell lines, apical PTH1Rs are more efficient at inhibiting NPT2a function when compared to basolateral receptor subpopulations, revealing compartment-dependent effects on downstream regulatory pathways. In LLC-PK1 cells grown on permeant membrane filters, apical application of PTH readily activates the PLC pathway with robust increase in inositol phosphates (IPs); in contrast, basolateral applications of ligand are far less effective at activating this pathway [14]. Conversely, differences in polarized signaling through the Gs/cAMP pathway are not evident [14]. These findings reveal that compartment dependent signaling mechanisms exist in the LLC-PK1 cell model for the PTH1R.
In order to investigate these phenomena, one must look at the signal transduction pathways linked to the PTH1R that are a step beyond coupling to G proteins to the existence of cell- and compartment-specific signaling complexes. A possible mechanism is the ability of the PTH1R to interact with intracellular constituents via cytoplasmic domains of the receptor. In recent years, the formation of signaling complexes or “signalsomes” directed by scaffold proteins has come to the forefront as a means of providing additional levels of signal transduction regulation beyond ligand-receptor interactions and G protein coupling [15, 16]. Using a yeast two-hybrid screen, a direct interaction between the C-terminal tail of the PTH1R and sodium-hydrogen exchanger regulatory factor 2 (NHERF-2) was discovered [7]. NHERF-2 and the closely related NHERF-1 are scaffold proteins that contain two PDZ interaction modules, which direct interactions with the PTH1R [7, 17], and are prominently expressed in the apical brush border of the kidney [18]. In a fibroblast cell model, NHERF-2 markedly enhances PTH1R signaling through the PLC pathway [7]. Notably all four isoforms of PLCβ contain conserved PDZ interaction motifs on their C-termini, motifs that direct NHERF co-immunoprecipitation [19] and receptor coupling [16, 20, 21]. In pull-down assays, NHERF-2 is capable of indirectly linking the C-terminal tails of the PTH1R and PLCβ1, thus coupling a downstream effector molecule to the activating receptor [7]. Furthermore, NHERF-1 directs complexes between the PTH1R, PLCβ1, PLCβ3 and the actin cytoskeleton in apical domains of OK cell models, a process that promotes PTH-mediated signaling through PLC [17]. NHERF-1 (also known as EBP50; ezrin-binding phosphoprotein of 50 kDa) and NHERF-2 contain a second binding module on their C-termini that directs strong interactions with the ERM (ezrin, radixin, moesin) family of proteins [22].
ERMs are keystone proteins that play crucial roles in the formation of specialized membranes, including the brush border membranes of the proximal tubule [23]. ERM proteins effectively link transmembrane proteins to the underlying cytoskeleton through an N-terminal FERM domain [24-26] and a C-terminal actin-binding motif [27, 28], respectively. In combination with NHERFs, ERM proteins, such as ezrin, readily form multi-faceted scaffold complexes. Through a polybasic amino acid motif located on the juxtamembrane region of the C-terminal tail, the PTH1R directly binds to the FERM domain of ezrin on a site that is mutually exclusive from the NHERF-1 binding domain [14]. Thus, the PTH1R, ezrin and NHERF-1 are capable of forming a ternary complex. In the LLC-PK1 cell model, formation of this complex is necessary for apical localization of the PTH1R [14]. However, in order to exist within these complexes, ezrin must be in an activated state. Through an auto-regulatory mechanism, the N-terminal FERM domain of ezrin binds to its own C-terminal region, forming a closed conformation that masks both FERM-domain-mediated interactions and actin cytoskeleton binding [29, 30]. Ezrin activation occurs in a two-step process; the FERM domain binds to phosphatidyinositol-4,5-bisphosphate (PIP2) in the membrane and via phosphorylation of threonine-567 located near the C-terminus [31]. In the activated state, ezrin assumes an open conformation, unmasking interaction domains and allowing for full participation in the formation of scaffold complexes linked to the cytoskeleton.
The requirement for PIP2 during ezrin activation and subsequent scaffold complex formation provided an intriguing link to the preferential PLC signaling mediated by apical PTH1R subpopulations, described above. Not only is PIP2 the substrate for PLC, phosphpoinositides differentially segregate to apical and basolateral membranes of polarized cell types, such as kidney epithelia [32]. Importantly, PIP2 localizes to apical membranes, thus raising the possibility that this lipid not only promotes the formation of activated ezrin scaffold complexes containing the PTH1R, but also is itself the substrate for PLC. Herein, the role of PIP2 during polarized PTH1R signaling via PLC and the regulation of ezrin activation in the LLC-PK1 cell model is investigated.
2. Materials and Methods
2.1. Materials
Total ERK and phospho-ERK antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Total ERM, phospho-ERM, β-catenin and monoclonal HA antibodies were from Cell Signaling Technology (Danvers, MA). Rho-tag-specific antibodies (1D4) were from National Cell Culture Center (Minneapolis, MN). Polyclonal HA antibodies were from Abcam (Cambridge, MA). Western Lightning ECL and 3H-myo-inositol were from Perkin-Elmer (Waltham, MA). DNA restriction enzymes were from either Promega (Madison, WI) or from New England Biolabs (Ipswich, MA). Pfu polymerase was from Agilent Technologies (Santa Clara, CA). All cell culture reagents, Fura2-AM, ZO-1 antibodies, Alex-Fluor-labeled secondary antibodies and adenoviral vector constructs and reagents were from Invitrogen (Carlsbad, CA). FuGene6 transfection reagent was from Roche (Indianapolis, IN). Matrigel was from Becton-Dickinson (Franklin Lakes, NJ). All remaining laboratory reagents were from Sigma (Saint Louis, MO).
2.2. Expression vector constructs
The intracellular PIP2 sensor consisting of the PLCδ1 pleckstrin homology domain fused to YFP was a generous gift from Dr. Tamas Balla. Using pfu polymerase and nested PCR, the pleckstrin homology domain of AKT (amino acids 1-167) was amplified from a human kidney cDNA library and directionally cloned onto the amino terminus of YPF in the pcDNA3.1 vector. The cDNA for the type IV phosphoinositide-5-phosphatase (PIPase) was another generous gift from Dr. Tamas Balla. Using PCR-mediated cloning, the catalytic domain of PIPase, lacking its native C-terminal lipid modification site, was cloned downstream of an HA tag. To generate the PIPaseKR construct, the polybasic, Kras membrane targeting sequence (SKKKKKKSKTKCVIM) [33] was appended to the C-terminus. The PTH1R-YFP [13, 17] and rho-tagged ezrin [14] constructs were as previously described. Adenoviruses expressing PLC-PH-YFP, AKT-PH-YFP, PIPase and PIPaseKR were generated using the ViraPower system from Invitrogen, following the manufacturers protocols as previously described for PTH1R-YFP and ezrin-rho.
2.3. Florescence microscopy
LLC-PK1 cells, a generous gift from Dr. Monique Arpin, and HEK293 cells were routinely cultured in DMEM supplemented with 10% fetal bovine serum with antimycotic and antibiotic agents from Invitrogen. Cells were plated in 4-well culture slides and either transduced with adenoviruses at an M.O.I. of ∼10 (LLC-PK1) or transfected with FuGene6 (HEK293). Forty-eight hours later, cells were fixed in 3.7% paraformaldehyde and thoroughly washed with phosphate buffered saline (PBS). Cells requiring immunological detection were permeabilized in PBS containing 0.1% Triton X-100 and Superblock from Thermo Scientific. Following incubations with the desired primary antibody, additional incubations with the appropriate, species-specific secondary antibodies, labeled with either Alexa-Fluor-488 (green) or Alexa-Fluor-546 (red), were performed. Samples were then analyzed by confocal microscopy using a Radiance 2100 confocal microscope and the associated LaserSharp 2000 software (Bio-Rad Laboratories, Inc., Hercules, CA). For assays requiring compartment-specific PTH applications, LLC-PK1 cells were grown on permeant membrane filters coated with a 1 to 30 dilution of Matrigel. Membrane filters were carefully excised from the baskets using a scalpel and placed on microscope slides for confocal analysis described above.
Formation and analysis of LLC-PK1 cysts is as follows. Permeant membrane filter baskets for 12-well plates, all tubes and pipets were cooled to 4°C, in order to prevent premature Matrigel polymerization. To reduce clumping, cells were put through a 40-μm cell strainer. Approximately 500 cells in 20 μl of media were mixed with 80 μl of Matrigel and immediately applied to the surface of the permeant membrane basket. The matrigel was allowed to polymerize at 37°C for 15 minutes, followed by the addition of complete media to the tops and bottoms of the baskets. Forty-eight hours later, cells were transduced with adenovirus at an M.O.I. of ∼100 in order to enhance the penetration of the viral particles through the mixed matrix. After another forty-eight hours, cysts were fixed with paraformaldehyde and immunostained as described earlier except that incubation times were doubled and the washing steps were increased. Membranes and the gelled matrix were excised from the baskets with a scalpel, placed on microscope slides with Secure-Seal spacers (Sigma) and immediately analyzed with a confocal microscope. The polyclonal HA antibodies used to detect the PIPase proteins bound excessively to the Matrigel matrix, preventing the production of publication quality images. Despite this background, PIPase expression within the cysts was discernable.
2.4. Immunoblotting and ERK phosphorylation
Total ezrin was extracted from LLC-PK1 cells using a lysis buffer consisting of 25 mM HEPES pH 7.4, 10% glycerol, 150 mM NaCl, 0.5% Triton X-100 and 1.0% SDS supplemented with protease and phosphatase inhibitor cocktails from Sigma. The viscous cell pellets were repeatedly vortexed during a 10 minute extraction period in order to shear the genomic DNA. The viscous clumps were removed from the lysate with a pipet tip. Lysates were analyzed using standard SDS-PAGE and immunoblotting on PVDF membranes. Bands were visualized with secondary antibodies conjugated with horseradish peroxidase and enhanced chemiluminescence. Following this immunoblotting procedure, analysis of the MAPK pathway using phospho-ERK antibodies was performed. For the compartment-specific PTH application assays, LLC-PK1 cells were grown on permeant membrane baskets designed for standard 6-well plates. Forty-eight hours post-transduction of the appropriate adenoviruses, cells were serum-starved for four hours prior to the application of ligand. Post-stimulation, cells were scraped and pelleted in pre-chilled tubes and extracted with a HEPES-based lysis buffer containing 1% Triton X-100 supplemented with protease and phosphatase inhibitor cocktails for 15 minutes on ice. Duplicate immunoblots were generated, one for total ERK and one for phospho-ERK. Detection and analysis was as described earlier.
2.5. PTH1R cell surface analysis
A florescence-based assay for the analysis of PTH1R cell surface expression was employed. Cells, either LLC-PK1 or HEK293, were plated in 12-well dishes and either transduced or transfected with PTH1R-YFP, respectively, and the vectors specific to the particular assay. For each assay condition, an equal number of wells were treated with either vehicle (acetic acid) or 100 nM PTH(1-34) labeled with tetramethylrhodamine (PTH-TMR) for 5 minutes, followed by washing with Hank's balanced salt solution. Cells were scraped, pelleted in microfuge tubes and extracted with a HEPES-based lysis buffer containing 1% Triton X-100 and 0.1% SDS. Supernatants were added to black 96-well plates and analyzed with an EnVision multilabel florescence plate reader (Perkin-Elmer, Waltham, MA) using YFP- and TMR-specific filter sets. This assay generates a surface expression index by quantifying surface receptors capable of binding to PTH-TMR divided by the total receptor expression determined from the PTH1R-YFP florescence. In the absence of receptor expression, PTH-TMR binding is negligible. Ratios derived in the absence of PTH-TMR, which represents YFP florescence bleed through into the red channel, are subtracted from the PTH-TMR positive ratios to yield the corrected surface index.
2.6. Analysis of second messengers
Analysis of total inositol phosphates and cAMP were as previously described [34]. For the analysis of intracellular calcium, a Fura2-based methodology was employed. Briefly, HEK293 cells were plated in black, 96-well plates and transiently transfected with the appropriate plasmids using FuGene6. Forty-eight hours post-transfection, cells were cooled to room temperature and washed with a complete Hank's balanced salt solution (HBSS). Cells were then loaded with 5 μM fura-2/acetoxymethyl ester (fura-2/AM) with 0.05% pluronic acid F-127 for 45 min at room temperature, washed in HBSS and unloaded for 30 min. Following baseline measurements, PTH(1-34) was added to individual wells, immediately followed by florescence detection with dual excitations at 340 and 380 nm and an emission at 515 nm over a time course of 200 s. Data are reported as a change in the 340/380 nm ratio over time in seconds averaged from four separate wells from a given transfection and fura2 loading experiment.
2.7. Data analysis
All of the data presented herein was replicated with a minimum of three independent experiments to confirm reproducibility. Where appropriate, statistical significance was determined using the Student's T test.
3. Results
3.1. Phosphoinositides display polarized segregation in LLC-PK1 cells
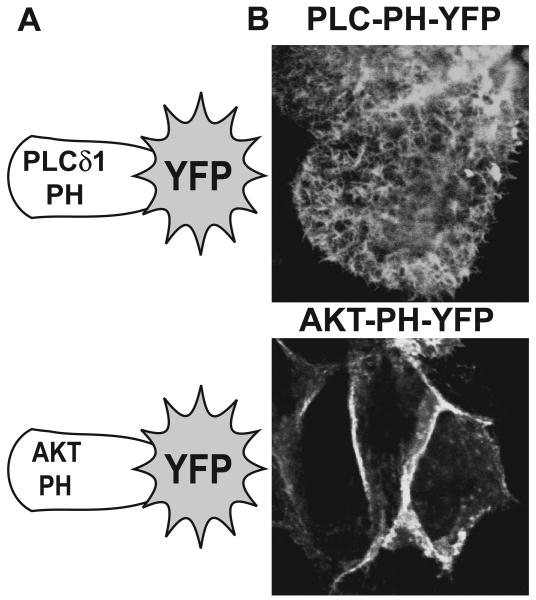
LLC-PK1 cells are a common, polarized, proximal tubule model system for the analysis PTH1R signaling. Proximal tubule-specific ezrin and NHERF-1 proteins are abundantly expressed and are appropriately localized to the apical domain within this model [13, 14]. LLC-PK1 cells readily form tight-junctions and domes, indicating the delineation of apical and basolateral domains and polarized transfer of solutes to the basal surfaces, respectively (data not shown). With respect to lipid-based signaling molecules, polarized localization of polyphosphoinositides has been demonstrated in several model systems with phosphatidylinositol-4,5-bisphosphate (PIP2) predominately localizing to apical surfaces and phosphatidylinositol-3,4,5-trisphosphate (PIP3) localizing to basolateral surfaces [32]. Cellular localization of PIP2 and PIP3 can be assessed using YFP fusions with pleckstrin homology domains from phospholipase Cδ1 (PLC-PH-YFP) and AKT (AKT-PH-YFP), respectively (Figure 1A) [35]. PLC-PH-YFP readily localizes to the apical brush-borders of LLC-PK1 cells, while the AKT-PH domain is primarily directed to the basolateral domains (Figure 1B), findings that are consistent with the polarized expression of these two prominent lipid-based signaling molecules.
Figure 1.
PIP2 and PIP3 predominantly localize to apical and basolateral membranes of LLC-PK1 cells, respectively. A. Schematics depicting the pleckstrin homology domains from PLCδ1 and AKT fused to YFP for the intracellular detection of PIP2 and PIP3, respectively. B. LLC-PK1 cells plated on culture slides were transduced with adenoviruses expressing either PLC-PH-YFP (PIP2 sensor, upper panel) or AKT-PH-YFP (PIP3 sensor, lower panel). Representative confocal images of the apical domains (PLC-PH-YFP, upper panel) or basolateral domains (AKT-PH, lower panel) are shown.
3.2. Apical PTH1R subpopulations preferentially signal via PLC
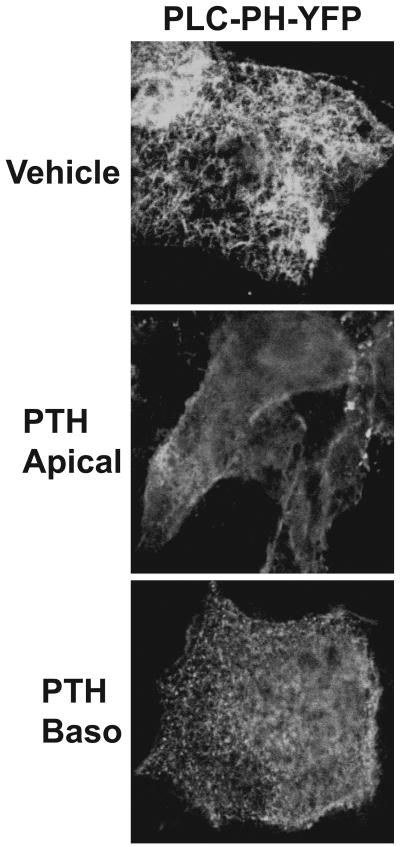
As previously mentioned, published findings from this lab demonstrate that apical PTH1R subpopulations are capable of generating substantially higher amounts of IPs when compared to basolateral receptors in LLC-PK1 cells [14]. Consistent with these findings, activation of the apical PTH1R induces a clear re-distribution of the PIP2 sensor (PLC-PH-YFP) to cytosolic and basolateral domains in LLC-PK1 cells grown on membrane filters, indicating PLC activation in this compartment (Figure 2A). In contrast, PLC-PH-YFP localization is only slightly altered when PTH is added to the basolateral side of the LLC-PK1 cells (Figure 2A). At this stage, preferential signaling via the PLC pathway from apical PTH1R subpopulations is likely due to the ample amount of substrate (i.e. PIP2) localized to this compartment. Next, the role of the apical PIP2 during ezrin activation and formation of the PTH1R-containing scaffold complex was examined.
Figure 2.
PIP2 hydrolysis and signaling via the MAPK pathway are primarily mediated by apical PTH1R subpopulations in LLC-PK1 cells. LLC-PK1 cells grown on perment membranes were doubly transduced with adenoviruses expressing the PTH1R and the PIP2 sensor, PLC-PH-YFP. Cells were treated with either vehicle (acetic acid, upper panel) or 10 nM PTH(1-34) applied to either the apical (middle panel) or basolateral (bottom panel) compartments for 10 minutes followed by fixation and analysis of YPF florescence using confocal microscopy.
3.3. PIP2 promotes ezrin activation and PTH1R apical localization in LLC-PK1 cells
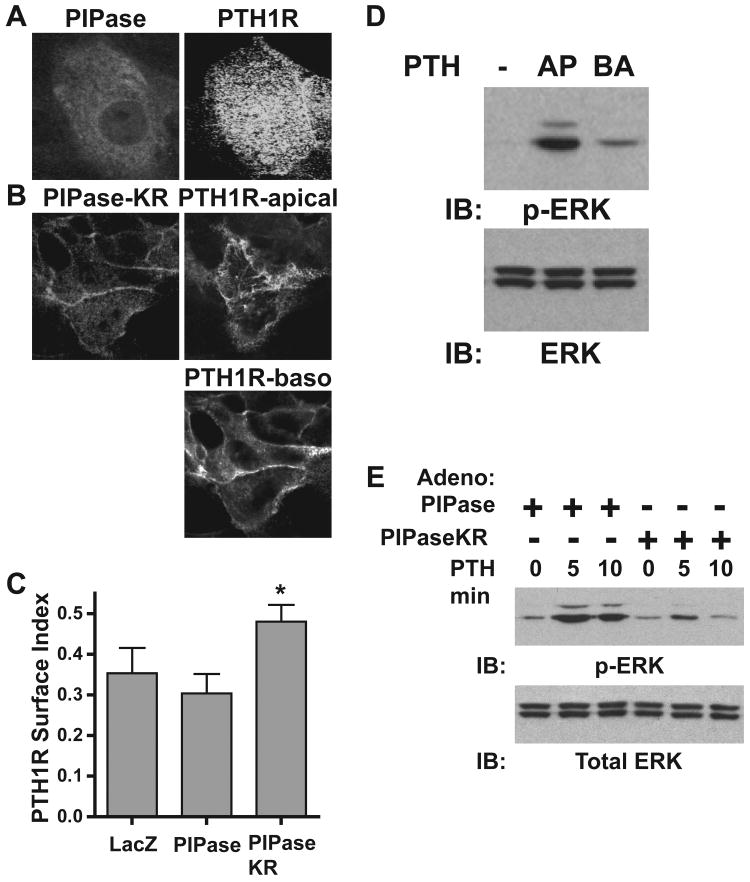
As previously mentioned, C-terminal phosphorylation and direct binding of PIP2 to the N-terminal, FERM domain synergize to promote the actin-membrane cross-linking activity of ezrin. To investigate the role of PIP2 during ezrin activation in LLC-PK1 cells, artificial hydrolysis of PIP2 was performed using the catalytic domain of the type IV 5-ptase (PIPase) targeted to the membrane, which removes the 5′ phosphate group yielding phosphatidylinositol-4-phosphate. To facilitate membrane targeting, the polybasic, CAAX motif of Kras, which becomes geranylgeranylated [33], was appended to the C-terminus of YFP. This membrane-targeting motif clearly promotes localization of YFP to the apical brush border of LLC-PK1 cells (Figure 3A). Likewise, the Kras targeting sequence anchors the PIPase (PIPaseKR) enzyme to the membranes of LLC-PK1 cells (Figure 3B, left panel). Furthermore, PIPaseKR completely dissociates the PIP2 sensor, PLC-PH-YFP, from the apical membranes and induces a marked localization to the nucleus, providing clear evidence for the functional removal of PIP2 from the plasma membranes (Figure 3B, right panel). Mason et al [36] when using a bacterial PIP2 phosphatase reported a similar localization pattern for PLC-PH-GFP.
Figure 3.
A PIP2 depleting enzyme targeted to the membrane (PIPaseKR) shifts the PIP2 sensor to the nucleus, indicating PIP2 hydrolysis. A. LLC-PK1 cells were transiently transfected with YFP containing a C-terminal membrane-targeting module from KRas (see schematic). Epiflorescent image of YFP-KRas efficiently targeted to the apical membrane domain is shown. B. LLC-PK1 cells were doubly transduced with adenoviruses expressing the membrane-targeted, PIP2 hydrolyzing enzyme, HA-PIPaseKR and PLC-PH-YFP. Cells were immunostained with HA-tag antibodies and secondary antibodies labeled with Alexa-Flour-546. Localization of HA-PIPaseKR (left panel) and PLC-PH-YFP (right panel) are shown in the representative confocal images.
Previously published findings demonstrate that ezrin directly binds to PIP2, an interaction that facilitates membrane localization and activation [31, 37, 38]. As shown in Figure 4A, PLC-PH-YFP and ezrin co-localize to apical membrane microdomains of LLC-PK1 cells, indicating an association between PIP2 and ezrin. PIPase targeting to the cytosolic compartment has no effect on the apical localization of ezrin in LLC-PK1 cells (Figure 4B); however, targeting PIPase to the membrane (PIPaseKR) induces a marked shift of ezrin to the cytosol (Figure 4C). Phosphorylation of the C-terminal threonine-567 is a second component associated with ERM protein activation. In the basal state, LLC-PK1 cells possess abundant levels of two phospho-ERM proteins, which consist of ezrin and possibly moesin (note the doublet shown in the immunoblots in Figure 4D). Transduction of an adenovirus expressing PIPase has no discernable effect on phosho-ERM levels in LLC-PK1 cells; in contrast, adeno-PIPaseKR promotes ERM dephosphorylation (Figure 4D). Cytosolic translocation and dephosphorylation clearly indicate that PIP2 depletion induces ezrin inactivation in LLC-PK1 cells.
Figure 4.
The activation state of ezrin is dependent upon PIP2 in LLC-PK1 cells. A. LLC-PK1 cells were doubly transduced with adenoviruses expressing the PIP2 sensor (PLC-PH-YFP) and the rho-tagged ezrin. Following immunostaining with rho-tag-specific antibodies (1D4) and Alexa-Flour-546-labeled secondary antibodies, confocal images of PLC-PH-YFP (left panel), ezrin (red, middle panel) and an overlayed image displaying co-localization (right panel) are shown. LLC-PK1 cells transduced with ezrin expressing adenoviruses and either adeno-PIPase (B) or adeno-PIPaseKR (C) were immunostained with HA-tag antibodies, as before. Confocal images displaying ezrin localization in the cells co-expressing the control PIPase (B) or the membrane-targeting PIPaseKR are shown (C). D. LLC-PK1 cells were transduced with adenoviruses expressing either beta-glucoronidase (GUS; control), PIPase or PIPaseKR, as indicated. Whole-cell, SDS lysates were immunoblotted with antibodies directed towards phospho-ERM (upper panel) or total ERM (lower panel). (color reproduction in print)
Through direct interactions, ezrin promotes apical localization of the PTH1R in LLC-PK1 cells [14]. Thus, the effect of PIP2 hydrolysis on PTH1R localization was examined. Cytosolic PIPase does not alter the apical PTH1R localization pattern (Figure 5A); however, PIPaseKR markedly impairs apical receptor localization, inducing a shift to the basolateral membranes (Figure 5B). Analysis of the surface index for the PTH1R reveals an increase in apical surface expression upon depletion of PIP2 in LLC-PK1 cells transduced with adeno-PIPaseKR when compared to adeno-PIPase expressing cells (Figure 5C). Transduction of adeno-PIPaseKR induces slight cell rounding and a reduction in cell-to-cell contacts, as revealed by the prevention of dome formation (data not shown). Therefore, quantification of apical versus basolateral PTH1R subpopulations cannot be achieved due to the ligand's ability to freely diffuse between apical and basolateral compartments. Therefore, the enhanced PTH1R surface expression is likely due analysis of total surface expression not just the apical membranes. With respect to signaling, PIPaseKR completely prevents PTH-mediated accumulation of IP3, as expected (data not shown). However, PIP2 depletion had no effect on PTH-mediated generation of cAMP (data not shown). Further evidence for the polarized, compartmental signaling of the PTH1R is demonstrated by a clear preference for activation of the MAPK pathway from apical receptors, as demonstrated by phospho-ERK analysis (Figure 5D). Membrane hydrolysis of PIP2 blunts apical PTH1R-mediated induction of ERK phosphorylation, demonstrating that signaling via MAPK is primarily PLC-dependent in the LLC-PK1 cell model (Figure 5E).
Figure 5.
PIP2 depletion disrupts apical localization of the PTH1R and blocks PTH-mediated activation of the MAPK pathway in LLC-PK1 cells. LLC-PK1 cells transduced with adenoviruses expressing PTH1R-YFP and either PIPase (A) or PIPaseKR (B) were immunostained with HA-tag antibodies and localization patterns of the indicated proteins were analyzed using confocal microscopy. C. LLC-PK1 cells were with transduced with PTH1R-YFP adenoviruses and either LacZ, PIPase or PIPaseKR, as indicated. The PTH1R surface index is reported, as described in the Materials and Methods section. (mean ±s.d.; n=4; * p < 0.05 from controls). D. LLC-PK1 cells grown on 6-well permeant membrane baskets were transduced with PTH1R adenoviruses and treated with 10 nM PTH(1-34) to either the apical (AP) or basolateral (BA) compartments for 5 minutes. Whole cell lysates were analyzed by immunoblotting with antibodies directed towards either phospho-ERK (upper panel) or total ERK (lower panel). E. LLC-PK1 cells expressing the PTH1R and either PIPase or PIPaseKR, as indicated, were treated with 10 nM PTH(1-34) for the times indicated. Whole-cell extracts were immunoblotted with antibodies directed towards either total ERK (lower panel) or phospho-ERK (p-ERK; upper panel).
3.4. PIP2 is required for lumen formation and polarity in LLC-PK1 cysts
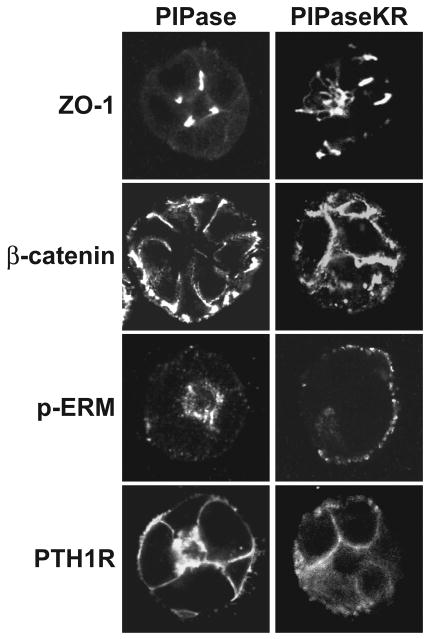
When grown in 3-dimensional, collagen matrices, LLC-PK1 cells form cysts, similar to MDCK cells [39]. Next, the effect of PIP2 hydrolysis on the localization patterns of key proteins was examined in these cysts. Unfortunately, the excessive background binding of the polyclonal HA-tag antibodies to the Matrigel components impaired production of publication quality images of the transduced PIPase enzymes (data not shown). Upon transduction of the control, cytosolic adeno-PIPase, LLC-PK1 cysts readily form lumens and tight cell junctions, as demonstrated by distinct localization of ZO-1 at cell-to-cell contact sites (Figure 6). LLC-PK1 cells within the cysts appear to be polarized due to the localized basolateral expression of β-catenin and apical expression of the endogenous phospho-ERM (p-ERM) proteins (Figure 6). Transduction of adeno-PTH1R-YFP reveals both apical and basolateral receptor subpopulations (Figure 6), localization patterns that are consistent with LLC-PK1 cells grown in monolayers. In general, LLC-PK1 cysts transduced with adeno-PIPaseKR appear disorganized with no discernable lumen formation. ZO-1 localization and the presence of tight junctions are disordered and β-catenin is no longer restricted to defined basolateral membranes (Figure 6). PIPaseKR also decreases the immunoreactivity of the phosph-ERM proteins and induces a distinct shift from the apical/luminal surface to the outer membranes of the LLC-PK1 cysts (Figure 6). Consistent with these findings, PTH1R localization is equally distributed among the entire membrane surface without pronounced expression on an apical domain (Figure 6). Overall, artificial hydrolysis of PIP2 appears to de-polarize LLC-PK1 cysts, revealing an important role for this lipid in establishing and/or maintaining polarized epithelia.
Figure 6.
PIP2 depletion disrupts the overall polarity of LLC-PK1 cysts grown in Matrigel. LLC-PK1 cysts were grown in 3-dimensional culture of Matrigel as described in the Materials and Methods section. After forty-eight hours, cysts were transduced with adenoviruses expressing either PIPase (left panels) or PIPaseKR (right panels) and adenoviruses expressing the PTH1R-YFP for only the bottom panels, as indicated. Representative confocal images of cysts immunostained for ZO-1, β-catenin, phopho-ERM and YPF florescence from PTH1R-YFP are shown.
3.5. Ezrin promotes PLC signaling via formation of PIP2/PTH1R complexes in HEK293 cells
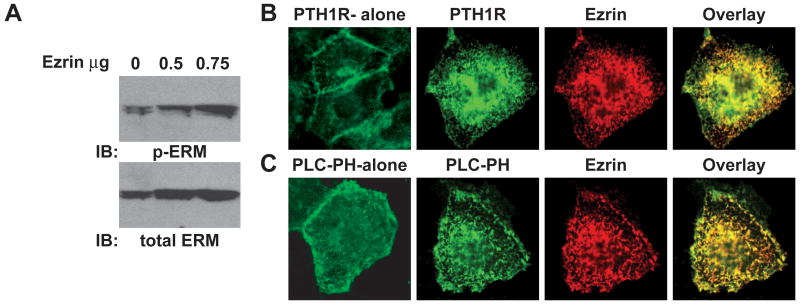
Attempts to knockdown endogenous ezrin from LLC-PK1 cells met with limited success (data not shown). Furthermore, the presence of a second ERM protein, which is likely moesin, would complicate this approach. Due to low levels of endogenous ezrin, the HEK293 cell model was examined. Previous findings from this laboratory revealed that transient expression of ezrin in HEK293 cells generates microvilli-like projections, containing ezrin and the PTH1R [14]. This phenomenon was examined further. HEK293 cells express detectable levels of ERM proteins (Figure 7A, lower panel), although at a level that is substantially lower than LLC-PK1 cells, as determined qualitatively by the differential exposure times required to detect the antigens on an immunoblot (data not shown). Expectedly, HEK293 cells express lower levels of the active, phospo-ERM proteins (Figure 7A, upper panel). Transient transfection of increasing amounts of ezrin results in correspondingly higher levels of phospho-ERM, specifically the upper band in the doublet (Figure 7A, upper panel), demonstrating a increase in activation that parallels the higher expression levels.
Figure 7.
Exogenous expression of ezrin in HEK293 cells promotes co-localization of ezrin, PTH1R and PIP2 within microvilli-like projections. A. HEK293 cells grown in 6-well plates were transiently transfected with the indicated amounts of ezrin expression plasmid, followed by immunoblotting of whole-cell extracts with antibodies specific for total ERM (lower panel) and phospho-ERM (upper panel). HEK293 cells grown in culture slides were transfected with either 100 ng of PTH1R-YFP alone (B; 1st panel) or PLC-PH-YFP alone (C; 1st panel) and in the presence of 100 ng of an ezrin expression plasmid (B and C; panels 2 to 4), as indicated. Ezrin positive wells were immunostained with 1D4 antibodies and Alex-Fluro-546-labeled secondary antibodies. Representative confocal images of the indicated proteins and overlayed images are shown. (color reproduction in print)
As shown in Figure 7B, PTH1R expression alone in HEK293 cells displays a typical plasma membrane localization pattern with some intracellular florescence due to unprocessed receptors. Co-expression of the PTH1R and ezrin, however, reveals strong co-localization to the microvilli-like projections (Figure 7B), yielding a pattern that is remarkably similar to that demonstrated in LLC-PK1 cells [13, 14]. Due to the distinct localization pattern displayed by ezrin, PIP2 expression was analyzed next. The PIP2 sensor expressed in HEK293 cells alone reveals diffuse localization to the plasma membrane with some punctate-like expression patterns (Figure 7C). Transient co-expression of the PIP2 sensor with ezrin, in contrast, reveals sharply defined co-localization patterns on the surface of HEK293 cells (Figure 7C). These findings suggest that ezrin influences PIP2 localizaton patterns, forming specialized membrane domains.
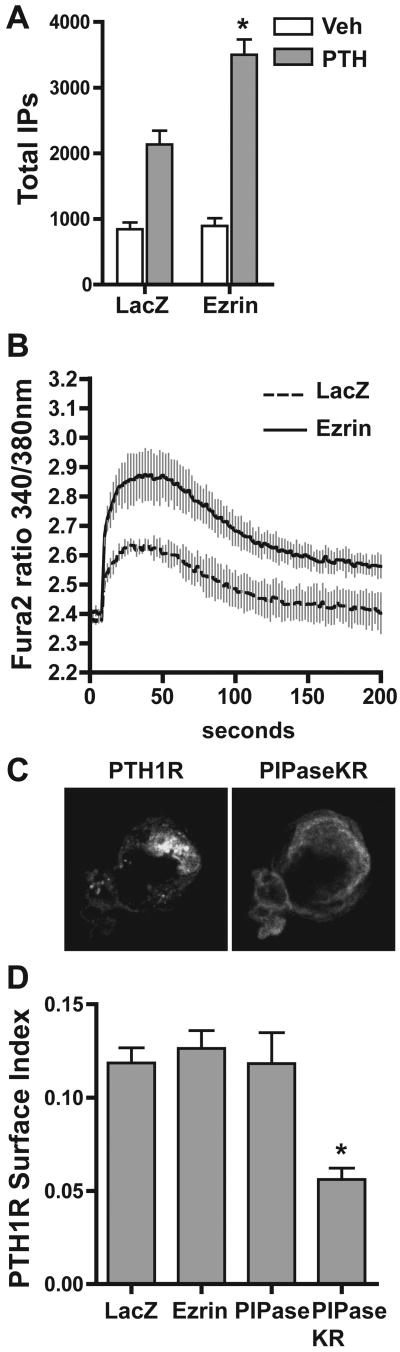
If ezrin acts to form specialized membrane domains rich in PIP2, then co-localization of the PTH1R in these regions is likely to influence the level of PLC signaling due to a concentrated source of substrate, i.e. PIP2. Consistent with this hypothesis, co-expression of the PTH1R and ezrin in HEK293 cells enhances PTH-mediated accumulation of total inositol phosphates (Figure 8A) and release of intracellular calcium (Figure 8B). These findings are consistent with the enhanced PLC signaling from apical PTH1Rs in LLC-PK1 cells, a process that is dependent upon the ezrin-based scaffold [14]. In contrast, no discernable effects on PTH-mediated signaling through the Gs/cAMP pathway were found (data not shown). Ezrin influences PTH1R expression patterns in a manner that does not increase the total receptor surface expression in HEK293 cells, as determined by the surface index (Figure 8D), revealing receptor relocation to the microvilli-like projections is responsible for the enhanced PLC signaling and not due to an increase in membrane surface expression. Surprisingly, hydrolysis of PIP2 from the plasma membrane markedly reduces PTH1R surface expression in HEK293 cells (Figures 8C and D), an effect not demonstrated in LLC-PK1 cells (Figure 5C).
Figure 8.
Ezrin promotes PTH1R mediated signaling via PLC and PIP2 depletion impedes receptor cell surface expression in HEK293 cells. HEK293 cells grown in either 24-well plates and labeled with 3H-myo-inositol (A) or 96-well plates (B) were transiently transfected with the PTH1R and either LacZ or ezrin, as indicated, at 100 ng for each plasmid/well in A and 20 ng for each plasmid/well in B. A. Cells were treated with either vehicle (acetic acid; white bars) or 100 nM PTH(1-34) (gray bars) for 15 minutes, followed by analysis of total inositol phosphates (mean ±s.d.; n=4; * p < 0.05 from LacZ transfected controls). B. Cells were loaded with Fura2 and PTH-mediated increase of intracellular calcium analyzed as described in the Materials and Methods section (mean ±s.d.; n=4; * p < 0.05 from LacZ transfected controls). C. HEK293 cells transiently transfected with PTH1R-YFP and PIPaseKR were immunostained with HA-tag-specific antibodies. Confocal images of the indicated proteins are shown. D. HEK293 cells grown in 12-well plates were transiently transfected with the PTH1R-YFP and either LacZ, ezrin, PIPase or PIPaseKR, as indicated, at 200 ng for each plasmid/well. The PTH1R cell surface index is reported (mean ±s.d.; n=3; * p < 0.05 from PIPase transfected controls).
4. Discussion
Herein, several new aspects have been put forth regarding PTH1R signaling in a polarized cell model and the role of an apical domain-specific signalsome. The primary goal for this current research was to examine mechanisms that promote robust PLC signaling from apical PTH1R subpopulations expressed in LLC-PK1 cells. Three additional findings support apical domain-specific signaling through PLC. First, the intracellular PIP2 sensor, which consists of the pleckstrin homology domain of PLCδ1, readily localizes to the brush border of LLC-PK1 cells, indicating ample amounts of spatially localized substrate. Second, apical application of PTH clearly shifts localization of this PIP2 sensor from the brush border to intracellular/basolateral sites, indicating substrate hydrolysis. PIP2 sensor localization patterns were largely unaffected upon activation of basolateral PTH1Rs. Third, signaling via MAPK is substantially greater when apical PTH1R subpopulations are activated when compared to basolateral receptors; a pathway that is primarily PLC-dependent in LLC-PK1 cells due to the marked inhibition upon artificial PIP2 depletion. A simple interpretation of these findings is that apical PTH1R subpopulations robustly activate the PLC pathway because the effector's substrate is localized to this compartment. However, several key facets associated with the apical signalsome must be considered.
Over the past several decades, it is clear that the PTH1R can signal through many pathways, processes that reflect the complex physiology regulated by this GPCR. How these many pathways are regulated in a spatial and temporal manner is an exciting area of research. Signaling via adenlyl cyclase(AC)/cAMP and PLC/inositol phosphates are the two major pathways elicited by the PTH1R. Are these pathways influenced by compartment-specific signalsomes? With respect to the cAMP pathway, a membrane-bound PTH1R activates a membrane-bound effector, AC, in the presence of an abundant level of soluble substrate, ATP. Using fluorescently labeled forskolin, which tags AC, Ferrandon et al [40] reported co-localization of the PTH1R, Gαs and AC within intracellular vesicles, revealing the existence of a signalsome directing activation of the cAMP/PKA pathway. In contrast to the cAMP pathway, second messenger generation from the PLC pathway requires activation of a soluble effector (i.e. PLC) from a membrane-bound receptor using a limited, spatially localized, membrane-bound substrate (i.e. PIP2). Considering these parameters, productive signaling via the PLC pathway likely requires the formation of specialized signaling complexes.
Several key attributes of the ezrin/NHERF-based scaffold within the apical brush border membranes likely promote PTH1R-mediated signaling via the PLC pathway. First, as previously mentioned, all four isoforms of PLCβ contain conserved PDZ interaction motifs that readily bind NHERF proteins, promoting localization and coupling to GPCRs [16, 19-21]. Mechanistically, the scaffold complex concentrates a particular PLCβ to PTH1R-containing membrane domains, thus making the effector insoluble and limiting the effects of cytosolic diffusion. Second, previous findings from this laboratory reveal that a ternary complex consisting of direct interactions between activated ezrin, NHERF-1 and C-terminal tail of the PTH1R function to anchor the receptor to the apical domains [14]. Loss of apical PTH1R subpopulations through ezrin inactivation via enzyme-directed PIP2 hydrolysis is corroborative evidence as to the role of the ezrin-based scaffold during brush border receptor localization. Lastly, several key pieces of evidence reveal that PIP2 itself is a critical co-factor in the ezrin-based scaffold and the existence of the apical PTH1R signalsome.
Several published findings demonstrate that phosphoinositides play a key role during epithelial morphogenesis and establishment of membrane polarity with PIP2 segregating to apical domains and PIP3 to the basolateral compartment (reviewed in [32]). Notably, the current model system, LLC-PK1 cells, displays polarized phosphoinositide expression patterns that are consistent with polarized epithelia with PIP2 and PIP3 localizing to the apical and basolateral domains, respectively. Chronic, artificial hydrolysis of PIP2 using PIPaseKR prevents apical membrane localization and phosphorylation of the C-terminal threonine-567 of ezrin. Loss of apical PTH1R localization upon PIP2 depletion directly correlates with ezrin inactivation, an effect that is consistent with ezrin-dependent receptor localization to this compartment [14]. Interestingly, acute, PTH1R activation promotes ezrin internalization into vesicles and a concomitant increase of ezrin in the Triton X-100 soluble fraction in LLC-PK1 cells [13], effects that reflect ezrin inactivation, but through differing mechanisms. These differential effects are likely due to chronic versus acute hydrolysis of PIP2. For example, cytosolic localization of ezrin due to chronic depletion of PIP2 likely enhances dephosphorylation mediated by phosphatases within this compartment over time. Furthermore, unlike PIPaseKR, PTH1R activation also leads to activation of additional pathways (i.e. cAMP/PKA), recruitment of β-arrestin and the formation of endocytic vesicles.
Evidence continues to accumulate regarding the essential role(s) of PIP2 in the formation and maintenance of epithelial cell polarity. In LLC-PK1 cysts, PIP2 depletion readily disrupts lumen formation and the localization patterns of key polarity marker proteins, such as ZO-1, β-catenin and phospho-ERMs. Importantly, preferential localization of the PTH1R to apical surfaces is also disrupted. In a seminal work by Martin-Belmonte et al [41], the essential role of PIP2 localization to the apical membranes in the formation of polarized Madin-Darby canine kidney (MDCK) cell cysts was demonstrated, involving a complex regulatory network incorporating phosphatase and tensin homolog on chromosome 10 (PTEN), the small GTP-binding protein, cdc42 and actin cytoskeletal elements. Qualitatively, luminal size appears larger for the MDCK cysts when compared the LLC-PK1 cells, however, localization patterns for ZO-1 and β-catenin are indistinguishable for both models in the basal state and upon depletion of PIP2 levels. Notably, ectopic basolateral membrane insertion of exogenous PIP2 in these MDCK cysts induces a rapid relocalization of ezrin from the apical membranes to the basolateral surface, indicating that this lipid is plays a role in membrane targeting [41]. In the current work, PIP2 depletion induces cytoplasmic localization and dephosphorylation. Combined, these data demonstrate that membrane localization of ezrin, in general, requires PIP2 interactions and that appropriate apical localization of this lipid establishes a properly formed ezrin-based scaffold complex. Analogous to the current approach, Grinstein and co-workers [36] induced PIP2 depletion using a bacterial phoshatase in various epithelial cell models. Disruption of cell-to-cell contacts and mislocalization of ZO-1 reported in this work are consistent with the findings herein.
It is clear that PIP2 within the LLC-PK1 apical domain functions to promote ezrin activation, but does the ezrin-based scaffold reciprocate and function to maintain the segregated PIP2 expression pattern? As noted above, PTEN, a phosphatidyl-3,4,5-trisphosphate-3-phosphatase, is localized to apical surfaces of MDCK cysts where it maintains PIP2 levels through hydrolysis of PIP3 [41]. Notably, several reports from Georgescu and co-workers [42-44] demonstrate that NHERF-1 directly binds to and regulates the activity of PTEN, including the down regulation PI3-kinase signaling and the up regulation of tumor suppressor activity. Although not investigated in this current work, direct association of PTEN with the apical, ezrin/NHERF scaffold possibly aids in maintaining PIP2 in the brush border domains of LLC-PK1 cells. These findings suggest that the ezrin scaffold maintains PIP2 levels through the actions of a phosphatase, however, other key pieces of evidence suggest that this scaffold also promotes the formation of PIP2 via associated kinase actions.
De novo synthesis of PIP2 arises from the phosphorylation of phosphatidylinositol-4-phosphate catalyzed by various isoforms of phosphatidylinositol-4-phosphate-5-kinase (PI4P5K) (reviewed in [45]). Analogous to the PTEN/NHERF-1 interaction, the type I phosphatidylinositol-4-phosphate-5-kinase isoform beta (PIPKIbeta) binds to NHERF-1 via a PDZ domain-mediated interaction [46], a process that regulates neutrophil chemotaxis [47]. Several reports demonstrate that ezrin and the small, GTP-binding protein RhoA participate in a reciprocal activation cycle [48-52]. A downstream effector of RhoA-GTP is PI4P5K [53], leading to an increase in PIP2 levels, an effect that would certainly promote ezrin activation. Furthermore, the active forms of ERM proteins readily bind to RhoGDI, (GDP dissociation inhibitor), a negative regulator of RhoA activity [48, 54]. Thus, through sequestration of RhoGDI, ezrin promotes RhoA activity, leading to more PIP2 synthesis and so on. This ongoing activation cycle, aided by the ezrin/NHERF scaffold, likely establishes a functional PLC signalsome for the PTH1R by concentrating receptor, effector and substrate within a defined apical compartment.
Several compelling observations arose from the analysis of the HEK293 cell model. First, straightforward, transient expression of wild type ezrin yielded distinct microvilli-like projections on the surface of HEK293 cells that are remarkably similar to microvilli normally found on LLC-PK1 cells. This finding illustrates that ERM proteins are capable of organizing complex membrane domains. Second, images of the PIP2 sensor revealed diffuse, membrane localization of PIP2 in HEK293 cells. In contrast, ezrin appeared to readily concentrate PIP2 to these microvilli-like membrane structures, promoting a PIP2 expression pattern that is consistent with a polarized cell model. Notably, the PTH1R migrated from a typical plasma membrane expression pattern to these surface projections upon ezrin expression, generating a specialized membrane domain in this otherwise generic cell model. Notably, the resulting complex promoted PTH1R-mediated signaling via the PLC pathway without increasing total surface expression of the receptor, suggesting that ezrin aided signal transduction by localizing substrate with receptor. Unexpectedly, PIP2 depletion induced by PIPaseKR markedly reduced surface expression of the PTH1R in HEK293 cells, an effect that was not demonstrated in LLC-PK1 cells. This finding suggests two possibilities. First, the low level, endogenous expression of activated ezrin is possibly required for PTH1R membrane expression, a process that would be markedly impaired through PIPaseKR-mediated ezrin inactivation. Second, normal surface PTH1R expression may require PIP2-containing membranes through direct lipid-receptor interactions or from an intermediate linking protein(s) or alternate scaffold not expressed in HEK293 cells.
Ezrin specifically and ERM proteins in general play critical roles in the formation of specialized membrane domains, such as the brush border membranes of the kidney proximal tubule. The ability to organize and maintain these specialized domains imparts the phenotypic characteristics attributed to these cell types. These complexes pull together receptors and downstream effectors in close proximity, allowing for a domain-specific level of regulation and specificity of elicited generation of second messengers. With respect to PTH1R-mediated PLC signaling, the work herein demonstrates a clear role for PIP2 in maintaining the ezrin-based scaffold complex and providing the required substrate for signal propagation in a compartment-dependent manner.
5. Conclusions
Phosphoinositides display a polarized localization pattern in LLC-PK1 cells with PIP2 and PIP3 segregating to apical and basolateral membranes, respectively. These patterns are consistent with a well-polarized cell model.
The apical PTH1R subpopulation preferentially signals via the PLC pathway when compared to the basolateral receptors, as demonstrated by PTH-mediated redistribution of the PIP2 sensor, PLC-PH-YFP, indicating PIP2 hydrolysis. This data corroborates previously published findings demonstrating enhanced accumulation of inositol phosphates upon activation of apical PTH1Rs.
PIP2 depletion, mediated by a membrane-targeted PIP2 phosphatase, fully inactivates ezrin in LLC-PK1 cells, as indicated by cytosolic localization and dephosphorylation of threonine-567.
Induced ezrin-inactivation via the phosphatase disrupts PTH1R apical localization and the apical-domain-specific activation of the MAPK pathway elicited by PTH, establishing a vital role of the ezrin-based scaffold for compartmental signaling of the PTH1R. PIP2 hydrolysis also disrupts lumen formation and polarity of LLC-PK1 cysts grown in 3D culture, demonstrating a vital role for this phosphoinositide in establishing and maintaining polarity.
Exogenous ezrin expression in HEK293 cells generates PTH1R-containing complexes that co-localize with PIP2, resulting in enhanced signaling via the PLC pathway.
Acknowledgments
This study was funded by a program project grant from the NIH (P01 DK073911) to Dr. John Potts. Thanks to Dr. Tamas Balla and Monique Arpin for providing invaluable reagents.
Abbreviations
- PTH
parathyroid hormone
- PTHrP
parathyroid hormone-related protein
- PTH1R
parathyroid hormone 1 receptor
- NPT2a
type IIa sodium-phosphate co-transporter
- OK
opossum kidney cell line
- PLC
phospholipase
- IPs
total inositol phosphates
- NHERFs
sodium hydrogen exchanger regulatory factors
- EBP50
ezrin-binding phosphoprotein of 50 kDa
- PIP2
phosphatidyinositol-4,5-bisphosphate
- PIP3
phosphatidyinositol-3,4,5-trisphosphate
- ERM
ezrin-radixin-moesin family of proteins
- p-ERM
ERM proteins phosphorylated on C-terminal threonines
- PLC-PH-YFP
pleckstrin homology domain of PLCδ1 fused to yellow florescent protein (PIP2 sensor)
- AKT-PH-YFP
pleckstrin homology domain of AKT protein kinase fused to yellow florescent protein (PIP3 sensor)
- PIPase and PIPaseKR
cytosolic and membrane-targeted catalytic domain of the type IV phosphoinositide-5-phosphatase, respectively
- ZO-1
zonula occludens tight-junction protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kronenberg HM, Lanske B, Kovacs CS, Chung UI, Lee K, Segre GV, Schipani E, Jüppner H. Recent Prog Horm Res. 1998;53:283–301. [PubMed] [Google Scholar]
- 2.Mannstadt M, Jüppner H, Gardella T. Am J Physiol. 1999;277:F665–675. doi: 10.1152/ajprenal.1999.277.5.F665. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg HM, Karaplis AC, Lanske B. Ann N Y Acad Sci. 1996;785:119–123. doi: 10.1111/j.1749-6632.1996.tb56249.x. [DOI] [PubMed] [Google Scholar]
- 4.Jüppner H, Schipani E, Bringhurst FR, McClure I, Keutmann HT, Potts JT, Jr, Kronenberg HM, Abou-Samra AB, Segre GV, Gardella TJ. Endocrinology. 1994;134:879–884. doi: 10.1210/endo.134.2.8299582. [DOI] [PubMed] [Google Scholar]
- 5.Bringhurst FR, Jüppner H, Guo J, Urena P, Potts JT, Jr, Kronenberg HM, Abou-Samra AB, Segre GV. Endocrinology. 1993;132(5):2090–2098. doi: 10.1210/endo.132.5.8386606. [DOI] [PubMed] [Google Scholar]
- 6.Reshkin S, Forgo J, Murer H. J Membr Biol. 1991;124:227–237. doi: 10.1007/BF01994356. [DOI] [PubMed] [Google Scholar]
- 7.Mahon M, Donowitz M, Yun C, Segre G. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- 8.Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PH. Endocrinology. 2005;146(5):2171–2175. doi: 10.1210/en.2004-1283. [DOI] [PubMed] [Google Scholar]
- 9.Tenenhouse HS. J Steroid Biochem Mol Biol. 2007;103(3-5):572–577. doi: 10.1016/j.jsbmb.2006.12.090. [DOI] [PubMed] [Google Scholar]
- 10.Kong XF, Zhu XH, Pei YL, Jackson DM, Holick MF. Proc Natl Acad Sci U S A. 1999;96:6988–6993. doi: 10.1073/pnas.96.12.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amizuka N, Lee HS, Khan MY, Arazani A, Warhawsky H, Hendy GN, Ozawa H, White JH, Goltzman D. Endocrinology. 1997;138:469–481. doi: 10.1210/endo.138.1.4845. [DOI] [PubMed] [Google Scholar]
- 12.Ba J, Brown D, Friedman PA. Am J Physiol Renal Physiol. 2003;285(6):F1233–1243. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 13.Mahon MJ. Am J Physiol Renal Physiol. 2008;294(3):F667–675. doi: 10.1152/ajprenal.00276.2007. [DOI] [PubMed] [Google Scholar]
- 14.Mahon MJ. Mol Endocrinol. 2009;23(10):1691–1701. doi: 10.1210/me.2009-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luttrell LM. J Mol Neurosci. 2005;26(2-3):253–264. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- 16.Kim JK, Lim S, Kim J, Kim S, Kim JH, Ryu SH, Suh PG. Adv Enzyme Regul. 2010 doi: 10.1016/j.advenzreg.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Mahon M, Segre G. J Biol Chem. 2004;279:23550–23558. doi: 10.1074/jbc.M313229200. [DOI] [PubMed] [Google Scholar]
- 18.Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Am J Physiol Cell Physiol. 2001;280(1):C192–198. doi: 10.1152/ajpcell.2001.280.1.C192. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. J Biol Chem. 2000;275(48):37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- 20.Suh PG, Hwang JI, Ryu SH, Donowitz M, Kim JH. Biochem Biophys Res Commun. 2001;288(1):1–7. doi: 10.1006/bbrc.2001.5710. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Lim S, Oh YS, Kim EK, Kim SH, Kim YH, Heo K, Kim J, Kim JK, Yang YR, Ryu SH, Suh PG. Cell Signal. 2010;22(7):1153–1161. doi: 10.1016/j.cellsig.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Reczek D, Berryman M, Bretscher A. J Cell Biol. 1997;139(1):169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fehon RG, McClatchey AI, Bretscher A. Nat Rev Mol Cell Biol. 2010;11(4):276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bretscher A, Reczek D, Berryman M. J Cell Sci. 1997;110(Pt 24):3011–3018. doi: 10.1242/jcs.110.24.3011. [DOI] [PubMed] [Google Scholar]
- 25.Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Jr, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Hoover KB, et al. Trends Biochem Sci. 1998;23(8):281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- 26.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S. J Cell Biol. 1998;140(4):885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. J Cell Biol. 1993;120(1):129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turunen O, Wahlstrom T, Vaheri A. J Cell Biol. 1994;126(6):1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gary R, Bretscher A. Mol Biol Cell. 1995;6(8):1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reczek D, Bretscher A. J Biol Chem. 1998;273(29):18452–18458. doi: 10.1074/jbc.273.29.18452. [DOI] [PubMed] [Google Scholar]
- 31.Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. J Cell Biol. 2004;164(5):653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Belmonte F, Mostov K. Cell Cycle. 2007;6(16):1957–1961. doi: 10.4161/cc.6.16.4583. [DOI] [PubMed] [Google Scholar]
- 33.Hancock JF, Cadwallader K, Paterson H, Marshall CJ. Embo J. 1991;10(13):4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iida-Klein A, Guo J, Xie LY, Jüppner H, Potts JT, Jr, Kronenberg HM, Bringhurst FR, Abou-Samra AB, Segre GV. J Biol Chem. 1995;270:8458–8465. doi: 10.1074/jbc.270.15.8458. [DOI] [PubMed] [Google Scholar]
- 35.Balla T, Varnai P. Sci STKE. 2002;2002(125):pl3. doi: 10.1126/stke.2002.125.pl3. [DOI] [PubMed] [Google Scholar]
- 36.Mason D, Mallo GV, Terebiznik MR, Payrastre B, Finlay BB, Brumell JH, Rameh L, Grinstein S. J Gen Physiol. 2007;129(4):267–283. doi: 10.1085/jgp.200609656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niggli V, Andreoli C, Roy C, Mangeat P. FEBS Lett. 1995;376(3):172–176. doi: 10.1016/0014-5793(95)01270-1. [DOI] [PubMed] [Google Scholar]
- 38.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. J Cell Biol. 2000;151(5):1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. J Cell Biol. 1997;138(2):423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Nat Chem Biol. 2009;5(10):734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K. Cell. 2007;128(2):383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi Y, Morales FC, Kreimann EL, Georgescu MM. Embo J. 2006;25(4):910–920. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgescu MM. Breast Cancer Res. 2008;10(2):106. doi: 10.1186/bcr1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molina JR, Morales FC, Hayashi Y, Aldape KD, Georgescu MM. Cancer Res. 2010;70(17):6697–6703. doi: 10.1158/0008-5472.CAN-10-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Bout I, Divecha N. J Cell Sci. 2009;122(Pt 21):3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 46.Manes S, Fuentes G, Peregil RM, Rojas AM, Lacalle RA. Faseb J. 2010;24(9):3381–3392. doi: 10.1096/fj.09-153106. [DOI] [PubMed] [Google Scholar]
- 47.Lacalle RA, Peregil RM, Albar JP, Merino E, Martinez AC, Merida I, Manes S. J Cell Biol. 2007;179(7):1539–1553. doi: 10.1083/jcb.200705044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. J Biol Chem. 1997;272(37):23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 49.Kotani H, Takaishi K, Sasaki T, Takai Y. Oncogene. 1997;14(14):1705–1713. doi: 10.1038/sj.onc.1200998. [DOI] [PubMed] [Google Scholar]
- 50.Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S. J Cell Biol. 1996;135(1):37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. J Cell Biol. 1998;140(3):647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yonemura S, Matsui T, Tsukita S. J Cell Sci. 2002;115(Pt 12):2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 53.Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. Cell. 1994;79(3):507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 54.Maeda M, Matsui T, Imamura M, Tsukita S. Oncogene. 1999;18(34):4788–4797. doi: 10.1038/sj.onc.1202871. [DOI] [PubMed] [Google Scholar]