Abstract
Background
The quality of polyp level data in a population-based registry depends on the ability to match each polypectomy recorded by the endoscopist to a specific diagnosis on the pathology report.
Objective
To review impediments encountered in matching colonoscopy and pathology data in a population-based registry.
Design
New Hampshire Colonoscopy Registry data from August 2006 to November 2008 was analyzed for prevalence of missing reports, discrepancies between colonoscopy and pathology reports, and the proportion of polyps that could not be matched because of multiple polyps submitted in the same container.
Setting
New Hampshire Colonoscopy Registry.
Patients
All consenting patients during the study period.
Interventions
Develop an algorithm for capturing number, size, location, and histology of polyps and for defining and flagging discrepancies to ensure data quality.
Main Outcome Measurements
The proportion of polyps with no assumption or discrepancy and the proportion of patient records eligible for determining the adenoma detection rate (ADR) and the number of patients with ≥3 adenomas.
Results
Only 50% polyps removed during this period were perfectly matched with no assumption or discrepancy. Records from only 69.9% and 29.7%% of eligible patients could be used to determine the ADR and the number of patients with ≥3 adenomas, respectively.
Limitations
Rates of missing reports may have been higher in the early phase of establishment of the registry.
Conclusions
This study highlights the impediments in collecting polyp level data in a population-based registry and provides useful parameters for evaluating the quality and accuracy of data obtained from such registries.
INTRODUCTION
Colorectal cancer (CRC) is the second most common cause of death from cancer in men and women, and the leading cause of cancer death in non-smokers, in the United States. Colonoscopy is the most commonly used screening method for CRC because it interrupts adenoma to carcinoma progression by polypectomy. Colonoscopy screening rates have increased 3- to 8-fold over the past two decades in the United States,1, 2 and the declining incidence of CRC has been attributed to the increasing use of screening colonoscopy and polypectomy.3–7 Improvements in colonoscopy screening and surveillance guidelines can be achieved by an assessment of the performance characteristics of the current system in a population based setting and this requires collection and matching of colonoscopy and pathology data on a large scale.
Polyp size, number, and histology are used to estimate risk for subsequent CRC and determine appropriate surveillance intervals8–12 and the adenoma detection rate (ADR) is considered to be an important quality indicator for screening colonoscopy.13–18 To capture these polyp characteristics accurately in a population based registry, polypectomy data from a colonoscopy procedure needs to be matched to a specific pathologic diagnosis for each specimen. This process can be complicated by missing, incomplete, and inaccurate colonoscopy or pathology reports. In addition, the common practice of submitting more than one polyp in a single container for pathologic evaluation precludes an exact diagnosis for each removed polyp. Clinically, this may result in an inappropriate surveillance recommendation and an adverse outcome. In epidemiologic studies that must rely on accurate polyp level data for each enrolled patient, these polyps may need to be dropped from analysis and, therefore, prove to be a potential source of study bias.
The New Hampshire Colonoscopy Registry (NHCR) was piloted in 2004 to determine the feasibility of establishing and maintaining a statewide registry of colonoscopy data. Originally piloted at two clinical sites with 13 practicing gastroenterologists,19 the NHCR now includes 20 sites with 114 participating endoscopists including gastroenterologists, surgeons and family practitioners and several more sites are being currently enrolled. Trainees in gastroenterology or surgery or family practice do not perform independent, unobserved colonoscopies at any of the currently enrolled NHCR sites. Data collected for each patient enrolled in the NHCR includes a patient questionnaire, a colonoscopy procedure form and a pathology report. Each polypectomy reported on the colonoscopy form is matched to a specific histologic diagnosis on the pathology report. The intent behind providing polyp-level data for each patient is to support a broad range of studies, including predisposing factors for different polyp subtypes; their underlying genetic abnormalities; the efficacy of current surveillance interval recommendations based on polyp type, size, number and location; and the development of new improved guidelines for prevention of CRC.
We present a detailed analysis of an algorithm developed at the NHCR for capturing number, size, location and histology of polypectomy specimens, our protocols for defining and flagging discrepancies to ensure data quality, and the impediments encountered in this process from our initial experience. Our findings may help others in establishing similar registries and provide a basis for developing quality indicators that can be used to assess strengths and limitations of studies performed in the setting of a population based polyp registry.
MATERIAL & METHODS
All patients who presented for screening, surveillance or diagnostic colonoscopy at NHCR participating sites were offered an opportunity to participate in the registry. Consenting patients were asked to complete a questionnaire that included personal and family history of colon polyps and cancer, prior screening tests and endoscopic examinations for CRC, smoking habits, and the use of dietary and other nutritional supplements. Endoscopists performing colonoscopy filled out a colonoscopy procedure form to record indication(s) for the procedure; quality of preparation; withdrawal time; findings on colonoscopy; polyp appearance (flat or polypoid), number, location, and size; and the technique and adequacy of polyp removal. The patient questionnaire and colonoscopy forms were scanned and the data stored in the NHCR database. The study was approved by the Dartmouth College Committee for the Protection of Human Subjects.
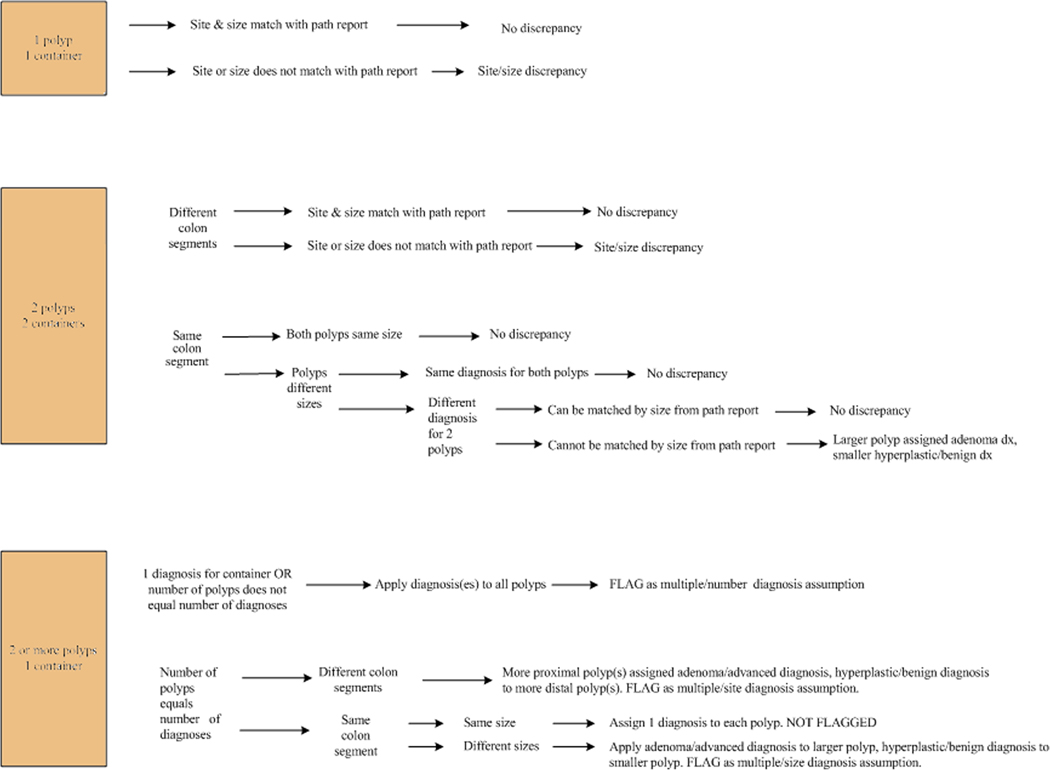
Pathology reports for all consenting patients were retrieved from the pathology laboratories at each participating site. Polyp number, location, size and diagnosis were collected in a pathology database. We attempted to match each polyp recorded on the colonoscopy form to a specific diagnosis on the pathology report with the intent of providing complete information for each polypectomy specimen. An algorithm was established for polyps in which the colonoscopy data was either missing, discrepant with findings on the final pathology report, or when an exact match could not be made due to multiple polyps being submitted for evaluation in the same container. The major issues addressed by our algorithm include discrepancies or ambiguities of polyp number, size and location. A protocol was also developed for cases in which multiple polyps from the same colonic segment, contiguous colonic segments or noncontiguous colonic segments were submitted together in a single container. This approach was devised so that investigators could set their inclusion and exclusion criteria appropriate to the hypothesis being tested. The salient features of our approach to matching colonoscopy and pathology data were as follows (see also Figure 1):
In cases where colonoscopy forms were missing or incomplete but there was data available from the pathology report, this was recorded in the database and the record flagged as a missing or incomplete colonoscopy form.
Site discrepancies between the colonoscopy and pathology reports were flagged as contiguous or non-contiguous. We defined contiguous sites as cecum and ascending colon, ascending colon and hepatic flexure, hepatic flexure and transverse colon, transverse colon and splenic flexure, splenic flexure and descending colon, descending and sigmoid colon, and sigmoid colon and rectum.
Polyp size from the pathology report was not recorded if the polyp was submitted as multiple fragments. Polyps submitted as a single fragment, where the polyp size on the pathology report varied by more than 3mm from the polyp size category (<5mm, 5–9 mm, 10–20 mm or >20 mm) marked on the colonoscopy form, were flagged as a size discrepancy. For example, a polyp marked as 5–9mm on the endoscopy form was flagged if the pathology size was < 2mm or > 12mm. The 3mm size difference was accepted as being within the expected range due to tissue shrinkage that occurs during formalin fixation and tissue processing for pathologic evaluation, as well as due to known inaccuracies in endoscopic polyp size estimation.20
When two polyps from the same colonic segment submitted in separate containers could not be matched individually (e.g., 2 sigmoid polyps at 15cm and 20cm on the colonoscopy form but both reported simply as “sigmoid polyp” on the pathology report) and the polyps were of different sizes, the more advanced diagnosis was assigned to the larger polyp and the record flagged as a diagnosis assumption. An adenoma was considered a more advanced diagnosis than a hyperplastic polyp whereas an adenoma with a villous component or high-grade dysplasia was regarded as more advanced than a tubular adenoma.
Normal colonic tissue fragments are seldom mentioned separately in pathology reports if any lesional tissue is identified. Normal mucosal fragments may be present due to excision of normal mucosa surrounding a polyp to ensure complete excision or due to a polypoid mucosal fold being mistaken for a polyp. When multiple polyps are submitted in one container it is impossible for the pathologist to know whether the normal fragments in a specimen represent polyp margins or prominent mucosal folds misinterpreted as a polyp by the endoscopist. Therefore, when multiple (>1) polyps were submitted in the same container and only one diagnosis was mentioned in the pathology report (e.g. fragments of tubular adenoma or fragments of hyperplastic polyp), all polyps were given the same diagnosis and the records were flagged as having a diagnosis assumption. This enables us to include or exclude these polyps from analysis depending on the hypothesis being tested. Thus, if the hypothesis involves the most advanced diagnosis in a patient, these cases can be included in the study, whereas if the hypothesis relates to the number of adenomas or serrated polyps per patient, the data from these patients needs to be excluded from primary analysis.
When multiple (>1) polyps were submitted in the same container and more than one diagnosis was mentioned on the pathology report (e.g. fragments of tubular adenoma AND hyperplastic polyp), the more advanced diagnosis was assigned to the larger or more proximal polyp. The records were flagged as having a diagnosis assumption based on size and site, respectively.
Figure 1.
Algorithm for assigning flags to polyp records
All polyp records from August 2006-November 2008 were retrieved and analyzed for the percentage of polyps in which a precise match was possible between the colonoscopy forms and pathology reports; the prevalence of size and site discrepancies; and the proportion of polyps flagged as diagnosis assumptions because of multiple polyps being submitted in the same container. We also determined the proportion of patients that had to be excluded from analysis while estimating the adenoma detection rate (ADR) and the number of patients with three or more adenomas detected during screening colonoscopy. Patients with history of inflammatory bowel disease were excluded from this analysis.
RESULTS
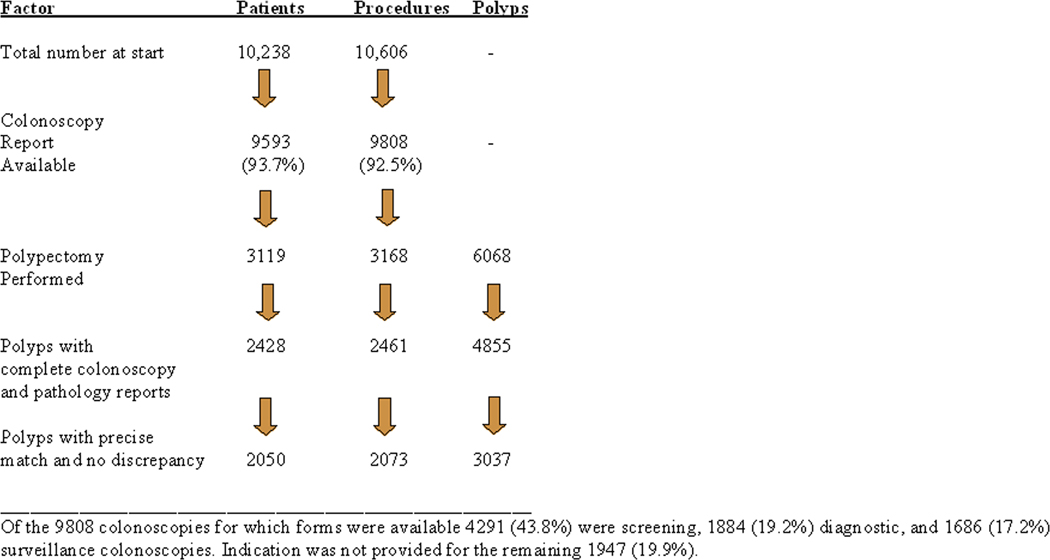
The NHCR consented and enrolled 10,238 patients undergoing 10,606 colonoscopies during the study period. Colonoscopy forms were received for 9808 colonoscopies (92.5%) from 9593 patients. Patients’ age range was 18–94 years, with a mean of 57.3 (11.8) years; there were 4070 males (42.4%), 4878 females (50.9%), with gender not recorded for 645 (6.7%) patients. Polyps were found in 3119/9593 (32.5%) patients and CRC was detected in 16 (0.2%). A total of 6068 polyps were removed from these 3119 patients in 3168 procedures and corresponding pathology reports were available for 2480/3168 (78.2%) of these colonoscopies. Missing pathology reports were due to omission of NHCR codes from the reports in the early phase of the registry. This prevented their retrieval during the monthly pathology data feed into the registry. Procedures with missing pathology reports (688/3168; 21.7%) and those in which incomplete polyp information was provided on the colonoscopy forms (19/3168; 0.6%) were excluded from further analysis. None of the polyps were removed by endoscopic mucosal resection during the study period. The results of the final matching process reported herein are for 4855 polyps removed in 2461 colonoscopies during the study period for which complete information was available (Fig. 2).
Figure 2.
Frequency of polyps with no missing or discrepant data compared to the total number of polypectomies during the study period.
A polyp diagnosis on the pathology report could be matched to a corresponding polypectomy listed on the procedure form for 65.7% (3191/4855) polyps. For the remaining 1664 (34.3%) polyps, a matching diagnosis could not be retrieved from the pathologic report because multiple polyps being submitted in one container (1421/1664; 85.4%); because no corresponding polyp was mentioned in the pathology report (227/1664; 13.6%) possibly as a result of the polyp not being retrieved during colonoscopy; or because of missing or discrepant information on the colonoscopy form (16/1664; 1%). No discrepancies between endoscopic and pathologic data were found in 3037/4855 (62.6%) polyps which represents only 50.0% (3037/6068) of all polyps removed during the study period.
The overall adenoma detection rate (ADR) was 17.9% (25.4% for men, 14.6% for women). However, data from 30.1% (3088/10,238) procedures had to be discarded from the ADR calculation because of missing indication for examination on the colonoscopy form (1947), missing colonoscopy forms (798), or missing pathology reports (343). A size discrepancy > than 3mm was found in 54/2410 polyps (2.2%) for which polyp sizes were recorded on both the colonoscopy form and the pathology report. A site discrepancy was detected for another 143/4855 (2.9%) polyps. The site discrepancy did not affect categorization into right versus left colon for 85.3% of these polyps (122/143).
More than one polyp was detected in 1185 (48.2%) of 2461 colonoscopies for which complete colonoscopy forms were available. Each polyp was submitted in a separate container in only 616 (51.9%) of these 1185 procedures. Clustering of polyps into a single container accounted for 29.5% (1432/4855) of polyps which could not be analyzed further for the number of adenomas or serrated polyps per patient. Table 1 shows details of the 569 procedures in which multiple polyps were submitted together in one container. The endoscopic size range for multiple polyps submitted in a single container varied from <5mm to greater than 20mm. Among the 660 containers with multiple polyps submitted for pathologic evaluation, 528 (80.0%) had only one diagnosis per container. Only hyperplastic polyps were diagnosed in 263 (39.9%) containers, only adenomas in 217/660 (32.9%), both adenomas and hyperplastic polyps in 110 (16.7%), adenomas with advanced histology (villous component or high grade dysplasia) in 35 (5.3%), other benign diagnoses in 32 (4.8%), and an adenocarcinoma in 3 (0.5%) containers that contained more than one polyp. Three or more polyps were removed in 694 procedures during the study period. Patients with three or more adenomas, who might be candidates for more intensive surveillance, could be determined with certainty in only 206/694 (29.7%) of these procedures because of missing pathology reports or submission of multiple polyps in the same container. In 200 patients, three or more polyps were detected, and each submitted in a separate container. Three or more adenomas were present in 69/200 (34.5%) patients and only one or two adenomas in another 107/200 (53.5%) patients.
Table 1.
Procedures with more than one polyp detected during screening or surveillance colonoscopy (n=1185)
| Feature | Number of procedures | % | |
|---|---|---|---|
| Procedures with >1 polyp submitted in same container for pathologic evaluation | 569 | 48.0 | |
| 2 polyps per container | 412 | 34.8% | |
| 3 polyps per container | 103 | 8.7% | |
| 4 polyps per container | 33 | 2.8% | |
| ≥5 polyps per container | 21 | 1.8% | |
| Polyp Location in procedures with >1 polyp submitted in same container for pathologic evaluation | |||
| Left colon only | 350 | 29.5% | |
| Right colon only | 207 | 17.5% | |
| Right AND Left colon | 12 | 1.0% | |
DISCUSSION
Population-based registries can play a pivotal role in evaluating the quality of current colonoscopy practice and generating evidence-based guidelines for CRC screening and surveillance. However, the quality of recorded data depends largely on prevailing clinical practices and information systems used for recording and disseminating clinical information21–23. Because the accuracy of recorded polyp information is critical in generating clinically useful guidelines, a well-defined algorithm to guide data collection is necessary for polyp research in a population-based setting. Discrepancies noted during data collection must be flagged so they can be identified during analysis and included or excluded based on their relevance to the hypothesis being tested.
To generate high quality data in a population-based polyp registry, each polypectomy recorded by the endoscopist must be matched to a specific diagnosis on the pathology report. This seemingly straightforward process is a significant challenge for a variety of reasons. In addition to missing reports, the matching process can be hampered by discrepancies in polyp size, location or number between the colonoscopy and pathology reports. Endoscopists often submit multiple polyps in the same container making it even more difficult to infer the diagnosis for each individual polyp from the pathology report and to determine the exact number of adenomas resected. Pathologists often use varying terminology to describe the same lesion, a practice that may cause confusion during data extraction from the pathology reports. Involvement of a pathologist to develop a data dictionary addressing this variance is critical in setting up a colonoscopy registry. Polyps with discrepant findings and those in which assumptions are made regarding number, site, size or diagnosis must be appropriately flagged. This gives investigators an option to test their hypotheses on the entire database or to use only those polyps where no assumptions or discrepancies were recorded.
Of the 6068 polypectomies performed during the study period, complete colonoscopy forms and pathology reports were available for 4855 (80%) polyps. However, only 3191 polypectomies could be matched to a specific diagnosis on the pathology report, which accounts for only 50.0% of the total number of polyps removed during the study period. Nearly 30% (1432/4855) of polyps could not be analyzed for an exact number of adenomas or serrated polyps per patient due to submission of multiple polyps in a single container. Thus, despite high rates of patient consent, endoscopist participation and availability of pathology reports, only half of all polyps removed at colonoscopy may be eligible for testing any polyp-level hypotheses. The practice of submitting multiple polyps in the same container is a significant impediment that can severely degrade the quality of data generated in population-based registries.
This practice is not confined to diminutive left-sided polyps which are more likely to be hyperplastic and of minor clinical significance. Of the 1185 procedures in which more than one polyp was detected, multiple polyps from the right colon were also submitted in the same container in 207 (17.5%) procedures. Moreover, the practice is not confined to diminutive polyps. Of the 1571 polypectomies submitted as multiple polyps in a single container, 536 (34.1%) were 5–9mm and 42 (2.7%) were greater than 10mm in size. Among 660 containers submitted with multiple polyps, 35 contained polyps with advanced histology, and three contained a cancer. Although rare, this may carry significant clinical implications if polyps from different colon segments were submitted in the same container, so that the exact location of the adenocarcinoma would be unknown. When multiple polyps are lumped together, determining the precise number of adenomas can be difficult. This may also affect recommended surveillance intervals, because current guidelines include the number of adenomas as a significant risk factor for CRC and suggest more frequent surveillance.24 In our study, the number of patients with three or more adenomas could be determined in only 29.7% of procedures (206/694) in which more than three polyps were removed because of missing pathology reports or multiple submissions in a single container.
Professional societies in endoscopy and pathology need to play a larger role in improving routine clinical practices to significantly improve the quality of data being reported to population-based registries. The practice of submitting multiple polyps in the same container is partly based on reimbursement patterns for pathologic evaluation that require separate billing for each container submitted. The technical and professional charge for pathologic evaluation for CPT code 88305 is about $164 and $328, respectively, whereas Medicare reimbursement per specimen for the technical (Part A) component, and the professional (Part B) component, is $38.68 and $36.72, respectively. Submitting each polyp separately does incur a higher charge for patients. As discussed above, when multiple polyps are submitted together, endoscopists have no way to determine the exact number of adenomas because some fragments may show a hyperplastic polyp or normal colonic mucosa. In this scenario, the endoscopist may choose a three year follow-up examination (per guideline recommendations for 3 or more adenomas) whereas an accurate adenoma count may have led to a five year follow-up recommendation (per guideline recommendation for one or two adenomas). In our study, 200 patients had three or more polyps which were submitted in a separate container. Of these, only 34.5% had three or more adenomas whereas another 53.5% patients had only one or two adenomas. It is reasonable to assume that in patients with three or more polyps, where multiple polyps are lumped in a single container, slightly over half the patients may end up with unnecessary surveillance at three years. This substantially increased cost related to inappropriate surveillance must also be taken into consideration while performing a cost-benefit analysis. There is clearly a need to develop a “standard practice” that ensures an accurate adenoma count and addresses the needs of both clinicians and researchers. There have been recent proposals to “resect and discard” diminutive colon polyps without pathologic evaluation because some of the newer endoscopy techniques such as chromoendoscopy and NBI may allow distinction of benign hyperplastic polyps from neoplastic adenomas. However, these techniques are not widely used in daily practice at the present time and there is an additional cost related to these methods too. Their precise role in polyp screening and surveillance in a population based setting remains to be defined.
Determining the quality of data recorded in a colonoscopy registry includes evaluating the percentage of consenting patients; the proportion of procedures for which complete patient information, colonoscopy and pathology reports are available; the percentage of polypectomies that can be matched to a specific diagnosis; the percentage of polyps submitted as multiple polyps in a single container; and the percentage of polyps in which size, site, or number discrepancies are noted between the colonoscopy and pathology reports. The algorithm presented here is designed to address some of these variables and provides a way to ensure greater consistency across polyp research studies. These quality indicators of colonoscopy outcome data should be reported in publications from population-based registries. This will eventually lead to more accurate data collection and development of better screening and surveillance protocols for prevention of CRC.
Acknowledgments
The National Cancer Institute R21CA100553 and R01CA131141 supported this work.
Glossary
- NHCR
New Hampshire Colonoscopy Registry
- CRC
Colorectal cancer
- ADR
Adenoma detection rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Meza R, Jeon J, Renehan AG, et al. Colorectal cancer incidence trends in the United States and United kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res. 2010 Jul 1;70:5419–5429. doi: 10.1158/0008-5472.CAN-09-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, White MC, Peipins LA, et al. Increase in screening for colorectal cancer in older Americans: results from a national survey. J Am Geriatr Soc. 2008 Aug;56:1511–1516. doi: 10.1111/j.1532-5415.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010 Jan 20;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009 Mar;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 5.Sedjo RL, Byers T, Barrera E, Jr, et al. A midpoint assessment of the American Cancer Society challenge goal to decrease cancer incidence by 25% between 1992 and 2015. CA Cancer J Clin. 2007 Nov–Dec;57:326–340. doi: 10.3322/CA.57.6.326. [DOI] [PubMed] [Google Scholar]
- 6.Thiis-Evensen E, Hoff GS, Sauar J, et al. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999 Apr;34:414–420. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 7.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006 May–Jun;56:143–159. doi: 10.3322/canjclin.56.3.143. quiz 84-5. [DOI] [PubMed] [Google Scholar]
- 9.Kristjansdottir S, Jonasson JG, Cariglia N, et al. Colonic adenomas found via colonoscopy: yield and risk factors for high-grade dysplasia. Digestion. 2010;82:252–257. doi: 10.1159/000297161. [DOI] [PubMed] [Google Scholar]
- 10.Kulling D, Christ AD, Karaaslan N, et al. The presence of more than two index adenomas is the strongest predictor of metachronous colon adenomas. Swiss Med Wkly. 2002 Mar 23;132:139–142. doi: 10.4414/smw.2002.09877. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien MJ, Winawer SJ, Zauber AG, et al. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990 Feb;98:371–379. [PubMed] [Google Scholar]
- 12.Saini SD, Kim HM, Schoenfeld P. Incidence of advanced adenomas at surveillance colonoscopy in patients with a personal history of colon adenomas: a meta-analysis and systematic review. Gastrointest Endosc. 2006 Oct;64:614–626. doi: 10.1016/j.gie.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 13.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006 Dec 14;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 14.Church J. Adenoma detection rate and the quality of colonoscopy: the sword has two edges. Dis Colon Rectum. 2008 May;51:520–523. doi: 10.1007/s10350-008-9239-y. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010 May 13;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 16.Millan MS, Gross P, Manilich E, et al. Adenoma detection rate: the real indicator of quality in colonoscopy. Dis Colon Rectum. 2008 Aug;51:1217–1220. doi: 10.1007/s10350-008-9315-3. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002 Jun;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006 Apr;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 19.Carney P, Butterly L, Goodrich M, et al. Design and development of a population-based colonoscopy registry. J Registry Manag. 2006;33:91–99. [Google Scholar]
- 20.Schoen RE, Gerber LD, Margulies C. The pathologic measurement of polyp size is preferable to the endoscopic estimate. Gastrointest Endosc. 1997 Dec;46:492–496. doi: 10.1016/s0016-5107(97)70002-6. [DOI] [PubMed] [Google Scholar]
- 21.Logan JR, Lieberman DA. The use of databases and registries to enhance colonoscopy quality. Gastrointest Endosc Clin N Am. 2010 Oct;20:717–734. doi: 10.1016/j.giec.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Dreyer NA, Garner S. Registries for robust evidence. JAMA. 2009;302:790–791. doi: 10.1001/jama.2009.1092. [DOI] [PubMed] [Google Scholar]
- 23.Butterly LF, Goodrich M, Onega T, et al. Improving the quality of colorectal cancer screening: assessment of familial risk. Dig Dis Sci. 2010 Mar;55:754–760. doi: 10.1007/s10620-009-1058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008 May–Jun;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]