Abstract
Background
The incidence of neurologic injury after proximal humerus fractures is variable, ranging from 6.2% to as much as 67%. However, it is unclear what factors might contribute to these injuries or whether they can be prevented by intraoperative nerve monitoring.
Questions/purposes
Therefore, using intraoperative nerve monitoring, we assessed the incidence, pattern of nerve involvement, and predisposing factors for nerve injury before and during shoulder fracture fixation.
Patients and Methods
We used continuous intraoperative monitoring of the brachial plexus in 37 patients undergoing open operative treatment of proximal humerus fractures. Impending intraoperative compromise of nerve function was signaled by sustained neurotonic EMG activity or greater than 50% amplitude attenuation of transcranial electrical motor evoked potentials (MEPs) (or both). When a nerve alert occurred, current surgical activity and arm and retractor position were recorded and adjustments were made to relieve tension.
Results
The intraoperative affected nerves included axillary (46%), combined (mixed plexopathy) (23%), radial (23%), musculocutaneous (4%), and ulnar (4%). Postoperatively, three patients had transient nerve palsies, which fully resolved within 3 weeks of surgery. Low body mass index (BMI) (22.7 ± 2.8), history of cervical spine disease, diabetes mellitus, and delay in surgical treatment (14 ± 2.9 days from time of injury) were associated with an increased incidence of nerve dysfunction.
Conclusions
Our observations suggest transcranial electrical MEPs are sensitive indicators of impending iatrogenic injury to the brachial plexus or peripheral nerves (or both) during open operative treatment of proximal humerus fractures. The use of intraoperative nerve monitoring during these procedures may be considered for the prevention of nerve injury, particularly in patients with underlying cervical spine disease, low BMI, diabetes mellitus, and/or delay in surgical treatment greater than approximately 14 days.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Proximal humeral fractures are the second most common upper-extremity fracture, after distal radius fractures. In patients older than 65 years, they are the third most common fracture, trailing only hip fractures and distal radius fractures [1, 17, 18, 22, 23]. The risk of neurologic injury has been well documented after displaced proximal humeral fractures. Stableforth reported a 6.2% incidence of brachial plexus injuries after proximal humerus fractures [28]. The axillary nerve is reportedly the most susceptible to injury after fractures with and without dislocation of the proximal humerus [27]. Several studies have reported using nerve monitoring in the asessment of proximal humerus fractures [6, 28, 30]. de Laat et al. found electrophysiologic evidence of nerve injury in 45% of cases (n = 101) [6]. Visser et al. reported axonal nerve lesions in proximal humeral fractures were much more common than has been reported in the literature: EMGs showed axonal denervation in 67% (96 of 143) of the patients and solitary nerve injury was seen in 21 of 143 cases [30].
Continuous intraoperative nerve monitoring has been used in spine surgery for some time to help predict and prevent neurologic complications [3, 4, 11, 23]. The literature on intraoperative nerve monitoring for shoulder surgery is limited as is the aforementioned literature regarding nerve injuries after proximal humerus fractures. The axillary nerve has been monitored during thermal capsulorrhaphy to help prevent nerve injury [7]. In 2007, Nagda et al. used intraoperative nerve monitoring for shoulder arthroplasty and reported having 17 nerve alerts during the course of 30 surgeries necessitating a surgical pause and adjustment of the retractors [20]. This report showed that, in the absence of preoperative trauma, intraoperative nerve dysfunction can occur during elective shoulder surgery [20]. Therefore the additive effect of preoperative trauma (ie, fracture) and surgery could contribute to the risk of nerve injury during proximal humeral fracture fixation.
We therefore used intraoperative nerve monitoring to assess the incidence, pattern of nerve involvement, and predisposing factors for nerve injury during shoulder fracture fixation.
Patients and Methods
We prospectively performed intraoperative nerve monitoring of all 39 patients undergoing surgical fixation of the proximal humerus at our institution between September 2007 and March 2009. Inclusion criteria included all patients 18 years of age or older with a displaced fracture or fracture-dislocation of the proximal humerus as defined by previously defined criteria [2, 5, 8, 13, 14, 25, 26]. We excluded patients with preexisting nerve deficits before their current proximal humerus fracture. Two patients were excluded because of nerve monitoring equipment failure during the surgery that precluded complete nerve monitoring. The remaining 37 patients (30 females, seven males) comprised the study population with a mean (± SD) age at the time of surgery of 66.0 ± 9.3 years (range, 32–83 years). The minimum followup was 7 months (mean ± SD, 12.7 ± 4.3 months; range, 7–22 months). Twenty-three patients had surgery on their dominant extremity. Using the Neer classification system [21], fractures were classified as two-part (29%), three-part (58%), and four-part (13%). There were four patients in this group who had an associated dislocation (two [two-part], one [three-part], one [four-part]). We had prior approval by our Institutional Review Board.
On consent for surgery, patients were given a questionnaire regarding demographic information, diagnosis, and medical history, including history of nerve or spinal cord injury, underlying history of cervical spine disease, history of rotator cuff injury, history of prior shoulder or elbow surgery, prior level of shoulder function, current medications, and history of comorbidities, among other questions. Patients also received a detailed preoperative examination with specific attention given to current neurologic status. Muscle strength of the deltoid, biceps, triceps, flexor carpi radialis, and abductor digiti minimi were graded according to the Medical Research Council scale [19]. Patient demographics such as age, gender, time (in days) between injury and surgery, BMI, and medical comorbidities were gathered from patient records (Table 1).
Table 1.
Patient Demographics
| Parameter | Mean |
|---|---|
| Age (years) | 67.2 (+/− 12.1) |
| Body mass index (kg/m2) | 29.7 (+/− 7.4) |
| Operative time (minutes) | 127 (+/− 22) |
| Time between injury and surgery (days) | 11 (+/− 6) |
Intraoperative neuromonitoring was performed throughout the surgical course of repair of the proximal humerus fracture by a dedicated neurophysiologist. The following muscles were tested: deltoid—anterior, middle, and posterior parts (axillary), biceps (musculocutaneous), triceps (radial), flexor digitorum superficialis and flexor carpi radialis (median), and adductor pollicis (ulnar nerve) [30].
All surgeries were performed by one fellowship-trained shoulder surgeon (JAA). The patient was induced with propofol and was administered a short- to medium-acting neuromuscular blocking agent to facilitate intubation. Total intravenous anesthesia was maintained with a propofol/opioid infusion. Patients underwent surgery in the beach chair position using a deltopectoral approach. After patient positioning, stimulation and recording leads were placed on the scalp and upper extremities, respectively (Fig. 1). Brachial plexus and peripheral nerve function was monitored by recording spontaneous EMG activity and transcranial electrical MEPs from six upper-extremity muscles on the operative side (Table 2). Myotome selection was based on innervation patterns by major divisions of the brachial plexus and peripheral nerves considered at risk for injury during the procedure (Table 2) [10, 12]. Control recordings also were obtained from a subset of muscles on the nonoperative side. Dermatomal somatosensory evoked potentials (SEPs) were obtained by stimulation of an appropriate dermatome. Stimulation current was begun at 10 mA, 200-μs duration, and 4.7 per second. The current was increased until a reliable response was seen from the scalp. Notation was made of any amplitude or latency change in the SEP and the stage of surgical procedure.
Fig. 1.
An intraoperative image shows characteristic lead placement along the operative upper extremity.
Table 2.
Pattern of brachial plexus and peripheral nerve monitoring
| Region Assessed | C5 & C6 | C5 & C6 | C5, C6, C7, C8 | C6, C7, C8, T1 | C5, C6, C7, C8, T1 | C8 & T1 |
|---|---|---|---|---|---|---|
| Trunk | Upper | Upper | Upper and middle | Middle and lower | Upper, middle, and lower | Lower |
| Cord | Posterior | Lateral | Posterior | Posterior | Lateral and medial | Medial |
| Nerve | Axillary | Musculocutaneous | Radial | Radial | Median | Ulnar |
| Muscle | Deltoid | Biceps | ECRL | Triceps | FDS, FCR | First dorsal interosseous |
ECRL = extensor carpi radialis longus; FDS = flexor digitorum superficialis; FCR = flexor carpi radialis.
Before the incision was made, baseline EMG and transcranial electrical MEP recordings were obtained. Global functional integrity of the ipsilateral brachial plexus and peripheral nerves was assessed repeatedly throughout the surgical course by recording MEPs to transcranial electrical stimulation. EMG activity also was recorded continuously to provide early warning of excessive traction on one or more neural elements.
Intraoperatively, the duration of the surgery and any complications were documented. A major nerve event was defined by sustained neurotonic EMG activity or greater than 50% amplitude reduction in the transcranial electrical MEPs from one or more muscles (or both). When this occurred, the neurophysiologist first repeated the motor test 1 minute after the decrease. If it remained consistent, an alert was signaled, prompting a surgical pause. After a nerve alert, the surgeon first removed all retractors, and the MEP was repeated for 2 minutes. If there was no improvement, the patient’s arm was moved from its operative position to a neutral position, with the arm at the side, for 5 minutes. A separate member of the research team, not involved with the surgical procedure, noted the stage of the procedure and the position of the surgical arm at the time of the alert. Notation also was made of the nerves involved, percent amplitude reduction of the transcranial electrical MEPs, and subsequent repositioning performed by the surgical team to alleviate the problem. Any placement of instruments that alerted the monitor was documented. Modifications made to relieve the nerve from irritation were documented. After a nerve alert, the procedure continued with an attempt to limit or completely avoid the provocative position.
After completion of the procedure, the patient received a complete neurologic evaluation of the extremity at the first available opportunity. If a nerve deficit was noted, documentation was made and the patient was followed postoperatively. Patients with notable intraoperative nerve dysfunction that did not resolve by the conclusion of the case underwent EMG evaluation at 3 months to document persistence or resolution of the nerve dysfunction. If no deficit was noted, the patient was followed at regular intervals (1 week, 3 weeks, 6 weeks, 12 weeks, 6 months, 9 months, and 12 months) for 1 year after surgery to monitor rehabilitation progress.
For the purpose of this study, a “neurologic event” was defined as the occurrence of a postoperative neurologic deficit or an intraoperative marked decrease of transcranial electrical MEP amplitude. Patients in the latter category were assumed to have experienced neurologic events, although this could not be verified, as there was no alternative intraoperative testing and because the patients had no postoperative neurologic deficit. In this study, we identified 14 patients in whom we believed true neurologic events had occurred.
We used a paired two-tailed Student t-test to determine differences in a number of variables (patient age, height, weight, BMI, cervical spine disease, fracture pattern, time between fracture and fixation, operative time, history of diabetes mellitus, and surgical procedure performed) between patients who experienced a neurologic event versus those who did not. Each variable was assessed to determine whether it was significantly related to the variable of interest (neurologic event). In cases where the two variables were continuous, the Pearson correlation coefficient was used to determine whether the two variables were significantly related. In cases where the patients experiencing a neurologic event were compared with the patients who did not experience a neurologic event to determine whether some characteristic of interest was present or absent, a Yates correction for continuity test or Fisher exact test was used. All medical, surgical, and demographic variables were considered potential confounders. All variables were considered individually. If they were related to the outcome of interest (neurologic event), they were placed in the regression model. After this, regression analysis was performed using all variables found to be related to either the outcome of interest or the variable of interest. We used the Computer Program for Epidemiological Analysis software package (PEPI Version 2.03; USD, Stone Mountain, GA, USA) and Statistics Calculator (Version 5.0; Statpac, Minneapolis, MN, USA) for all analyses.
Results
Of the 37 patients, six (16%) had sustained decreased baseline transcranial electrical MEP amplitude in their operative extremity before incision (Table 3). Fourteen patients (38%) had nerve alerts during the course of their surgical procedure. There were a total of 26 different nerve alerts in the 14 patients. Of the 26 nerve alerts, 22 (85%) were based on major attenuation of transcranial electrical MEP amplitude. Four alerts (15%) were based on an episode of sustained neurotonic EMG activity (Table 4).
Table 3.
Patients with preoperative decreased baselines
| Affected nerves | Number |
|---|---|
| Combined | 2 |
| Musculocutaneous | 0 |
| Axillary | 3 |
| Ulnar | 0 |
| Radial | 1 |
| Median | 0 |
| Total alerts | 6 |
Table 4.
Intraoperative nerve events
| Affected nerves | Number (%) |
|---|---|
| Combined | 6 (23) |
| Musculocutaneous | 1 (4) |
| Axillary | 12 (46) |
| Ulnar | 1 (4) |
| Radial | 6 (23) |
| Median | 0 (0) |
| Total alerts | 26 (100) |
| Breakdown of combined events | |
| Upper trunk | 3 (50) |
| Posterior cord | 2 (33.3) |
| Lateral cord | 1 (16.6) |
| Total | 6 |
Nerve dysfunction occurred during various portions of the procedure (Table 5). Of the 26 nerve events, 15 (58%) occurred while the extremity was positioned in abduction, external rotation, and flexion. Eleven nerve (42%) events occurred while the patient’s shoulder was positioned in adduction, internal rotation, and extension. Removal of retractors without repositioning the arm resulted in a return to the baseline nerve signal in nine cases. Repositioning of the surgical extremity to the neutral position and removal of all retractors from the operative field resulted in a return of the nerve signal within 5 minutes in 10 patients. In all patients for whom the signal returned completely, the surgery was completed successfully with the knowledge of the positioning that had caused the nerve event. Avoidance of these positions along with attenuation of fracture reduction maneuvering resulted in no additional compromise in eight instances. For seven patients with alerts, repositioning and removal of retractors did not result in full return of transcranial electrical MEP amplitude to baseline range by the end of the surgery. There was complete return of transcranial electrical MEP amplitude to baseline by the end of the procedure in nine of 14 patients (64%) who had an alert during the procedure. Of the five patients who did not have a complete return of transcranial electrical MEP amplitude at the completion of the procedure, three had evidence of weakness on examination postoperatively (two radial nerve distribution, one axillary nerve distribution). These postoperative findings were consistent with intraoperative alerts. All three of these patients had complete resolution of symptoms by 3 weeks postoperatively and underwent followup EMG 3 months postoperatively with normal findings. Of the six patients who had decreased baseline transcranial electrical MEP amplitude in their operative extremity as compared with the contralateral arm before incision, two had nerve alerts during the surgical procedure. Five of six had no notable weakness postoperatively whereas one had weakness of the radial nerve and was one of the three patients in the study who showed signs of nerve dysfunction after surgery. This patient postoperatively had weakness in a pattern consistent with findings noted on the preincision transcranial electrical MEP amplitude.
Table 5.
Point during procedures of nerve event
| Time of event | Number (%) |
|---|---|
| Exposure | 1 (4%) |
| Fracture reduction | 17 (65%) |
| Plate application | 8 (31 %) |
Comparison of demographic data for patients who had a nerve alert with data for those who had no event showed no difference in age, preoperative diagnosis, or operative time (Table 6). We found that patients who experienced a delay in treatment (> 14 days), had a low BMI (< 22), a history of underlying cervical spine disease (Fig. 2), or diabetes mellitus were statistically more likely to experience a neurologic event.
Table 6.
Possible risk factors
| Parameter | No neurologic event group (mean +/− SD) | Neurologic event group (mean +/− SD) | Statistical significance |
|---|---|---|---|
| Demographics | |||
| Age | 67.8 (+/− 13.7) | 66.4 (+/− 11.8) | p = .67 |
| Body mass index (kg/m2) | 31.8 (+/− 3.4) | 22.7 (+/− 2.8) | p = .03* |
| Operative time (minutes) | 126 (+/− 17.4) | 131 (+/− 23.8) | p = .55 |
| Time between injury and surgery (days) | 8 (+/− 2.4) | 14 (+/− 2.9) | p = .04* |
| Comorbities (number/%) | |||
| Diabetes mellitus | 3 (15.7) | 7 (38.9) | p = .04* |
| Alcohol abuse | 2 (10.5) | 2 (11.1) | p = .55 |
| Smoking | 3 (15.7) | 4 (22.2) | p = .66 |
| Heart disease | 6 (31.5) | 6 (33.3) | p = .75 |
| Cervical spine disease | 1 (5.3) | 7 (38.9) | p = .001* |
| Fracture type (%) | |||
| 2-part | 27 % | 31 % | p = .57 |
| 3-part | 63 % | 53 % | p = .38 |
| 4-part | 10 % | 16 % | p = .44 |
| With associated dislocation | 50 % | 50 % | p = .99 |
* Significant difference.
Fig. 2.
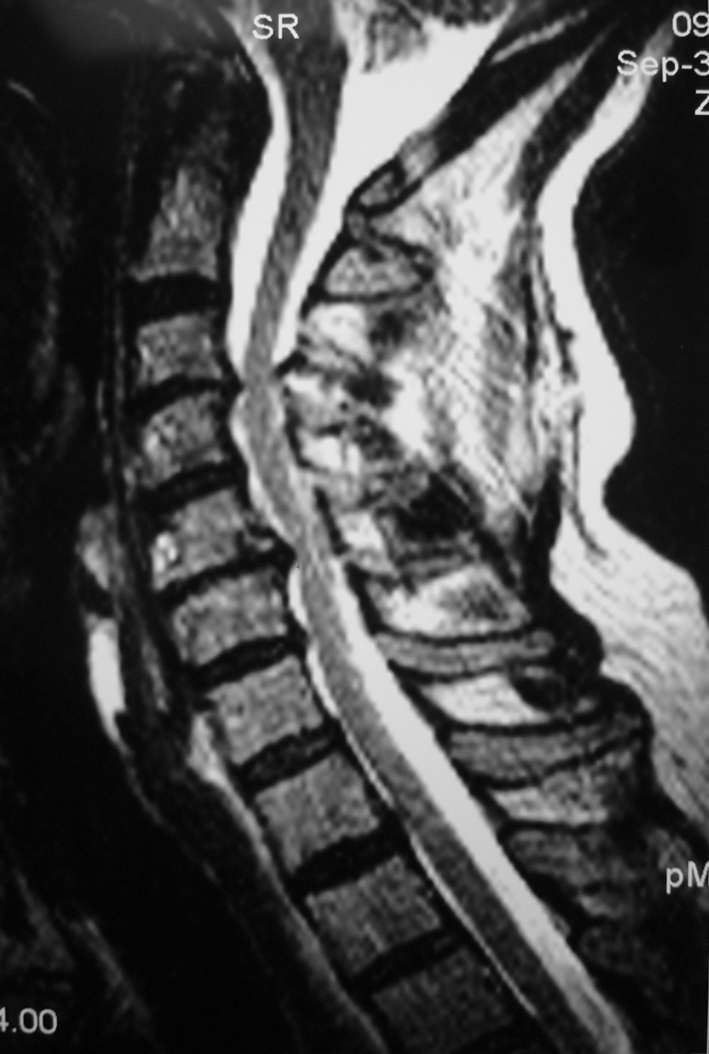
A coronal T2-weighted cervical spine MR image of a patient shows multilevel disc degeneration and spinal stenosis. This patient notably also had diabetes mellitus and a transient postoperative radial nerve palsy.
Discussion
The incidence of neurologic injury after proximal humerus fractures is variable, reportedly anywhere from 6.2% to as much as 67% [28, 30]. We used intraoperative nerve monitoring to identify the incidence, pattern, and predisposing factors for nerve injury during shoulder surgery for proximal humerus fractures.
We acknowledge limitations of our study. First, we had a relatively small sample size. Although this is a relatively small cohort of patients, we did look specifically at age, gender, and fracture type to minimize the risk of confounding variables. There was no evidence to suggest the two groups in this study were disparate enough to account for the differences we found. Second, we had a variety of fracture types. However, there was only one treating surgeon using one method of proximal humeral fracture fixation and we presumed this would help minimize the variability. Third, all surgeries were performed at a tertiary referral center using the patient population of one fellowship-trained shoulder surgeon. Although this may represent a skewed subset of more difficult patients to treat, our rate of nerve injury in this series actually might be understated as most shoulder surgeries are not performed by shoulder specialists [9]. Finally, we could not verify the validity of our intraoperative nerve alerts. Although nerve monitoring does have limits to its accuracy [24], the combined SEP and MEP monitoring we used in this study has several theoretical advantages over single modality methods, including increased accuracy provided by complementary information from two independent systems with a reduced risk of false negatives. However, some intraoperative nerve alerts that do occur, although physiologically valid, have no postoperative clinical relevance.
In our study, 18 of 37 patients (49%) had either a preoperatively diminished transcranial electrical MEP amplitude or an intraoperative nerve alert. Six of 37 patients (16%) had sustained decreased baseline transcranial electrical MEP amplitude before surgical incision, suggesting a previously undiagnosed nerve injury after proximal humerus fracture. Moreover, 14 of 37 patients (38%) had intraoperative nerve alerts necessitating a surgical pause, removal of retractors, and change to a more neutral surgical position. Three patients had a transient nerve injury (all clinically resolved within 3 weeks) that had fully resolved on postoperative EMG at 3 months. Although our rate of preoperatively diminished transcranial electrical MEP amplitude (16 %) was lower than we had anticipated, the overall rate of nerve compromise (49%) was high.
In our series, 65% of the nerve events occurred during fracture reduction whereas 31% occurred during plate application. During these portions of the procedure, the humeral shaft and tuberosities were subjected to traction to aid in fracture reduction. This lends support to the theory that injury was likely a result of traction on the nerves. Moreover, these data provide a rationale for removing retractors and placing the arm in a more neutral position if there is an intraoperative delay for any reason. The findings of this study are supported by biomechanical data from Kwaan and Rappaport [15], who used strain gauge testing to delineate that an arm placed in 90° abduction, external rotation, and slight extension produced traction on the brachial plexus. We found approximately 23% of the events involved portions of the brachial plexus, whereas 77% of nerve alerts involved a distinct nerve pattern.
We did find factors that placed the patient at increased risk for a nerve alert, including history of diabetes, lower BMI (approximately 22), history of cervical spine disease (spinal stenosis, herniated nucleus pulposus, cervical spine surgery), and delay in treatment (approximately 2 weeks). There were seven patients with diabetes in the subgroup of those who experienced neurologic compromise, which is consistent with a previous study showing an increased risk for nerve injury in diabetics undergoing orthopaedic surgical procedures [31]. In analyzing our patients with cervical spine disease, we have postulated a double-crush phenomenon may be contributing to this subset of patients having a higher rate of nerve dysfunction [29]. In analyzing our patients with low BMI and their potential for increased prevalence of nerve alert, we have no clear explanation for this finding. However, the literature does support that patients with lower BMIs can be at increased risk for neurologic compromise [16]. This may be related to differences in conduction velocity in patients with high versus low BMI and relative margin of safety for nerve excursion while the patient is placed in traction before neurologic abnormalities are recorded. Finally, it appears a delay in treatment (approximately 14 days) was correlated with an increased risk for nerve alert in our study. Although the reason for this is unknown, one may postulate displaced fractures left to settle in position for a longer period of time with associated edema and scar/callous formation may require increased traction at the time of surgery to achieve an adequate reduction. However, this cannot be concluded based solely on our data.
Our data suggest transcranial electrical MEPs are sensitive indicators of impending iatrogenic injury to the brachial plexus or peripheral nerves (or both) during operative shoulder fracture fixation. The value of intraoperative neurophysiologic monitoring with transcranial electrical MEPs is underscored by the fact that, in all patients in this study in whom transcranial electrical MEP changes were reversed by corrective surgical action, there were no postoperative sequelae. In cases in which intraoperative transcranial electrical MEP changes could not be reversed, it is likely the extent of injury was minimized by quickly identifying and, to the extent possible, avoiding repetition of the offending surgical maneuvers.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at 3B Orthopaedics, Philadelphia, PA.
References
- 1.Baron JA, Barrett JA, Karagas MR. The epidemiology of peripheral fractures. Bone. 1996;18(3 suppl):209S–213S. doi: 10.1016/8756-3282(95)00504-8. [DOI] [PubMed] [Google Scholar]
- 2.Bjorkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate: a retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthop Scand. 2004;75:741–745. doi: 10.1080/00016470410004120. [DOI] [PubMed] [Google Scholar]
- 3.Blom S, Dahlbäck LO. Nerve injuries in dislocations of the shoulder joint and fractures of the neck of the humerus: a clinical and myographical study. Acta Chir Scand. 1970;136:461–466. [PubMed] [Google Scholar]
- 4.Boardman ND, 3rd, Cofield RH. Neurologic complications of shoulder surgery. Clin Orthop Relat Res. 1999;368:44–53. doi: 10.1097/00003086-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Charalambous CP, Siddique I, Valluripalli K, Kovacevic M, Panose P, Srinivasan M, Marynissen H. Proximal humeral internal locking system (PHILOS) for the treatment of proximal humeral fractures. Arch Orthop Trauma Surg. 2007;127:205–210. doi: 10.1007/s00402-006-0256-9. [DOI] [PubMed] [Google Scholar]
- 6.Laat EA, Visser CP, Coene LN, Pahlplatz PV, Tavy DL. Nerve lesions in primary shoulder dislocations and humeral neck fractures: a prospective clinical and EMG study. J Bone Joint Surg Br. 1994;76:381–383. [PubMed] [Google Scholar]
- 7.Esmail AN, Getz CL, Schwartz DM, Wierzbowski L, Ramsey ML, Williams GR., Jr Axillary nerve monitoring during arthroscopic shoulder stabilization. Arthroscopy. 2005;21:665–671. doi: 10.1016/j.arthro.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Fankhauser F, Boldin C, Schippinger G, Haunschmid C, Szyszkowitz R. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop Relat Res. 2005;430:176–181. doi: 10.1097/01.blo.0000137554.91189.a9. [DOI] [PubMed] [Google Scholar]
- 9.Hasan SS, Leith JM, Smith KL, Matsen FA., 3rd The distribution of shoulder replacement among surgeons and hospitals is significantly different than that of hip or knee replacement. J Shoulder Elbow Surg. 2003;12:164–169. doi: 10.1067/mse.2003.23. [DOI] [PubMed] [Google Scholar]
- 10.Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86:1248–1253. doi: 10.2106/00004623-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Hwang RW, Bae DS, Waters PM. Brachial plexus palsy following proximal humerus fracture in patients who are skeletally immature. J Orthop Trauma. 2008;22:286–290. doi: 10.1097/BOT.0b013e31816b7898. [DOI] [PubMed] [Google Scholar]
- 12.Kendall FP, McCreary EK. Muscle Testing and Function. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- 13.Kettler M, Biberthaler P, Braunstein V, Zeiler C, Kroetz M, Mutschler W. [Treatment of proximal humeral fractures with the PHILOS angular stable plate: presentation of 225 cases of dislocated fractures] [in German] Unfallchirurg. 2006;109:1032–1040. doi: 10.1007/s00113-006-1165-7. [DOI] [PubMed] [Google Scholar]
- 14.Koukakis A, Apostolou CD, Taneja T, Korres DS, Amini A. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop Relat Res. 2006;442:115–120. doi: 10.1097/01.blo.0000194678.87258.6e. [DOI] [PubMed] [Google Scholar]
- 15.Kwaan JHM, Rappaport I. Postoperative brachial plexus palsy: a study on the Mechanism. Arch Surg. 1970;101:612–615. doi: 10.1001/archsurg.1970.01340290068015. [DOI] [PubMed] [Google Scholar]
- 16.Landau ME, Barner KC, Campbell WW. Effect of body mass index on ulnar nerve conduction velocity, ulnar neuropathy at the elbow, and carpal tunnel syndrome. Muscle Nerve. 2005;32:360–363. doi: 10.1002/mus.20345. [DOI] [PubMed] [Google Scholar]
- 17.Lanting B, MacDermid J, Drosdowech D, Faber KJ. Proximal humeral fractures: a systematic review of treatment modalities. J Shoulder Elbow Surg. 2008;17:42–54. doi: 10.1016/j.jse.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Dargent-Molina P. Breart G; EPIDOS Group. Epidemiologie de l’Osteoporose Study. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res. 2002;17:817–825. doi: 10.1359/jbmr.2002.17.5.817. [DOI] [PubMed] [Google Scholar]
- 19.Medical Research Council. Aids to the Examination of the Peripheral Nervous System. Memorandum No. 45. London, UK: Her Majesty’s Stationery Office; 1967.
- 20.Nagda SH, Rogers KJ, Sestokas AK, Getz CL, Ramsey ML, Glaser DL, Williams GR., Jr Neer Award 2005: Peripheral nerve function during shoulder arthroplasty using intraoperative nerve monitoring. J Shoulder Elbow Surg. 2007;16(3 suppl):S2–S8. doi: 10.1016/j.jse.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Neer CS., 2nd Displaced proximal humeral fractures: I. Classification and evaluation. J Bone Joint Surg Am. 1970;52:1077–1089. [PubMed] [Google Scholar]
- 22.Nguyen TV, Center JR, Sambrook PN, Eisman JA. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology Study. Am J Epidemiol. 2001;153:587–595. doi: 10.1093/aje/153.6.587. [DOI] [PubMed] [Google Scholar]
- 23.Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15:12–26. doi: 10.5435/00124635-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pelosi L, Lamb J, Grevitt M, Mehdian SM, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082–1091. doi: 10.1016/S1388-2457(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 25.Plecko M, Kraus A. Internal fixation of proximal humerus fractures using the locking proximal humerus plate. Oper Orthop Traumatol. 2005;17:25–50. doi: 10.1007/s00064-005-1120-8. [DOI] [PubMed] [Google Scholar]
- 26.Rose PS, Adams CR, Torchia ME, Jacofsky DJ, Haidukewych GG, Steinmann SP. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg. 2007;16:202–207. doi: 10.1016/j.jse.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Schlegel TF, Hawkins RJ. Displaced proximal humeral fractures: evaluation and treatment. J Am Acad Orthop Surg. 1994;2:54–78. doi: 10.5435/00124635-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Stableforth PG. Four-part fractures of the neck of the humerus. J Bone Joint Surg Br. 1984;66:104–108. doi: 10.1302/0301-620X.66B1.6693466. [DOI] [PubMed] [Google Scholar]
- 29.Upton AR, McComas AJ. The double crush in nerve entrapment syndromes. Lancet. 1973;2:359–362. doi: 10.1016/S0140-6736(73)93196-6. [DOI] [PubMed] [Google Scholar]
- 30.Visser CP, Coene LN, Brand R, Tavy DL. Nerve lesions in proximal humerus fractures. J Shoulder Elbow Surg. 2001;10:421–427. doi: 10.1067/mse.2001.118002. [DOI] [PubMed] [Google Scholar]
- 31.Yacub JN, Rice JB, Dillingham TR. Nerve injury in patients after hip and knee arthroplasties and knee arthroscopy. Am J Phys Med Rehabil. 2009;88:635–641; quiz 642–634, 691. [DOI] [PMC free article] [PubMed]



