Abstract
Background
The reverse total shoulder arthroplasty was introduced to treat the rotator cuff-deficient shoulder. Since its introduction, an improved understanding of the biomechanics of rotator cuff deficiency and reverse shoulder arthroplasty has facilitated the development of modern reverse arthroplasty designs.
Questions/purposes
We review (1) the basic biomechanical challenges associated with the rotator cuff-deficient shoulder; (2) the biomechanical rationale for newer reverse shoulder arthroplasty designs; (3) the current scientific evidence related to the function and performance of reverse shoulder arthroplasty; and (4) specific technical aspects of reverse shoulder arthroplasty.
Methods
A PubMed search of the English language literature was conducted using the key words reverse shoulder arthroplasty, rotator cuff arthropathy, and biomechanics of reverse shoulder arthroplasty. Articles were excluded if the content fell outside of the biomechanics of these topics, leaving the 66 articles included in this review.
Results
Various implant design factors as well as various surgical implantation techniques affect stability of reverse shoulder arthroplasty and patient function. To understand the implications of individual design factors, one must understand the function of the normal and the cuff-deficient shoulder and coalesce this understanding with the pathology presented by each patient to choose the proper surgical technique for reconstruction.
Conclusions
Several basic science and clinical studies improve our understanding of various design factors in reverse shoulder arthroplasty. However, much work remains to further elucidate the performance of newer designs and to evaluate patient outcomes using validated instruments such as the American Society for Elbow Surgery, simple shoulder test, and the Constant-Murley scores.
Introduction
The term “cuff tear arthropathy” (CTA) was first coined by Neer et al. in 1983 to describe patients with massive rotator cuff tears in addition to various structural changes in the glenohumeral joint [50] (Fig. 1). Since his original description of CTA, various investigators have documented that rotator cuff dysfunction leads to a wide spectrum of glenohumeral joint disease (Fig. 2) [12, 17, 18, 20]. Neer et al. advocated hemiarthroplasty as the initial surgical treatment [51]. However, subsequent published literature revealed hemiarthroplasty for CTA had unpredictable and often poor function based on low American Society for Elbow Surgery, simple shoulder test, and Constant-Murley scores [12, 20, 50, 51, 55, 57, 66, 67].
Fig. 1.
True AP (Grashey view [46]) of a shoulder with cuff tear arthropathy is shown. The film demonstrates glenohumeral arthritis, superior glenoid wear, proximal humeral migration, and acetabularization of the acromion.
Fig. 2.

This figure demonstrates the wide spectrum of disease that can be seen in rotator cuff tear arthropathy.
Reverse design total shoulder arthroplasty was developed to address the need for a treatment with better and more predictable function for CTA. Initial devices were constrained and had high rates of clinical failure, mostly from component loosening [2, 54]. These early failures led to the development of a semiconstrained prosthesis by Grammont and Baulot in 1985 [29]. Over time, this was subsequently modified and evolved, in 1991, to the Delta III prosthesis (DePuy International Ltd, Warsaw, IN) (Fig. 3) [28]. A 2-year followup study on his initial group of patients demonstrated decreases in pain and improvement in function [28]. Similar findings were reported by Boulahia et al. in their series of 16 patients [8].
Fig. 3.

This figure shows the Delta 3 reverse arthroplasty.
The Delta III was designed to transfer the center of rotation of the humerus medially and to lengthen the humerus. In theory, this would tension the deltoid and allow for compression between the humerosocket and glenosphere, thus stabilizing the prosthetic articulation. Furthermore, medializing the glenoid center of rotation could allow for enhanced recruitment of deltoid muscle fibers, which would allow for a more efficient deltoid lever arm [53]. Additionally, it was believed that a humeral center of rotation located as close as possible to the bone-glenosphere junction would decrease baseplate loosening [6].
Despite the clinical improvements seen with the Delta III [29], several mechanical problems remained. First, scapular notching reportedly occurs in between 44% and 96% of cases [6, 7, 42, 61, 63, 65]. Second, the device was associated with limited external rotation, often requiring muscle transfers for improvement [4, 5, 19, 27, 28, 64]. Finally, by medializing and moving the humerus distally, patients noted a loss of the normal deltoid contour leading not only to cosmetic concerns, but decreased deltoid efficiency resulting in a prosthesis with less inherent stability [26]. Recognition of these problems led to clinical and basic science studies aimed at improving the surgical technique and design of reverse shoulder arthroplasty.
In this article, we review (1) the basic biomechanical challenges associated with the rotator cuff-deficient shoulder; (2) the biomechanical rational for newer designs aimed at reducing complications related to reverse shoulder arthroplasty; (3) the current scientific evidence related to the function and performance of reverse shoulder arthroplasty; and (4) specific technical aspects of reverse shoulder arthroplasty, including glenoid baseplate fixation, glenoid component position, glenohumeral stability, glenohumeral ROM, and muscle function about the shoulder treated with a reverse prosthesis.
Methods
We conducted a PubMed search of the English language literature published between 1974 and 2010 using the following key words: reverse shoulder arthroplasty (128 results), rotator cuff arthropathy (2033 results), and biomechanics of reverse shoulder arthroplasty (12 results). These articles were reviewed for exclusion and crossreferenced to discard repeated references. Review articles and those outside the scope of reverse shoulder arthroplasty were excluded, leaving the 66 articles cited in this review.
Shoulder Biomechanics: The Basics of Normal and Cuff-deficient Shoulders
A key concept describing the mechanics of the normal shoulder relates to concavity-compression [48]. In general, Matsen et al. described this concept as a ball sitting in the concavity of a table [50]. The greater the depth of the concavity, the greater a displacing force must be to dislodge the ball for a given compressive load (Fig. 4). In the normal shoulder, the rotator cuff muscles provide the compressive load. This is lost in the rotator cuff-deficient shoulder, leading to instability resulting from unbalanced muscle forces in the glenohumeral joint [6, 14, 17, 21]. A second biomechanical point proposed by Matsen and Lippitt is the concept of the glenoid center line [49]. In a normal glenoid, the center line represents a line perpendicular to the articular surface of the glenoid and directed, on average, approximately 10° posterior (retroverted) to the plane of the scapula (Fig. 5A–B). The center line serves as the pillar under which the humeral head rests; glenohumeral and scapulothoracic motion are coupled to maintain the center line beneath the humeral head throughout the shoulder’s ROM. In the rotator cuff-deficient shoulder, unbalanced muscular forces disrupt this relationship. This mechanical alteration can lead to pathologic wear patterns on the glenoid consisting of a spectrum of disease from no wear to superior, inferior, anterior, posterior, or global wear [25]. Abnormal wear patterns are likely the result of the altered biomechanical and biochemical environment of the cuff-deficient shoulder and often appear in eccentric patterns [17, 50, 62]. Recognizing these patterns can assist in proper preoperative planning. Ideally, the glenoid component is placed along the glenoid center line. However, in some cases with glenoid bone loss, component placement in this plane is not possible. In cases of severe or eccentric glenoid wear, a stable baseplate fixation can only be achieved by placing the component along the alternate glenoid center line (Fig. 5C–D) [25]. This alternate center line is defined as a line aiming at the dense bone where the scapular spine meets the body of the scapula and is not necessarily perpendicular to the remaining glenoid face. Understanding the center line in the cuff-deficient shoulder with little glenoid wear can provide excellent fixation for the glenoid baseplate in reverse arthroplasty; knowing what glenoid wear patterns make the center line unusable allows surgeons to compensate for bone loss by achieving adequate fixation along the alternate center line.
Fig. 4.
This artist rendition uses billiard balls to explain concavity-compression. Deeper concavities require a larger displacing force for a given compressive load. Reprinted with permission from Chapter 8, Principles of glenohumeral stability. In: Matsen F, Lippitt S, eds. Shoulder Surgery: Principles and Procedures. Philadelphia: WB Saunders; 2003, Fig. 8-5.
Fig. 5A–D.
(A) This computer model demonstrates the anterior view of the glenoid center line. (B) This computer model demonstrates the inferior view of the glenoid center line. (C) This computer model demonstrates the anterior view of the alternate center line along the scapular spine. (D) This computer model demonstrates the inferior view of the alternate center line along the scapular spine.
A third important alteration from normal shoulder mechanics seen in the cuff-deficient shoulder involves the concept of impingement. This can occur between the greater tuberosity and the acromion and lead to acromial erosion or “acetabularization.” In patients who have lost the dynamic stabilizers of the rotator cuff muscles, the humeral head migrates superiorly and leads to abutment underneath the acromion (Fig. 1). In these cases, use of a fixed-fulcrum prosthesis such as a reverse prosthesis can neutralize this dynamic instability.
Total Joint Arthroplasty: The Basics
Basic principles of total joint arthroplasty, learned mostly from the total hip literature, can provide a conceptual framework to improve understanding of reverse shoulder arthroplasty. The key concepts relate to component fixation and prosthetic impingement.
Fixation in total joint arthroplasty has undergone considerable changes since Charnley introduced the THA. Initial implants were fixed with cement, which provides an initial stable prosthetic attachment by acting as a grout. Over time, the concept of osteointegration was introduced. In this case, various implant surface characteristics can be used to allow bone ingrowth or ongrowth to the prosthesis; this could theoretically provide a longer lasting attachment and a decreased incidence of mechanical failure. Osteointegration requires initial stable attachment of the prosthesis to the bone with micromotion at the bone-prosthetic interface of less than 150 μm and a surface with properties to allow osseous healing [40]. These properties depend on the type of coating used, the porosity of the material (typically 65%–80% for most orthopaedic applications), and the surface roughness of the coating [3].
Currently available reverse shoulder designs use a combination of cement for diaphyseal fixation of the humeral component and a proximal coating for metaphyseal osseointegration (Table 1). Although previous work on humeral stem fixation in an anatomic stem suggested both proximal and fully cemented humeral stems offer decreased micromotion and enhanced rotational stability when compared with a press-fit technique [37], the humeral component of the reverse shoulder arthroplasty may behave differently.
Table 1.
Manufacturers, humeral stem coating, and type of offset in reverse shoulder arthroplasty implant designs
| Manufacturer (location) | Humeral stem porous coating | Type of offset |
|---|---|---|
| DJO RSP (Vista, CA) | Very light | None and lateral |
| Zimmer Anatomical (Warsaw, IN) | Very light | None and inferior |
| Exactech Equinox (Gainesville, FL) | Very light | Inferior |
| Affinis Inverse (Switzerland) | Very light | Inferior |
| Universal Arrow System (Heimsbrunn, France) | Very light | None and lateral |
| Lima SMR (Udine, Italy) | Heavy | None and inferior |
| Zimmer Trabecular (Warsaw, IN) | Heavy | None |
| Biomet Comprehensive (Warsaw, IN) | Heavy | None, lateral, and inferior |
| DePuy (Warsaw, IN) | None | None and inferior |
| Tornier Aequalis (Edina, MN) | None | None |
Glenoid fixation in reverse arthroplasty universally uses cementless techniques (Table 1). The goal on the glenoid side is to prevent micromotion and allow for bony ongrowth to decrease glenoid failure rates. Often, this is achieved by the basic AO principles of bone healing: compression and neutralization. Compression is achieved through either a central axis screw or peripheral unlocked screws. Once the baseplate is properly compressed to the glenoid bone, forces can be neutralized with locking screws, thereby minimizing micromotion [36].
Positioning of the components in total joint arthroplasty is important for several reasons. First is to avoid instability by providing patients with a functional ROM within their native range [13]. Second is positioning can help avoid impingement. Impingement is a cause of polyethylene wear and component failure after THA [47]. Impingement between the humerosocket and the inferior lateral scapular pillar occurs in reverse shoulder arthroplasty and can lead to scapular notching, which is associated with inferior scapular wear, osteolysis, and polyethylene wear [54]. Impingement in reverse arthroplasty may also contribute to prosthetic instability [16], unexplained pain [59], and long-term prosthetic loosening [59]. Finally, component position is important to restore the function of the surrounding musculature of the joint. In THAs, malposition is one reason for postoperative dislocation [16]; these concerns are also present in reverse shoulder arthroplasty.
Glenoid Baseplate Fixation
To achieve secure fixation to the glenoid, it is important that the baseplate be well-fixed to the highest quality bone available. Failure at the glenoid baseplate has been attributed to the inability of the baseplate fixation to overcome the forces imparted to the interface before adequate bony ingrowth [23]. Chebli et al. emphasized the importance of glenoid fixation and peripheral screw placement into the highest quality glenoid bone stock [10]. This bone stock can be found in the three major columns of scapular bone, as described by Humphrey et al. They emphasize placing peripheral screws into the base of the coracoid, the spine of the scapula, or the scapular pillar [39]. However, as a result of the varied spectrum of disease seen in CTA, these optimal locations are not always readily available for fixation. Furthermore, pathologic changes in the glenoid often develop and may require alternate locations for baseplate fixation [25].
Bone growth into or onto the prosthesis can provide durable attachment of the component to the bone. To achieve bony ingrowth using a cementless prosthesis, it is important to minimize micromotion to less than 150 μm [40]. Hopkins et al. noted micromotion between the baseplate and glenoid could be decreased by increasing the length, diameter, and inclination of the peripheral screws in a reverse shoulder model [38]. Furthermore, they found micromotion increases if the stiffness of the surrounding bone decreases, as would occur in poor-quality or osteoporotic bone.
It is possible to apply the basic principles of bone healing to enhance bony ingrowth to the baseplate. All reverse designs advocate compression of the baseplate to the glenoid bone. This compression can be established by central axis or peripheral screws. For example, the DJO RSP uses a central lag screw to facilitate compression of the baseplate (Fig. 6), whereas the DePuy Delta III establishes compression through a central post and peripheral screws (Fig. 3). A biomechanical study using force sensors beneath the baseplates was conducted to evaluate the amount of compression achieved using these two designs. A 10-fold difference was observed between the DJO RSP (2000 N) and the Delta III (200 N) [21]. Using a load-to-failure model, the central screw design was compared with the central post design. A 2.3-fold increase in load to failure was observed between the central screw (629 N) and the central post (269 N) [21]. Although these studies suggest stronger fixation than the Delta III, early clinical studies using a central 6.5-mm lag screw in combination with 3.5-mm peripheral nonlocked screws for glenoid fixation showed an initial high glenoid failure rate of approximately 10% [23]. Further analysis of these failures using scanning electron microscopy revealed a failure of bone ingrowth between the baseplate and the glenoid bone. These early clinical glenoid-sided failures prompted further analysis of glenoid component fixation. In a study of construct stiffness, Harman et al. evaluated different baseplate constructs using locked (5.0 mm) and unlocked screw (3.5 mm) combinations. Baseplates fixed with 5.0-mm locked screws were observed to have 29% less micromotion than those fixed with 3.5-mm unlocked screws [36]. A subsequent clinical study using locked 5.0-mm peripheral screws found glenoid failure was reduced to 0.4% [14].
Fig. 6.
The DJO RSP has a central 6.5-mm lag screw.
Glenoid Position
Positioning of the glenoid component is currently an area of active research; the study of glenoid position may have important consequences for maximizing the impingement free arc of motion.
Grammont and Baulot [29] believed glenoid failure was related to the amount of shear stress across the glenoid component and that simply medializing the center of rotation of the glenosphere to the glenoid face would dramatically reduce this stress [6]. However, initially there was no information available to guide surgeons in positioning of the baseplate on the glenoid in a way that could best minimize these shear stresses. Understanding of how component position can alter glenoid survival was not appreciated until later. While analyzing the initial failures of lateral offset reverse arthroplasties implanted with 3.5-mm unlocked peripheral screws, it became apparent that they shared a common radiographic appearance of a superiorly tilted glenosphere [24]. This was not surprising, because patients initially treated with reverse shoulder arthroplasty often had superior glenoid wear, and baseplates were thus often implanted at the native glenoid inclination, superiorly tilting the component. Comparing different glenosphere implantation angles (15° of superior tilt, 15° of inferior tilt, and neutral tilt), Gutierrez et al. demonstrated inferior tilting the glenosphere 15°, by preferentially reaming the inferior glenoid, resulted in the most uniform compressive forces and the least amount of tensile forces and micromotion [32].
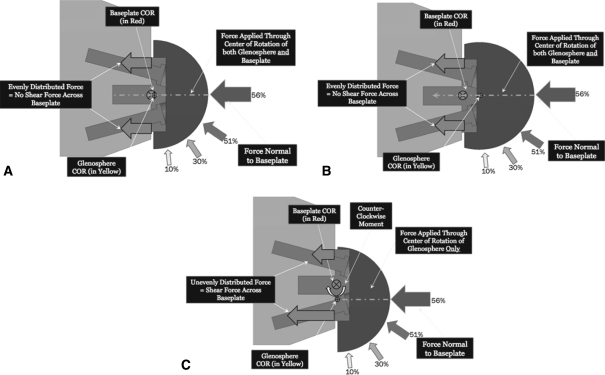
In a subsequent study, Gutierrez et al. investigated the forces at the baseplate-bone interface for three different glenosphere designs (concentric, lateral eccentric, and inferior eccentric) placed in three different positions (superior tilt, neutral, and inferior tilt) (Fig. 7) [35]. Using computer simulation, the authors showed a predictable pattern of forces at the baseplate-bone junction corresponding to the type of offset used and tilt of the glenoid (Fig. 8A–C). The most uniform distribution of forces occurred with a lateralized or concentric center of rotation placed in inferior tilt; the most nonuniform distribution of forces occurred with either a concentric or lateral center of rotation placed in superior tilt. Those glenospheres with inferior offset demonstrated more nonuniform forces beneath the baseplate when placed in inferior tilt as opposed to neutral or even superior tilt.
Fig. 7A–C.
(A) The figure shows the progression of joint forces throughout abduction for the concentric glenosphere. (B) This figure shows the progression of joint forces throughout abduction for the lateral eccentric glenosphere. (C) This figure shows the progression of joint forces throughout abduction for the inferior eccentric glenosphere. Reprinted with permission from Gutiérrez S, Walker M, Willis M, Pupello D, Frankle M. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. Figure 5. J Shoulder Elbow Surg. 2011 Jan 31 [Epub ahead of print].
Fig. 8.
This figure shows the effect of different forces at the baseplate-bone interface.
Stability
Instability is common after reverse prosthesis with rates of dislocation reported as high as 30% using the Grammont design [15]. Several factors contribute to postoperative instability: soft tissue tension, glenosphere diameter, humeral-socket constraint, impingement, and axillary nerve/deltoid function.
Restoring anatomic soft tissue tension can be achieved through a variety of prosthetic design and surgical technique factors (Table 2), each of which can help to retension the muscular envelope around the shoulder. Boileau et al. noted soft tissue can be tensioned with a Grammont-style device, but the vector of pull is no longer in an anatomic plane once the humerus is substantially elongated [6]. In fact, designs that medialize the center of rotation can place a distracting force on the humeral component in the case of glenoid bone loss, increasing the propensity of dislocation (Fig. 9A–B) [52]. An alternative method of improving stability of the reverse shoulder arthroplasty is by lengthening the humerus (Table 2) [6]. However, this is not without cost. Increasing humeral length carries the additional risk of acromion stress fracture, brachial plexopathy, deltoid overtensioning, and loss of motion. An increase in compressive force across the glenohumeral joint can also be achieved by increasing the glenoid tuberosity distance (Table 2). Potential problems with increasing the glenoid tuberosity distance may include greater component or bony impingement.
Table 2.
Surgical and implant factors that can help restore soft tissue tension
| Prosthetic factors |
| • Varying glenosphere offset and size |
| • Different humeral neck-shaft angles (a varus or valgus component) |
| • Varying thickness of humeral inserts |
| Surgical factors |
| • Altering the level of the humeral osteotomy |
| • Offsetting placement of the humerosocket |
| • Changing placement of the glenosphere |
| Techniques to restore soft tissue tension by lengthening the humerus |
| • Placement of the glenosphere with inferior translation |
| • Placement of the glenosphere with inferior tilt |
| • Using an inferiorly eccentric glenosphere |
| • Using a larger glenosphere with inferior overhang |
| • Using a valgus humeral neck shaft angle |
| • Humeral augments |
| Techniques to restore soft tissue tension through compressive force across the glenohumeral joint |
| • Placement of glenosphere with lateral offset |
| • Using a varus humeral component |
| • Translating humeral component so the intramedullary stem is lateral to socket |
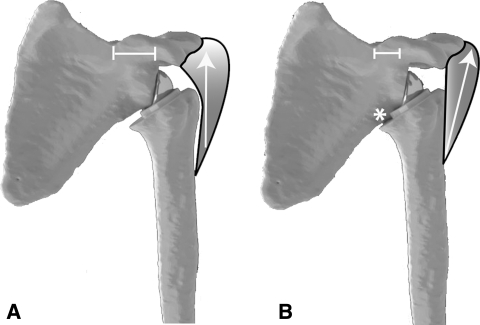
Fig. 9A–B.
(A) Devices with lateralized centers of rotation allow the proximal pull of the deltoid to pull the humerus toward the glenosphere, thus increasing the compression and stability of the implant. (B) Devices with medialized centers of rotation allow the deltoid to place a distraction moment on the prosthesis and increase the propensity for dislocation.
Gutierrez et al. evaluated several factors of prosthetic design, including the compressive force on the prosthesis, the prosthetic socket depth, and the glenosphere size and how these related to dislocation [32]. They found a hierarchy of importance among these factors; the most important parameter for achieving stability was increasing the compressive forces on the prosthesis followed by humerosocket depth. Furthermore, they developed a mathematical model based on the mechanical data that were collected and validated that a critical determinant in translational stability was the relationship of the diameter of the socket to the diameter of the sphere [30]. They called this the D/R ratio (diameter of the sphere/the radius of the humerosocket). The model suggested that devices with an increased D/R ratio have more inherent stability, or, for the same size ball, a deeper socket would be more stable.
Ultimately, this is a matter of constraint; a deeper humerosocket concavity means a more constrained device that is harder to dislocate [1, 41]. The essence of the D/R ratio rests in the basis of the theory of concavity-compression [48]. In the native shoulder, Matsen and Lippitt discussed the balance stability angle; this is the maximum “angle that the net force on the humeral head forms with the glenoid center line before dislocation occurs” [49]. They used this angle to calculate the stability ratio: the ratio of the translational force on the humerus to the compressive normal load on the humeral head (Fig. 10). Problematic to prostheses with a deeper socket, however, is a decreased impingement-free arc of motion, as shown by Gutierrez et al. [34].
Fig. 10.
The balance stability angle. This represents the maximum angle that the net force on the humeral head forms with the glenoid centerline before dislocation. Reprinted with permission from Chapter 9, Principles of the glenoid concavity. In: Matsen F, Lippitt S, eds. Shoulder Surgery: Principles and Procedures. Philadelphia: WB Saunders; 2003, Fig. 9-23.
Range of Motion
A patient’s shoulder function after reverse shoulder arthroplasty relates to the interaction of soft tissue tension, residual muscle function, and the potential motion provided by the prosthetic design used. With many prosthetic designs, the medial aspect of the humeral socket impinges on the inferior glenoid neck or scapular body; this often occurs in the resting position with the patient’s arm at their side in adduction and results in scapular notching.
Several authors have suggested an adaptation of surgical technique as a method of avoiding the adduction deficit. This involves positioning the baseplate more inferiorly on the glenoid [42, 53]. Nyffeler et al. studied the effects of different glenoid positions on impingement and ROM and found that inferior placement of the glenosphere had a major effect in improving adduction and abduction ROM [53]. Similar findings were reported by Chou et al., who showed eccentric glenospheres with larger diameters can improve impingement-free ROM [11]. Additionally, Roche et al. found that modification of the Grammont prosthesis by distalizing the glenoid and increasing the glenosphere thickness improved impingement-free ROM arc from 59° to 109° [56].
Other factors contributing to scapular notching were evaluated by Gutierrez et al. [30]. Using a virtual model, they determined that the factor that helped to best avoid an adduction deficit was a varus humeral neck-shaft angle (130°); next was using an inferior placement of the glenosphere, then a 10-mm lateral offset glenosphere, then inferiorly tilting the glenosphere, and finally using a larger glenosphere size.
In the same study, Gutierrez et al. used computer simulation to assess different combinations of surgical and implant-related factors when moving the humerus from 0° of abduction to impingement. The authors found the most important factor for maximizing impingement-free ROM was using a glenosphere with a center of rotation lateral to the glenoid. Additionally, Gutierrez et al. characterized the ability of available reverse arthroplasty designs to maximize arc of motion [33]. Using abduction of the humerus in the scapular plane, a linear correlation was found between abduction ROM and center of rotation offset relative to the glenoid; as center of rotation is lateralized, the ROM in abduction increased.
Maximizing impingement-free arc of motion is not only important in achieving ultimate ROM, but it may play a critical role in the survivability of the implant. Simovitch et al. found that the presence and size of a notch negatively correlated with Constant score, subjective shoulder value, active flexion, and active abduction [61]. Furthermore, Sirveaux et al. found that the presence of a notch in 50 of 77 cases not only had a significant impact on the Constant score, but in three of the cases, the glenoid component had actually loosened [58].
Muscle Function: Loss of External Rotation Strength
Clinical studies of reverse shoulder arthroplasty using the Grammont device repeatedly described pain relief and functional recovery, but a closer look revealed unreliable improvement in external rotation [7, 18, 60, 63, 65]. The altered anatomy often seen in cuff tear arthropathy decreases the efficiency of contraction of the remaining shoulder muscles and can explain the loss of external rotation strength. Furthermore, evidence suggests those patients who do not have improvements in external rotation are less satisfied with their function [60].
In contrast to the rotation seen using the Grammont device, return of external rotation using a prosthesis with a lateral center of rotation was observed more consistently [23]. The authors speculated that restoring the anatomic placement of the humerus would improve tension of the remaining rotator cuff muscles and hence provide for restoration of strength. They believed, in the setting of rotator cuff deficiency, the unstable, proximally migrated humerus prevents the remaining intact rotator cuff muscles from properly functioning. By restoring stability to the joint and tensioning the remaining muscles in a more anatomic fashion, external rotation could be improved. Furthermore, clinical studies have confirmed that restoring the lateral offset of the humerus leads to an increase in external rotation postoperatively in a variety of pathologic conditions [9, 14, 22, 43, 44]. In addition, these authors believed restoring muscle tension would also help restore the stability of the glenohumeral joint by increasing the compression on the humerus, a factor that was compromised with medialization and hence slackening of those rotator cuff muscles.
For patients with severe preoperative external rotational deficits, latissimus dorsi and teres major transfers can be beneficial [4, 5, 19, 27, 28, 64]. Gerber et al. speculated the absence of the teres minor was predictive of external rotational weakness and advocated transferring motor units, specifically the latissimus dorsi, to help restore rotational strength [19, 27, 28]. Boileau et al. also described a modification of the latissimus/teres minor tendon transfer to help improve rotational strength [4, 5]. Finally, Grammont has speculated the posterior deltoid could be recruited after reverse total shoulder arthroplasty to help improve rotation, but there remain little biomechanical data to support this speculation [29].
Discussion
The reverse total shoulder arthroplasty was introduced to better treat patients with rotator cuff-deficient shoulders. An improved understanding of the biomechanics of rotator cuff deficiency and design features of reverse shoulder arthroplasty has facilitated the evolution of reverse arthroplasty designs. The purposes of this article were to review (1) the biomechanics of the rotator cuff-deficient shoulder; (2) the biomechanical rational for newer reverse shoulder arthroplasty designs; (3) the current scientific evidence related to the function of reverse shoulder arthroplasty; and (4) specific technical aspects of reverse shoulder arthroplasty.
The limitations of this review relate mostly to the limitations in the information provided in the particular studies evaluated; readers should understand this point so conclusions are not overstated. Each article included has its own limitations related to the methodologies, which were used to reach the authors’ conclusions; for example, (1) some were basic science studies with no clinical studies to correlate; (2) some were small patient series; and (3) some had short clinical followup. The most effective way to improve treatment methods is to combine basic science research with well-designed clinical outcome studies to evaluate the effectiveness of the various available designs.
To gain insight into the rotator cuff-deficient shoulder, reviewing the function of the normal shoulder is helpful. Several concepts related to the stability of the normal shoulder come from Matsen’s theories of concavity-compression and glenoid center line [45, 48, 49]. These theories form the basis for understanding how the reverse shoulder arthroplasty is stabilized. Initial studies evaluated the stability of the reverse design based on the depth of the humerosocket and the size of the glenosphere as well as proper tensioning of the deltoid [7, 25, 32, 54]. However, in a later study, Gutierriz et al. illustrated the importance of other variables such as glenosphere placement, tilt, and offset as well as the humeral neck-shaft angle [34]. From these data it is clear that stability involves a complex interplay of both implant design-related factors and surgical implantation-related techniques. Both can be exploited to achieve improved stability depending on the clinical situation at hand. Future studies should focus on elucidating the effects of these various implant and technique factors to deal with different clinical scenarios, for example, a study designed to clarify the effectiveness of various glenoid offsets or bone graft techniques in the setting of glenoid bone loss.
Evolution in implant design is driven by the manufacturers who create different implants, but it is mandatory that these advances be adequately studied in both the laboratory and in clinical practice. For example, basic science studies have shown designs with a central screw have more compression and improved load to failure between the baseplate and the remaining glenoid bone than those with a central peg [21, 36]. Although this may be true, a more useful study design would focus on threshold levels for glenoid baseplate-bone compressive forces and loads to failure so that each device available on the market can be tested to ensure it meets these minimum requirements.
Other areas where further research is needed relate to glenosphere position. Several devices are available on the market (Table 1) with varying offsets. Manufacturers have advocated placing these glenospheres in different positions and in different angles of inclination on the glenoid, but little data exist evaluating the effects of these varying configurations. The little data available [31] suggest positioning of varying offset components in certain tilt angles on the glenoid may lead to a more uniform force distribution at the baseplate-bone junction, but better understanding could be obtained in the future with carefully designed clinical studies evaluating the survivorship of these different glenosphere designs based on the tilt in which they were placed.
Finally, the application of biomechanical principles to the design of reverse arthroplasties should work to benefit the patient. Studies suggest various implant and surgical techniques can maximize impingement-free ROM for patients; for example, moving the glenosphere inferiorly and distally [44, 55, 56], using eccentric glenospheres [11], and using lateral offset and a more varus humeral neck-shaft angle [23] are all available to the surgeon.
The currently available literature relating to the biomechanics of the rotator cuff-deficient shoulder and the reverse shoulder arthroplasty tend to support the following: first, using a central lag screw to increase the compression at the baseplate bone junction; second, using devices with centers of rotation closer to the anatomic center of rotation (lateral offset), because this improves not only ROM and external rotation strength, but may retension the remaining cuff musculature and lead to a more stable prosthesis; third, using a more varus (and hence, closer to anatomic) neck-shaft angle, which may reduce notching and the potential problems associated with this complication; and finally, implanting the glenoid component in positions that best use the particular system being used. Although the details of this final point are still being worked out, the literature supports that when using a device with a lateral offset, clinical failures are lower when the glenosphere is placed in slight inferior tilt [14].
Footnotes
One of the authors’ (JB) employer received support related to the work. One of the authors (MF) receives royalties that could be construed as related to this manuscript and receives personal payment from a commercial entity (eg, serves as a consultant) related to the work.
References
- 1.Anglin C, Wyss UP, Pichore DR. Shoulder prosthesis subluxation: theory and experiment. J Shoulder Elbow Surg. 2000;9:104–114. doi: 10.1016/S1058-2746(00)90038-7. [DOI] [PubMed] [Google Scholar]
- 2.Bayley J, Kessel L. The Kessel total shoulder replacement. In: Bayley I, Kessel L, editors. Shoulder Surgery. New York: Springer-Verlag; 1982. pp. 160–164. [Google Scholar]
- 3.Bobyn JD, Toh K-K, Hacking SA, Tanzer M, Krygier JJ. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty. 1999;14:347–354. doi: 10.1016/S0883-5403(99)90062-1. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466:584–593. doi: 10.1007/s11999-008-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16:671–682. doi: 10.1016/j.jse.2007.02.127. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Watkinson DJ, Hatzidakis A, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Near Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 9.Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91:119–127. doi: 10.2106/JBJS.H.00094. [DOI] [PubMed] [Google Scholar]
- 10.Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F., 3rd Factors affecting fixation of the glenoid component of a reverse total shoulder prosthesis. J Shoulder Elbow Surg. 2008;17:323–327. doi: 10.1016/j.jse.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Chou J, Malak SF, Anderson IA, Astley T, Poon PC. Biomechanical evaluation of different designs of glenospheres in the SMR reverse total shoulder prosthesis: range of motion and risk of scapular notching. J Shoulder Elbow Surg. 2009;18:354–359. doi: 10.1016/j.jse.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66:899–906. doi: 10.2106/00004623-198466060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Coventry MB, Beckenbaugh RD, Nolan DR, Ilstrup DM. 2, 012 total hip arthroplasties. A study of postoperative course and early complications. J Bone Joint Surg Am. 1974;56:273–284. [PubMed] [Google Scholar]
- 14.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 15.Wilde LF, Ovost E, Uyttendaele D, Verdonk R. Results of an inverted shoulder prosthesis after resection for tumor of the proximal humerus [in French] Rev Chir Orthop Reparatrice Appar Mot. 2002;88:373–378. [PubMed] [Google Scholar]
- 16.Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop Relat Res. 1998;355:144–151. doi: 10.1097/00003086-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Ecklund KJ, Lee TQ, Tibone J, Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007;15:340–349. doi: 10.5435/00124635-200706000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Favard L, Lautmann S, Sirveaux F, Oudet D, Kerjean Y, Huguet D. Hemi-arthroplasty versus reverse arthroplasty in treatment of osteoarthritis with massive rotator cuff tear. In: Walch GBP, Mole D, editors. 2000 Prostheses…2 to 10 Year Follow-up. Paris, France: Sauramps Medical; 2001. pp. 261–268. [Google Scholar]
- 19.Favre P, Loeb MD, Helmy N, Gerber C. Latissimus dorsi transfer to restore external rotation with reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg. 2008;17:650–658. doi: 10.1016/j.jse.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Field LD, Dines DM, Zabinski SJ, Warren RF. Hemiarthroplasty of the shoulder for rotator cuff arthropathy. J Shoulder Elbow Surg. 1997;6:18–23. doi: 10.1016/S1058-2746(97)90066-5. [DOI] [PubMed] [Google Scholar]
- 21.Frankle M. Rationale and biomechanics of the reverse shoulder prosthesis: the American experience. In: Frankle M, editor. Rotator Cuff Deficiency of the Shoulder. New York: Thieme; 2008. pp. 76–104. [Google Scholar]
- 22.Frankle M, Levy JC, Pupello D, Siegal S, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1):178–190. doi: 10.2106/JBJS.F.00123. [DOI] [PubMed] [Google Scholar]
- 23.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 24.Frankle MA, Siegal S, Pupello DR, Gutierrez S, Griewe M, Mighell M. Coronal plane tilt angle affects risk of catastrophic failure in patients treated with a reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16:e46. doi: 10.1016/j.jse.2007.02.096. [DOI] [Google Scholar]
- 25.Frankle MA, Teramoto A, Luo Z-P, Levy JC, Pupello D. Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg. 2009;18:874–885. doi: 10.1016/j.jse.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250–257. doi: 10.1097/00003086-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 27.Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am. 2006;88:113–120. doi: 10.2106/JBJS.E.00282. [DOI] [PubMed] [Google Scholar]
- 28.Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer. A preliminary report. J Bone Joint Surg Am. 2007;89:940–947. doi: 10.2106/JBJS.F.00955. [DOI] [PubMed] [Google Scholar]
- 29.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez S, Comiskey CA, Luo Z-P, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90:2606–2615. doi: 10.2106/JBJS.H.00012. [DOI] [PubMed] [Google Scholar]
- 31.Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE., 3rd Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16:S9–S12. doi: 10.1016/j.jse.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez S, Keller TS, Levy JC, Lee WE, 3rd, Luo Z-P. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:670–676. doi: 10.1007/s11999-007-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutiérrez S, Levy JC, Lee WE, 3rd, Keller TS, Maitland ME. Center of rotation affects abduction range of motion of reverse shoulder arthroplasty. Clin Orthop Relat Res. 2007;458:78–82. doi: 10.1097/BLO.0b013e31803d0f57. [DOI] [PubMed] [Google Scholar]
- 34.Gutiérrez S, Luo Z-P, Levy J, Frankle MA. Arc of motion and socket depth in reverse shoulder implants. Clin Biomech. 2009;24:473–479. doi: 10.1016/j.clinbiomech.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty journal of shoulder and elbow surgery. J Shoulder Elbow Surg. 2011 Jan 31 [Epub ahead of print]. [DOI] [PubMed]
- 36.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14:162S–167S. doi: 10.1016/j.jse.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Harris TE, Jobe CM, Dai QC. Fixation of proximal humeral prostheses and rotational micromotion. J Shoulder Elbow Surg. 2000;9:205–210. doi: 10.1016/S1058-2746(00)90056-9. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins AR, Hansen UN, Bull AMJ, Emery R, Amis AA. Fixation of the reversed shoulder prosthesis. J Shoulder Elbow Surg. 2008;17:974–980. doi: 10.1016/j.jse.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Humphrey CS, Kelly JD, 2nd, Norris TR. Optimizing glenosphere position and fixation in reverse shoulder arthroplasty, Part Two: the three-column concept. J Shoulder Elbow Surg. 2008;17:595–601. doi: 10.1016/j.jse.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 40.Jasty M, Bragdon C, Burke D, O’Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg Am. 1997;79:707–714. doi: 10.2106/00004623-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Karduna AR, Williams GR, Williams JL, Iannotti JP. Glenohumeral joint translations before and after total shoulder arthroplasty. A study in cadavera. J Bone Joint Surg Am. 1997;79:1166–1174. doi: 10.2106/00004623-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Siveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89:292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 44.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89:189–195. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 45.Lippitt SB, Vanderhooft JE, Harris SL, Sidles JA, Harryman DT, II, Matsen FA., III Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elbow Surg. 1993;2:27–35. doi: 10.1016/S1058-2746(09)80134-1. [DOI] [PubMed] [Google Scholar]
- 46.Long BW, Rafert JA. Orthopaedic Radiography. Philadelphia: WB Saunders; 1995. pp. 189–199. [Google Scholar]
- 47.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89:1832–1842. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 48.Matsen F, Lippitt S. Principles of glenohumeral stability. In: Matsen F, Lippitt S, DeBartolo S, editors. Shoulder Surgery: Principles and Procedures. Philadelphia: WB Saunders; 2004. p. 83. [Google Scholar]
- 49.Matsen F, Lippitt S. Principles of glenoid concavity. In: Matsen F, Lippitt S, DeBartolo S, editors. Shoulder Surgery: Principles and Procedures. Philadelphia: WB Saunders; 2004. p. 89. [Google Scholar]
- 50.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 51.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–337. [PubMed] [Google Scholar]
- 52.Norris TR, Kelly JDI, Humphrey CS. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Tech Shoulder Elbow Surg. 2007;8:37–46. doi: 10.1097/BTE.0b013e318030d3b7. [DOI] [Google Scholar]
- 53.Nyffeler RW, Werner CML, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14:524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Nyffeler RW, Werner CML, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86:1187–1191. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 55.Pollock RG, Deliz ED, McIlveen SJ, Flatow EL, Bigliani LU. Prosthetic replacement in rotator cuff-deficient shoulders. J Shoulder Elbow Surg. 1992;1:173–186. doi: 10.1016/1058-2746(92)90011-Q. [DOI] [PubMed] [Google Scholar]
- 56.Roche C, Flurin P-H, Wright T, Crosby LA, Mauldin M, Zuckerman JD. An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement and stability. J Shoulder Elbow Surg. 2009;18:734–741. doi: 10.1016/j.jse.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001;83:1814–1822. doi: 10.2106/00004623-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Sirveaux F, Favard L, Oudet D, Huguet D, Lautman S. Grammont. Inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive and non repairable cuff rupture. In: Walch G, Mole D, editors. 2000 Shoulder Prostheses . . . Two to Ten Year Follow-up. Montpellier: Sauramps Medical; 2001. pp. 247–252. [Google Scholar]
- 59.Shon WY, Baldini T, Peterson MG, Wright TM, Salvati EA. Impingement in total hip arthroplasty: a study of retrieved acetabular components. J Arthroplasty. 2005;20:427–435. doi: 10.1016/j.arth.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 60.Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89:934–939. doi: 10.2106/JBJS.F.01075. [DOI] [PubMed] [Google Scholar]
- 61.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 62.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(Suppl 2):35–40. [PubMed] [Google Scholar]
- 63.Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 64.Warner JJ. Management of massive irreparable rotator cuff tears: the role of tendon transfer. Instr Course Lect. 2001;50:63–71. [PubMed] [Google Scholar]
- 65.Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 66.Williams GR, Jr, Rockwood CA., Jr Hemiarthroplasty in rotator cuff-deficient shoulders. J Shoulder Elbow Surg. 1996;5:362–367. doi: 10.1016/S1058-2746(96)80067-X. [DOI] [PubMed] [Google Scholar]
- 67.Zuckerman JD, Scott AJ, Gallagher MA. Hemiarthroplasty for cuff tear arthropathy. J Shoulder Elbow Surg. 2000;9:169–172. doi: 10.1016/S1058-2746(00)90050-8. [DOI] [PubMed] [Google Scholar]