Abstract
Background
Cuff tear arthropathy is the primary indication for total reverse shoulder arthroplasty. In patients with pseudoparalytic shoulders secondary to irreparable rotator cuff tear, reverse shoulder arthroplasty allows restoration of active anterior elevation and painless shoulder. High rates of glenoid notching have also been reported. We designed a new reverse shoulder arthroplasty with a center of rotation more lateral than the Delta prosthesis to address this problem.
Questions/purposes
Does reduced medialization of reverse shoulder arthroplasty improve shoulder motion, decrease glenoid notching, or increase the risk of glenoid loosening?
Patients and Methods
We retrospectively reviewed 76 patients with 76 less medialized reverse shoulder prostheses implanted for pseudoparalytic shoulder with rotator cuff deficiency between October 2003 and May 2006. Shoulder motion, Constant-Murley score, and plain radiographs were analyzed. Minimum followup was 24 months (mean, 44 months; range, 24–60 months).
Results
The absolute Constant-Murley score increased from 24 to 59, representing an increase of 35 points. The range of active anterior elevation increased by 61°, and the improvement in pain was 10 points. The gain in external rotation with elbow at the side was 15°, while external rotation with 90° abduction increased by 30°. Followup showed no glenoid notching and no glenoid loosening with these less medialized reverse prostheses.
Conclusions
Less medialization of reverse shoulder arthroplasty improves external and medial rotation, thus facilitating the activities of daily living of older patients. The absence of glenoid notching and glenoid loosening hopefully reflects longer prosthesis survival, but longer followup is necessary to confirm these preliminary observations.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Cuff tear arthropathy is the primary indication for total reverse shoulder arthroplasty (RSA) [2, 9, 31]. Charles Neer, a pioneer of the anatomic prosthesis, proposed a reverse constrained prosthesis for this indication [19]. Analysis of Mark 1, 2, and 3 (3 M Healthcare Ltd, Loughborough, UK) prostheses shows the fixed, most external center of rotation, at some distance from the glenoid bone, creates considerable tilt forces in the glenoid cavity. These forces cause loosening of the glenoid component and fracture of the implant. In 1974, Neer abandoned these reversed constrained prostheses, insisting on the need to repair the rotator cuff to restore mobility, in particular, external rotation [20]. The concept of reversing the shoulder prosthesis reappeared in 1985 with Paul Grammont’s biomechanical work [12]. Grammont demonstrated medialization and distalization of the center of rotation on the glenoid bone increased the lever arm of the deltoid. RSA restores active anterior elevation up to 130° in pseudoparalytic shoulders secondary to irreparable rupture of the rotator cuff [1, 4, 11, 24, 26, 27, 30]. However, some studies [1, 3, 4, 17, 21, 22, 26] have documented drawbacks with medialization of the center of rotation, including glenoid notching and loss of shoulder rotation.
Four options can be considered to decrease the risk of glenoid notching and to improve rotation: (1) lowering the position of the metal baseplate [25, 26]; (2) increasing the inferior overhang of the glenosphere; (3) lateralizing the center of rotation (6–8 mm) with a bone graft interposed between the native glenoid bone and the baseplate (the bony increased-offset RSA [BIO-RSA]) [3]; and (4) less medialized design of the RSA [8]. We chose the last option and designed a new RSA prosthesis with a center of rotation more lateral than that of the Delta prosthesis to address the risk of glenoid notching and to improve rotation.
In this study, we asked: does a less medialized RSA prosthesis improve shoulder motion, decrease glenoid notching, or increase the risk of glenoid loosening?
Patients and Methods
Between October 2003 and May 2006, 126 RSAs were performed in our department. We retrospectively reviewed all 76 patients who had 76 less medialized RSA prostheses (Arrow® Anatomical Shoulder System; Fournitures Hospitalières Industrie, Mulhouse, France) for pseudoparalytic shoulder with massive rotator cuff tear. Fifty patients had RSAs for other indications: posttraumatic osteoarthritis (15 cases), recent dislocations-fractures (six cases), chronic anterior locked dislocations (two cases), rheumatoid arthritis (two cases), and revisions of a failed hemiarthroplasty or a total anatomic arthroplasty (25 cases). Contraindications were deltoid paralysis, history of chronic infection of the shoulder, massive glenoid bone defects, manual workers, and young active patients. All patients had previously received at least 6 months of physiotherapy; nevertheless, the active anterior elevation of the shoulder remained less than 60°. The mean age of the patients was 73 years (range, 52–90 years) with a female predominance (58 women versus 18 men). The dominant side was the most frequently impaired (56 dominant, 20 nondominant). No patients were lost to followup. No patients were recalled specifically for this study; all data were obtained from medical records and radiographs. The minimum followup was 24 months (mean, 44 months; range, 24–60 months).
The preoperative examination systematically included pain evaluation, shoulder motion, and Constant-Murley score evaluation. We obtained standard AP, lateral, and axillary view radiographs of the shoulder. We assessed the glenoid bone stock, the muscular trophicity of the deltoid and the remaining cuff, and the degree of fatty infiltration of the cuff using the classification of Goutallier et al. [10]. This classification describes four stages of fatty infiltration of the cuff muscles: Stage 1, no fatty tissue in the muscle; Stage 2, little fatty tissue; Stage 2, less fatty tissue than muscle; Stage 3, comparable muscle and fatty tissue; and Stage 4, more fatty tissue than muscle.
All patients had a complete rupture of the supraspinatus and infraspinatus tendon, which were retracted to the glenoid level. The mean preoperative fatty infiltration index was 2.96 for the supraspinatus and 2.91 for the infraspinatus. The superior third of the subscapularis muscle was altered in 30% of the cases with a fatty infiltration index of 2.20.
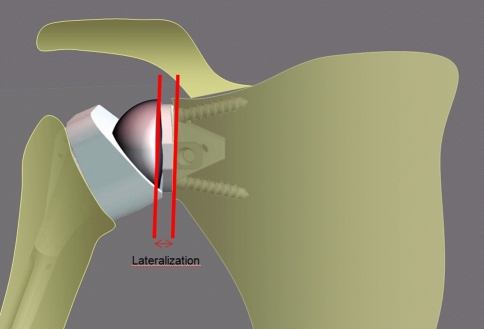
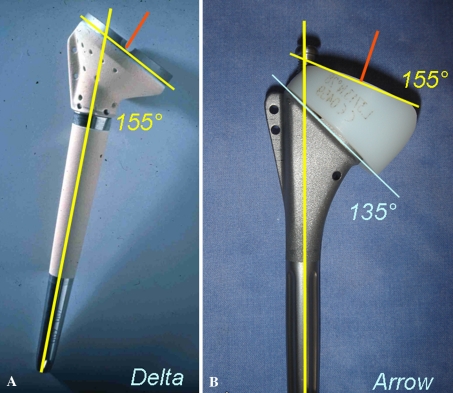
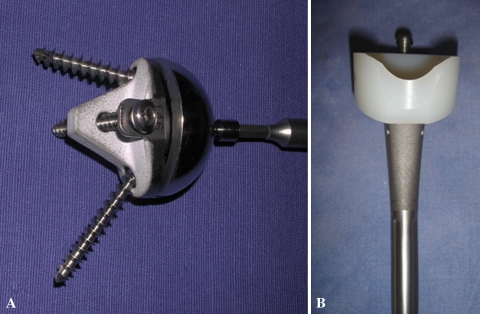
All surgery was performed by one of three surgeons (PV, DK, PS). All patients were operated on through a superolateral approach under complete general anesthesia with an interscalene block. After surgery, the catheter was left in place for 48 hours for analgesia. We performed tenodesis of the biceps tendon in the bicipital groove if the tendon had not ruptured. Wide resection of the subacromial bursa and degenerative fibrous tissues facilitated lowering the head of the humerus, especially in eccentric glenohumeral arthritis classified as Hamada Stage IV and V. Section of the superior third of the subscapularis allowed lowering the head of the humerus when the joint was stiff and the humeral head was fixed in the subcutaneous anterosuperior position. To expose the glenoid through this external superior approach, we performed a circumferential capsulectomy of the glenoid cavity to allow inferior and posterior translation of the humerus. Sectioning the bone at an angle of 135° was slightly more than sectioning when inserting an anatomic prosthesis. The retroversion angle chosen during the operation was 10° to 20°. In patients with osteoporosis and advanced osteopenia, the humeral stem was cemented (six cemented, 70 press-fit). The metal baseplate was positioned as low as possible in a plane perpendicular to the glenoid or slightly downward (inferior tilt). A superior or superoposterior defect of the glenoid must be preoperatively detected to avoid malpositioning of the metal baseplate into a superior tilt, which predisposes to an early glenoid failure. We reamed the glenoid cavity as conservatively as possible to retain subchondral bone. The convex-backed hydroxyapatite-covered metal baseplate consisted of a central keel and an anterior plate providing press-fit fixation to counter the shearing forces in the first degrees of abduction. Two divergent screws reproducing the Grammont concept provided definitive fixing for this metal baseplate. The glenosphere was fitted (not embedded) on the metal baseplate and fixed by impaction and one screw. This design of the glenosphere and metal baseplate allows a lateralization of 8.5 mm of the center of rotation (Fig. 1). Compared with the “trumpet”-shaped Grammont prosthesis, the shape of the metaphyseal part of the humeral stem, with a fixed angle inclination at 135° and a final angle at 155° with the polyethylene cup, creates a 4-mm lateralization of the humeral component (Fig. 2). The lateralization of the glenoid and humeral components of the Arrow® prosthesis afforded a more lateral axis of the humerus than that with the Delta III (Fig. 3).
Fig. 1.
The glenosphere of the Arrow® prosthesis is a half-sphere placed on the metal back. The center of rotation is lateralized by 8.5 mm (arrows) due to the thickness and the curve design of the metal back.
Fig. 2A–B.
Photographs illustrate the design of the humeral stem. (A) The Delta III humeral stem is less lateralized. (B) The metaphysis of the Arrow® reverse prosthesis stem is angled at 135° and is lateralized (+4 mm).
Fig. 3A–B.
AP views compare (A) the Delta III prosthesis with (B) the Arrow® prosthesis. Lateralization of both the center of rotation (point) and proximal metaphysis increases the lateral offset (arrow).
A shoulder splint was used during the first 4 weeks. Passive physiotherapy was started immediately during the first month, the only limitation to this being pain. Active mobilization was started at 1 month postoperatively. Elderly patients were transferred to a rehabilitation center from the fifth postoperative day on.
All patients were examined postoperatively at 3, 12, and 24 months. Followup evaluation consisted of a physical examination, Constant-Murley functional scores [5], and plain radiographs (AP, lateral outlet, and axillary view). We divided the patients into three groups using the classification of Hamada et al. [15]: Group A (17 patients) characterized by the head of the humerus being eccentric without glenohumeral arthritis (Hamada Stages I, II, and III); Group B (56 patients) characterized by an irreparable rupture of the rotator cuff associated with an eccentric humeral head with glenohumeral osteoarthritis (Hamada Stages IV and V); and Group C (three patients) characterized by medialized, centered arthrosis and a nonfunctional, thin, atrophic rotator cuff, with fatty infiltration of greater than 3.
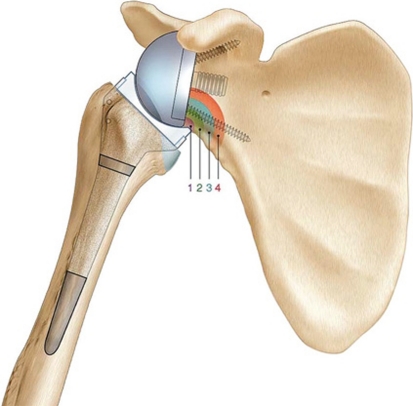
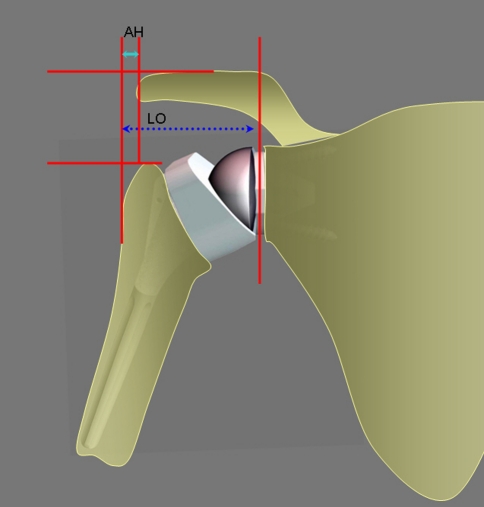
On plain radiographs, glenoid notching was identified according to the classification of Valenti et al. [27] (Fig. 4). Mechanical failures were assessed. Lateral offset (distance from the center of rotation to the great tuberosity) and acromiohumeral distance were also measured on plain radiographs (Fig. 5).
Fig. 4.
A diagram illustrates the classification of Valenti et al. [27]: (1) Grade 1, small notch, confined to the scapular pillar; (2) Grade 2, notch with condensation (stable), notch outline contacts lower; (3) Grade 3, notch over the lower screw (evolving notch); and (4) Grade 4, notch extending to baseplate.
Fig. 5.
Lateral offset (LO) (distance from the center of rotation to the great tuberosity) and acromion humeral distance (AH) were measured.
Results
The absolute Constant-Murley score increased from 24 to 59, representing an increase of 35 points (Table 1). According to the Constant-Murley score, postoperative pain was 14, representing an increase of around 10 points. The increase in active anterior elevation was 61° (range, 65°–126°). The increase in external rotation with elbow at the side was 15°; the increase in external rotation with 90° abduction was 30°; and the increase in internal rotation was 1 point according to the Constant-Murley score. In Group B, the increase in active anterior elevation was only 54° compared with 85° increase in Group A (Table 2).
Table 1.
Pre- and postoperative clinical results
| Variable | Preoperative | Postoperative | Gain |
|---|---|---|---|
| Constant-Murley score (points) | 24 (22–26) | 59 (56–61) | 35 (32–38) |
| Pain (points) | 4 (3–5) | 14 (13–14) | 10 (9–11) |
| Active anterior elevation (°) | 65 (58–72) | 126 (121–132) | 61 (52–70) |
| External rotation 1 (°) | 15 (11–18) | 30 (27–33) | 15 (10–20) |
| External rotation 2 (°) | 19 (14–24) | 50 (45–55) | 31 (24–38) |
| Internal rotation (points) | 5.5 (5–6) | 6.6 (6–7) | 1 (1–2) |
| Strength (points) | 1.6 (1–2.2) | 6.3 (5.7–7) | 4.8 (3.9–5.6) |
Values are expressed as mean, with range in parentheses; external rotation 1 = external rotation with elbow at the side; external rotation 2 = external rotation with 90° abduction.
Table 2.
Results according to the classification of Hamada et al. [15]
| Group | Subscapular fatty degeneration index | Infrascapular fatty degeneration index | Suprascapular fatty degeneration index | Gain in anterior elevation (°) | Gain in external rotation 1 (°) | Gain in external rotation 2 (°) | Gain in internal rotation (points) |
|---|---|---|---|---|---|---|---|
| All (n = 76) | 2.2 | 2.9 | 3 | +61 | +15.5 | +26 | +1.1 |
| Group A (Hamada I, II, III) (n = 17) | 2.17 | 3 | 3.1 | +80.5 | +13.3 | +43.8 | +0.56 |
| Group B (Hamada IV, V) (n = 56) | 2.22 | 2.89 | 2.93 | +57.3 | +15.1 | +27.6 | +1.31 |
External rotation 1 = external rotation with elbow at the side; external rotation 2 = external rotation with 90° abduction.
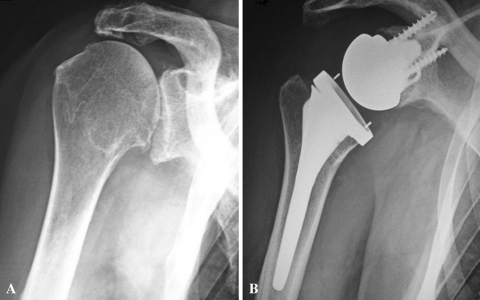
At the last followup, on AP and outlet views, no patient had developed a notch in the pillar of the scapula with this less medialized RSA prosthesis (Fig. 6). Lateral offset was 5.1 cm (range, 4–6.5 cm). Acromiohumeral distance was 1.4 cm (range, 0.5–1.8 cm).
Fig. 6A–B.
(A) A preoperative AP view shows the shoulder of a 74-old-woman with an irreparable rotator cuff tear with an eccentric arthritis (Hamada III). (B) A postoperative AP view shows the shoulder after implantation of the Arrow® prosthesis (second generation).
Fourteen of the 76 patients (18.4%) experienced complications. For 10 patients, the prosthesis came apart due to a mechanical failure in the first-generation prostheses implanted between April 2003 and June 2004. The glenospheres or the polyethylene separated from either the metal baseplate or the humeral stem, respectively. All of these patients underwent revision surgery. They were fitted with a second-generation prosthesis. In the second-generation implant, the glenosphere was impacted and screwed into the metal baseplate, and likewise the humeral insert was impacted and screwed into the humeral stem (Fig. 7). The mean Constant-Murley score of this group was 58.1 points after revision and was not different from the noncomplicated prostheses. Since starting to use this second-generation prosthesis (beginning of 2005), no mechanical failures have been reported. Among the four other patients, one patient presented less than 6 months after surgery with an early glenoid failure secondary to malpositioning of the glenosphere with a superior tilt. One patient presented with a stress fracture of the acromion. One patient suffered from transitory axillary palsy and one other had an anterior superolateral dislocation.
Fig. 7A–B.
Photographs illustrate the second-generation Arrow® prosthesis: (A) The metal back is designed with a quadrangular central quill and an anterior plate. The component is set with two glenoid screws (15° divergent) and an accessory AP screw against shear forces. The glenosphere is impacted and screwed on the metal back. (B) The humeral polyethylene is notched at the medial side. The component is impacted and screwed on the stem.
Discussion
RSA following Grammont’s principles has proven its efficiency for restoring active anterior elevation in patients presenting with a pseudoparalytic shoulder secondary to cuff tear arthropathy [23, 29]. However, the literature suggests the gain in external and internal rotation is limited, and from the first months, glenoid notching occurs in 40% to 70% of patients [18, 22, 23, 26, 28, 31]. To improve external and internal rotation and to decrease the rate of glenoid notching, we presumed such lateralization in the prosthesis would prevent these mentioned problems. In this study, we asked: does a less medialized RSA prosthesis improve shoulder motion, decrease glenoid notching, or increase the risk of glenoid loosening?
We acknowledge the limitations of our study, inherent to retrospective reviews. First, a longer followup is necessary to confirm these preliminary observations. Second, a CT evaluation would be more accurate in determining notching, especially posteroinferior. Third, we do not have a control group with a medialized RSA prosthesis.
The concept of Grammont (medialized RSA) will limit both external and internal rotation. Increased offset should improve both external and internal rotation by recruiting anterior and posterior fibers of the deltoid muscle and retensioning the remaining cuff (Table 3). Frankle et al. [8] reported improvements of external rotation from 12° preoperatively to 41° postoperatively with an increased-offset RSA. The authors reported 17% (10 patients) with complications, including seven cases of glenoid loosening, two cases of stress fracture of the metal baseplate, and one case of infection. No notching was found with this much more lateralized prosthesis [8]. For Boileau et al. [3], improvement in active rotation was 10° for external rotation and 1.3° for internal rotation and was inferior to that obtained with the Arrow® prosthesis (15° for external rotation with elbow at the side and 31° with the arm in abduction). With the BIO-RSA, there is only glenoid lateralization, in contrast to the Arrow®, which lateralizes both the glenoid and humeral components.
Table 3.
Results of RSA from the literature
| Study | Year | Followup (months) | Number of patients | Mean age (years) | Active anterior elevation (preop/postop) | Active external rotation (preop/postop) | Constant-Murley score (points) (preop/postop) | ASES score (points) (preop/postop) | Reoperation rate (%) | Notching (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Valenti et al. [27] | 2001 | 84 | 29 | 70 | 60°/120° | 21/63 | 15 | |||
| Frankle et al. [8] (increased-offset RSA) | 2005 | Minimum 24 | 60 | – /105° | 12°/41° | 34/68 | 12 | 0 | ||
| Sirveaux et al. [26] | 2004 | 44 | 80 | 72 | 73°/138° | 3°/11° | 22/65 | 5 | 63 | |
| Levigne et al. [18] | 2009 | 47 | 326 | 72 | 70°/125° | 7°/9° | 23/58 | 62 | ||
| Boileau et al. [3] (BIO-RSA) | 2011 | 28 | 45 | 86/146 | 12°/22° | 31/67 | 0 | 19 | ||
| Valenti et al. (increased-offset RSA) | 2011 | 44 | 76 | 73 | 65°/126° | 15°/30° | 24/59 | 18 | 0 |
RSA = reverse shoulder arthroplasty; preop = preoperative; postop = postoperative; ASES = American Shoulder and Elbow Surgeons; BIO-RSA = bony increased-offset RSA.
The clinical implications of glenoid notching have been discussed in the literature (Table 3). Guéry et al. [13] reported it did not affect the Constant-Murley score, while Sirveaux et al. [26] observed clinical deterioration with Valenti et al. [27] Type 3 and 4 notches. Delloye et al. [6] reported a decrease in Constant-Murley scores if the notch reached the central keel, with the risk of glenoid loosening. Many recommendations have been made in the literature concerning preventing this glenoid notching from occurring. De Wilde et al. [7] reported a series using a larger-diameter glenosphere (42 mm). All authors agree the metal baseplate should be placed as low as possible, flush with the inferior pole of the glenoid cavity [21, 25, 26, 30]. However, according to Guttierez et al. [14], if the pillar of the scapula is very vertical, the notch may still occur. Simovitch et al. [25] suggested inferior tilting of the metal baseplate would not in theory prevent notching. Boileau et al. [3] proposed the BIO-RSA lateralized the glenoid component up to 10 mm. The technique involves placing a bone graft (taken from the resected head of the humerus) between the metal baseplate and the native glenoid. With this procedure, they reported 19% notching for 42 patients. The BIO-RSA procedure with a center of rotation at the level of the new bone graft reproduces the concept of Grammont but cannot prevent glenoid notching definitively, and Boileau et al. [3] recommended implanting the baseplate in a lower position. In 1998, Frankle et al. [8] reported on a less medialized RSA prosthesis (center of rotation close to that of a nonconstrained anatomic prosthesis). In a series of 60 patients with a minimum followup of 2 years, the authors found no notching.
Lateralization of the center of rotation has the disadvantage of increasing torque and shearing forces applied to the glenoid component and potentially increasing the risk of glenoid component loosening [16]. Frankle et al. [8] recommended improving the quality of fixation for a baseplate with a convex bottom with a Size 6.5 central compressive screw and Size 5 peripheral screws locking into the plate. In addition, the authors proposed a more or less lateral center of rotation depending on the quality of the glenoid bone. In the Arrow® RSA prosthesis, two factors explain the lateralization: (1) the glenosphere is fitted to the metal baseplate (not embedded), creating a lateralization of 8.5 mm; and (2) the shape of the metaphyseal part of the humeral stem is angled at 155° and creates a 4-mm humeral lateralization. This lateralization, admittedly less than that in the prosthesis designed by Frankle et al. [8], has led us to propose an improvement in the primary fixation of the glenoid implant: the metal baseplate with a convex base, covered with hydroxyapatite, consequently comes in three different sizes to provide optimal contact with the subchondral bone of the glenoid cavity. A central keel and an anterior lug provide primary fixation, which opposes the forces of torsion. This plate is also fixed by two screws (one superior and one inferior in a divergent direction) to oppose tilting forces at the beginning of abduction. In patients with osteoporotic glenoid bone, a third AP screw can be used to increase the fixation of the metal baseplate. We did not detect any glenoid notches and only one episode of glenoid loosening occurred (one early technical fault).
In conclusion, modification of the initial concept of Paul Grammont with a moderate lateralization of the center of rotation around 8.5 mm provides good function. There is an increase in active rotation (especially in external but also in medial rotation), with considerable impact on the quality of life of these elderly people. The absence of glenoid notching suggests long prosthesis survival, but it is too early to make any assertion about the longevity of these “less medialized” RSA prostheses. A longer followup is needed to confirm the absence of glenoid notching and loosening of the baseplate before this solution can be proposed for the considerable challenge of younger patients.
Acknowledgments
The authors thank Doctor Peter Hughes, Royal Preston and Chorley Hospital, United Kingdom, for his advice.
Footnotes
One or more of the authors (PV, PS, DK) are consultants for Fournitures Hospitalières Industrie (Mulhouse, France) and have received royalties for a product related to this work. The remaining authors certify that they have no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that all investigations were conducted in conformity with ethical principles of research and that informed consent for participation in the study was obtained.
References
- 1.Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction: a propos of 16 cases] [in French. Acta Orthop Belg. 1995;61(Suppl 1):112–119. [PubMed] [Google Scholar]
- 2.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279–2292. doi: 10.2106/JBJS.F.00125. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. February 1, 2011 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 5.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 6.Delloye C, Joris D, Colette A, Eudier A, Dubuc JE. Mechanical complications of total shoulder inverted prosthesis] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2002;88:410–414. [PubMed] [Google Scholar]
- 7.Wilde LF, Audenaert EA, Berghs BM. Shoulder prostheses treating cuff tear arthropathy: a comparative biomechanical study. J Orthop Res. 2004;22:1222–1230. doi: 10.1016/j.orthres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Frankle M, Levy JC, Pupello D, Siegal S, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients: surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):178–190. doi: 10.2106/JBJS.F.00123. [DOI] [PubMed] [Google Scholar]
- 9.Franklin JL, Barrett WP, Jackins SE, Matsen FA., 3rd Glenoid loosening in total shoulder arthroplasty: association with rotator cuff deficiency. J Arthroplasty. 1988;3:39–46. doi: 10.1016/S0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 10.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 11.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 12.Grammont PM, Laffay JP, Deries X. Evaluation of a new shoulder prosthesis] [in French] Rhumatologie. 1987;39:407–418. [Google Scholar]
- 13.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE., 3rd Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg. 2007;16(3 Suppl):S9–S12. doi: 10.1016/j.jse.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: a long-term observation. Clin Orthop Relat Res. 1990;254:92–96. [PubMed] [Google Scholar]
- 16.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in “reverse” total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14(1 Suppl S):162S–167S. [DOI] [PubMed]
- 17.Jacobs R, Debeer P, Smet L. Treatment of rotator cuff arthropathy with a reversed Delta shoulder prosthesis. Acta Orthop Belg. 2001;67:344–347. [PubMed] [Google Scholar]
- 18.Levigne C, Boileau P, Favard L, Garaud P, Mole D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 20.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–337. [PubMed] [Google Scholar]
- 21.Nyffeler RW, Werner CM, Simmen BR, Gerber C. Analysis of a retrieved Delta III total shoulder prosthesis. J Bone Joint Surg Br. 2004;86:1187–1191. doi: 10.1302/0301-620X.86B8.15228. [DOI] [PubMed] [Google Scholar]
- 22.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 23.Rockwood CA., Jr The reverse total shoulder prosthesis: the new kid on the block. J Bone Joint Surg Am. 2007;89:233–235. doi: 10.2106/JBJS.F.01394. [DOI] [PubMed] [Google Scholar]
- 24.Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy. Oper Orthop Traumatol. 2005;17:1–24. doi: 10.1007/s00064-005-1119-1. [DOI] [PubMed] [Google Scholar]
- 25.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 26.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 27.Valenti PH, Bouttens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long term results (> 5 years) In: Walch G, Boileau P, Molé D, editors. 2000 Shoulder Prostheses: Two to Ten Year Follow-up. Montpellier, France: Sauramps Medical; 2001. pp. 253–259. [Google Scholar]
- 28.Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy: a retrospective study of 32 cases. Acta Orthop Belg. 2004;70:219–225. [PubMed] [Google Scholar]
- 29.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(Suppl 2):35–40. [PubMed] [Google Scholar]
- 30.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 31.Wirth MA, Rockwood CA., Jr Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78:603–616. doi: 10.2106/00004623-199604000-00018. [DOI] [PubMed] [Google Scholar]