Abstract
Background
Postganglionic neurons in the sympathetic nervous system reportedly are involved in lumbar radicular pain and release norepinephrine (NE), a neurotransmitter. Increased numbers of sympathetic nerve fibers have been found in dorsal root ganglion (DRG) neurons in a root constriction model. Whether this is a reasonable model for pain, however, is unclear
Questions/purposes
We asked whether: (1) painful behaviors occurred in the root constriction model; (2) NE enhanced the excitability of DRG neurons in the root constriction model; and (3) which adrenoceptors were related to the mediation of the NE effects.
Methods
The L5 root was sutured proximal to the DRG as the root constriction model. Behavioral tests were performed until 28 days after surgery. At 10 to 14 days after the root constriction, DRG neurons were quickly excised and digested with collagenase for electrophysiologic studies. Action potentials were recorded from single DRG neurons using a whole-cell patch clamp technique. NE (10 μmol/L) was directly applied to the DRG neurons. The adrenergic sensitivity was examined in combination with antagonists.
Results
The rats with root constriction exhibited painful behavior. NE increased the excitability of DRG neurons in the root constriction model. The effects of NE were inhibited by pretreatment with an α-antagonist and α2-antagonist but not an α1-antagonist.
Conclusions
Our observations suggest NE plays an important role in generating lumbar radicular pain mainly via α2-adrenoceptors.
Clinical Relevance
An α2-antagonist may be an appropriate agent for trials to treat lumbar radicular pain.
Introduction
Lumbar radicular pain with lumbar disc herniation and lumbar spinal canal stenosis are common. Nonoperative treatment and surgery can alleviate lumbar radiculopathy, but 10% to 42% of patients continue to experience pain resistant to conventional treatment [2, 3, 41]. Intractable pain frequently is believed to be of neuropathic origin [18, 19], a complex pain state typically accompanied by tissue and nerve injury. Neuropathic pain is defined as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system, commonly in lumbar radicular pain, diabetic neuropathy, and postherpetic neuralgia [47].
Animal models of neuropathic pain after peripheral nerve injury have been developed in the search for underlying mechanisms [4, 21]. Using such models, some studies have documented that sympathetic nerve fibers sprout in the DRG [9, 10, 28, 34, 35, 52]. Sympathetic sprouting is a phenomenon in which sympathetic postganglionic axons extend around DRG neurons after peripheral nerve injury. Mizuno et al. reported sympathetic nerve fibers increased in corresponding DRG neurons in the root constriction model [30]. Sympathectomy alleviates pain behaviors such as mechanical allodynia and thermal hyperalgesia in animal models [20, 24, 30, 31, 37]. In clinical cases, lumbar sympathetic block has been performed to relieve chronic pain, including intractable lumbar radicular pain [6, 32, 46]. Therefore, the sympathetic nervous system currently is considered to be involved in lumbar radicular pain.
NE is the neurotransmitter that is released from postganglionic neurons in the sympathetic nervous system. In the abnormal somatosensory-sympathetic connection through sympathetic sprouting in the DRG, NE may influence the excitability of primary afferent DRG neurons. Some electrophysiologic studies suggest NE application induces hyperexcitability of DRG after a chronic constriction injury (CCI) of the sciatic nerve [50, 53]. Kirita et al. used whole-cell patch clamp recording as an intracellular recording technique to evaluate electrophysiologic properties of single DRG neurons and showed that root constriction increased the excitability of DRG neurons [23]. However, the effects of NE on DRG neurons in the root constriction model have not been clear.
We therefore asked whether: (1) painful behaviors occurred in rats with root constriction; (2) NE enhanced the excitability of DRG neurons in the root constriction model using whole-cell patch clamp recording; and (3) which adrenoceptors were related mainly to the mediation of the NE effects.
Materials and Methods
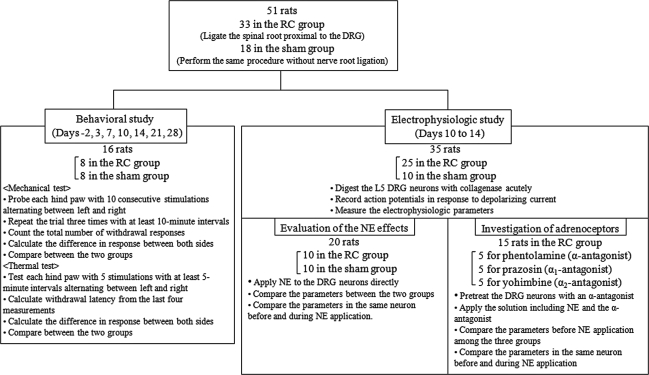
We created a root constriction model by suturing the L5 root proximal to the DRG. Fifty-one adult male Sprague-Dawley rats weighing 150 to 200 g at the beginning of the study were divided into two groups: (1) a root constriction group (n = 33), and (2) a sham group (n = 18) (Fig. 1). In the behavioral study, 16 rats (eight in each group) were used for 2 days before and on Days 3, 7, 10, 14, 21, and 28 after surgery. At each experimental day, we tested each hind paw with mechanical and thermal stimulations. In the electrophysiologic study, 35 rats (25 in the root constriction group and 10 in the sham group) were used on postoperative Days 10 to 14. The L5 DRG neurons were acutely digested with collagenase. Twenty rats (10 in each group) were used to evaluate the response to NE application. In addition, 15 rats in the root constriction group (five in each antagonist) were used to investigate adrenoceptor subtypes activated by NE application together with α-adrenergic antagonists: phentolamine (α-antagonist), prazosin (α1-antagonist), and yohimbine (α2-antagonist) (Fig. 1). All experimental protocols were approved by the Sapporo Medical University Animal Care and Use Committee.
Fig. 1.
The diagram of the study design shows the 51 rats were divided between the root constriction (RC) group (n = 33) and the sham group (n = 18). In the behavioral study, 16 rats (eight in each group) were used up to 28 days after surgery. In the electrophysiologic study, 35 rats (25 in the root constriction group and 10 in the sham group) were used on postoperative Days 10 to 14. Twenty rats (10 in each group) were used to evaluate the response to NE application. In addition, 15 rats in the root constriction group (five in each antagonist) were used to investigate adrenoceptor subtypes together with α-adrenergic antagonists.
Unilateral lumbar radiculopathy was induced as described previously [23]. Briefly, under sodium pentobarbital anesthesia (50 mg/kg, intraperitoneal), through a midline dorsal incision, we identified the left L5/L6 facet joint. A left L5 hemilaminectomy and L5/L6 partial facetectomy were performed. With a surgical microscope, we carefully exposed the L5 spinal root and DRG and the L5 spinal root was tightly ligated extradurally with 8-0 nylon suture proximal to the DRG as the root constriction group. The same surgical procedure without nerve root ligation was performed in a sham group.
An investigator (TI), blinded to the surgical protocols for each rat, performed behavioral tests using a previously described method [23]. For mechanical withdrawal response, we placed rats in a Plexiglas® chamber measuring 18 × 25 × 18 cm above a wire mesh floor that allowed full access to the hind paw. Before testing, behavioral accommodation was allowed for at least 20 minutes. We measured mechanical withdrawal response as the frequency of hind paw withdrawals elicited by a defined mechanical stimulus of 3.4 g using a calibrated nylon filament (Semmes-Weinstein Monofilaments; North Coast Medical Inc, San Jose, CA, USA). The mechanical stimulus was applied to the middle of the foot pad on the plantar surface of the hind paw. Each hind paw was probed with 10 consecutive tactile stimulations alternating between left and right. We repeated the trial three times in succession with at least a 10-minute interval, resulting in each foot receiving 30 mechanical stimulations. Mechanical sensitivity was assessed by counting the total number of withdrawal responses elicited for a total possible score of 30. The mechanical withdrawal frequency was expressed as the difference in responses between the two sides (= score on the constriction side - score on the contralateral side).
For thermal withdrawal response, we placed the rats in the Plexiglas® chamber on a glass platform. After accommodation, thermal withdrawal response was measured as the latency of hind paw withdrawals elicited by a radiant heat source (Tail Flick Analgesia Meter; IITC Inc, Woodland Hills, CA, USA), which was moved beneath a portion of the hind paw that could be seen in the glass. Thermal stimulation was delivered to the foot pad of the hind paw. Intensity of the heat stimulus was adjusted to elicit a quick withdrawal reflex at a latency of approximately 6 to 8 seconds in control rats and the intensity was kept constant at this setting throughout all experiments. A cutoff time of 10 seconds was set to prevent tissue damage. We tested each hind paw five times with at least 5-minute intervals alternating between left and right. Mean withdrawal latency was calculated from the last four measurements. We defined thermal withdrawal latency in each rat as the difference in latency between the two sides (= latency on contralateral side − latency on constriction side). For frequency and latency, positive scores indicated increased sensitivity on the constriction side.
At postoperative Days 10 to 14, 35 rats were deeply anesthetized with sodium pentobarbital (60 mg/kg intraperitoneal) and decapitated. Under a stereoscopic microscope, the left L5 DRG was quickly excised and placed in oxygenated normal Tyrode’s solution consisting of (in mmol/L) NaCl 143, KCl 5.4, CaCl2 1.8, MgCl2 0.5, NaH2PO4 0.33, glucose 5.5, and HEPES 5.0 at a pH of 7.4 with NaOH. Dissected DRG was immersed in Ca2+-free Tyrode’s solution at room temperature (greater than 20 minutes) to remove extracellular Ca2+. Tissue was digested with collagenase (8 mg/mL, Type II, 034-10533; Wako Pure Chemical Industries, Tokyo, Japan) for 90 minutes in a shaking incubator (37°C, 1.5 Hz) and rinsed with Ca2+-free Tyrode’s solution. The DRG neurons were dissociated in Ca2+-free Tyrode’s solution with a handmade pipette, which has a larger hole.
We recorded action potentials in response to depolarizing current using a whole-cell patch clamp apparatus (Axopatch 200B; Molecular Devices, Sunnyvale, CA, USA) at room temperature (22°–24°C). DRG neurons were placed in the recording chamber on the stage of an inverted microscope (IX-70; Olympus, Tokyo, Japan). Because small neurons may be involved in the transmission of nociceptive signals [43], we selected cells less than 40 μm in diameter, which corresponds to the size range of small neurons in rat DRG [13, 36, 44]. We used normal Tyrode’s solution as an external solution. Electrode impedance was 3 to 5 MΩ when filled with solution, consisting of (in mmol/L) K-asparate 110, KCl 20, MgCl2 1.0, ATP-K2 5.0, phosphocreatine-K2 5.0, EGTA 5.0, and HEPES 5.0 at a pH of 7.4 with KOH. Electrode position was controlled by a three-dimensional hydraulic micromanipulator under the inverted microscope.
We used two protocols to evaluate the excitability of DRG neurons. In one protocol (short stimulation), depolarizing currents of 0.2 to 4.0 nA (0.5 ms duration) in increments of 0.2 nA were injected into a DRG neuron until an action potential (AP) was evoked. We then examined the threshold current, resting membrane potential (RMP), amplitude, after hyperpolarization (AHP), threshold voltage, action potential duration 50 (APD50), and dV/dt max. The threshold current was defined as the minimum current required to evoke AP. RMP was taken 3 minutes after a stable recording was first obtained. We measured amplitude from the RMP to the peak. AHP was measured from the RMP to the valley peak. The threshold voltage was defined as the beginning of the upstroke of AP. APD50 was measured as the interval from AP onset to the point of 50% repolarization. The dV/dt max was the maximum rate of increase of AP.
In the other protocol (long stimulation), we used depolarizing currents of 0.01 to 0.39 nA (1000 ms duration) in increments of 0.02 nA. We confirmed the discharge pattern and examined the maximum number of spikes in each current (maximum spike count).
NE stock solution (10 mmol/L) was dissolved in distilled water with an equivalent amount of ascorbic acid and diluted with the external solution so that final concentration was 10 μmol/L. After baseline recording, we applied NE to DRG neurons by gravity through a valve connected to the chamber. Five minutes after NE application, data were recorded again.
We performed application of α-antagonists in the root constriction neurons. Phentolamine (α-antagonist), prazosin (α1-antagonist), and yohimbine (α2-antagonist) were used for this study. Fifteen DRG neurons (five in each antagonist) were used for recording. Phentolamine and yohimbine (10 mmol/L) were dissolved in distilled water. Prazosin (5 mmol/L) was made by dissolution in 10% ethanol in distilled water. Final concentration of all drugs was 10 μmol/L. DRG neurons had been pretreated with α-antagonists for 1 hour before recording. After electrophysiologic recording was performed in pretreatment with an antagonist, we applied a solution containing NE and the antagonist to DRG neurons.
Data were expressed as means ± standard error of mean and analyzed using SPSS 12.0 for Windows (IBM Corporation, Somers, NY, USA). Student’s t-test was used to determine the differences in the behavioral data and the electrophysiologic parameters between the sham and the root constriction groups. We used the paired t-test to determine the differences in electrophysiologic parameters evoked by current injection in the same neuron before and during NE application. ANOVA was used to compare the root constriction groups without adrenergic antagonists.
Results
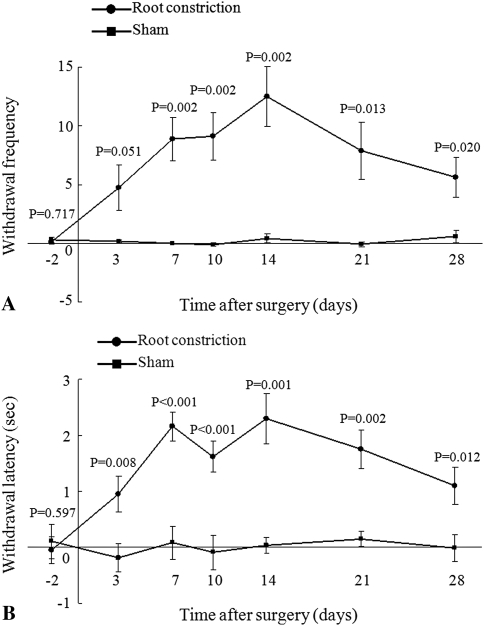
In the behavioral study, the rats with the root constriction showed mechanical allodynia and thermal hyperalgesia. The root constriction group exhibited an increase (p = 0.002) in tactile sensitivity from 7 days after surgery as compared with the sham group, and the hypersensitivity was maintained until 28 days after surgery (p10 = 0.002, p14 = 0.002, p21 = 0.013, p28 = 0.020) (Fig. 2A). The withdrawal latency in the root constriction group decreased from 3 to 28 days after surgery as compared with that in the sham group (p3 = 0.008, p7 < 0.001, p10 < 0.001, p14 = 0.001, p21 = 0.002, p28 = 0.012) (Fig. 2B).
Fig. 2A–B.
The responses to (A) mechanical and (B) thermal stimulation of the hind paw in the root constriction and the sham groups were evaluated. The root constriction group showed mechanical allodynia from 7 days after surgery and thermal hyperalgesia from 3 days after surgery. Both of the changes were maintained up to 28 days after surgery.
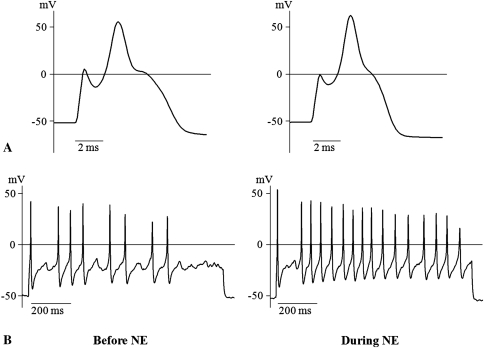
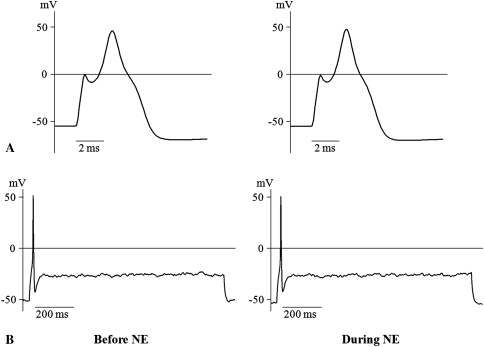
In the electrophysiologic study, NE enhanced the excitability of DRG neurons in the root constriction model. In the root constriction group, the mean threshold current was lower (p = 0.038) and the mean RMP was more depolarized (p = 0.008), compared with the sham group (Table 1). There were no major changes in the other parameters. With regard to discharge pattern, all the root constriction neurons showed a multiple spike pattern (Fig. 3), whereas seven of 10 sham neurons (70%) exhibited a single spike (Fig. 4). The maximum spike count of the root constriction group was greater (p < 0.001) than that of the sham group. In the root constriction group, NE application induced an increase (p = 0.007) in dV/dt max. The maximum spike count during NE stimulation was 15.9 ± 2.1, which was greater (p = 0.006) than the mean of 10.7 ± 1.8 before NE stimulation (Fig. 3). In contrast, there were no changes in the sham group (Fig. 4).
Table 1.
Effects of NE on electrophysiologic properties
| Electrophysiologic parameter | Sham group | Root constriction group | p Value | ||||
|---|---|---|---|---|---|---|---|
| Before NE | During NE | Before NE | During NE | Sham versus root constriction group (before NE) | Sham group Before versus during NE | Root constriction group Before versus during NE | |
| Threshold current, nA | 1.92 ± 0.12 | 1.80 ± 0.11 | 1.54 ± 0.12 | 1.46 ± 0.09 | 0.038 | 0.051 | 0.309 |
| RMP, mV | −55.4 ± 1.4 | −55.7 ± 1.4 | −49.0 ± 1.6 | −48.9 ± 1.5 | 0.008 | 0.632 | 0.948 |
| Amplitude, mV | 93.7 ± 4.4 | 96.2 ± 4.9 | 90.3 ± 5.3 | 96.1 ± 3.9 | 0.622 | 0.201 | 0.071 |
| AHP, mV | 8.9 ± 1.5 | 9.7 ± 1.4 | 9.8 ± 0.9 | 11.1 ± 1.3 | 0.647 | 0.659 | 0.647 |
| Threshold voltage, mV | −5.7 ± 3.1 | −7.0 ± 3.0 | −9.8 ± 2.6 | −10.7 ± 1.9 | 0.333 | 0.522 | 0.192 |
| APD50, ms | 3.4 ± 0.3 | 3.3 ± 0.3 | 4.6 ± 0.5 | 4.2 ± 0.5 | 0.072 | 0.475 | 0.602 |
| dV/dt max, M/second | 50.9 ± 7.8 | 56.8 ± 8.9 | 49.7 ± 8.6 | 63.0 ± 7.8 | 0.921 | 0.050 | 0.007 |
| Max spike count | 3.5 ± 1.4 | 3.1 ± 1.4 | 10.7 ± 1.8 | 15.9 ± 2.1 | 0.006 | 0.462 | 0.006 |
Values are means ± SEM; NE = norepinephrine; RMP = resting membrane potential; AHP = after hyperpolarization; APD50 = action potential duration at half width.
Fig. 3A–B.
Examples of action potentials generated from DRG neurons in the root constriction group are shown. NE enhanced the excitability of the root constriction neurons. In (A) short stimulation, NE induced the increase of dV/dt max, which represents the maximum rate of increase of action potential. In (B) long stimulation, the maximum spike count further increased during NE application.
Fig. 4A–B.
Examples of action potentials generated from DRG neurons in the sham group are shown for (A) 2 ms and (B) 200 ms before and during NE application. There were no changes by applying NE.
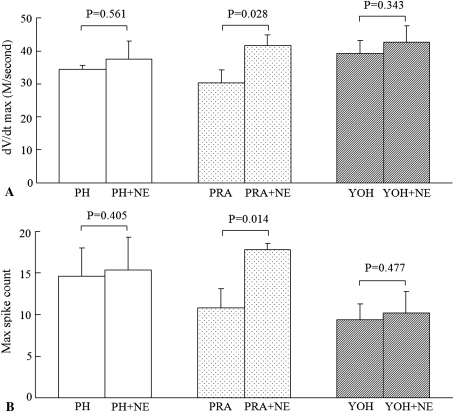
As for the α-adrenergic antagonists, there were no differences among the three groups before NE stimulation. The effects of NE were inhibited by pretreatment with the α-antagonist phentolamine or the α2-antagonist yohimbine. However, the α1-antagonist prazosin failed to abolish the responses to NE. The dV/dt max and the maximum spike count increased from 30.3 ± 4.0 to 41.7 ± 3.2 (p = 0.028) and from 10.8 ± 2.2 to 17.8 ± 0.8 (p = 0.014), respectively (Fig. 5).
Fig. 5A–B.
The results of pretreatment with the α-adrenergic antagonists in the root constriction neurons are shown for (A) dV/dt max and (B) maximum spike count. The effects of NE were inhibited by the α-antagonist (phentolamine = PH) and the α2-antagonist (yohimbine = YOH), not the α1-antagonist (prazosin = PRA).
Discussion
We suspected NE plays an important role in regulating the excitability of DRG neurons in the root constriction model, because the sympathetic nervous system reportedly is associated with lumbar radicular pain. Therefore, we asked whether: (1) painful behaviors occurred in rats with root constriction; (2) NE enhanced the excitability of DRG neurons in the root constriction model; and (3) which adrenoceptors were related mainly to the mediation of the NE effects.
There were limitations in the experimental settings in this study. First, the root constriction model was created by ligating the spinal root with a nylon suture, which may not reflect spinal disorders such as lumbar disc herniation and lumbar spinal canal stenosis. However, suture of the spinal root is considered a reliable and reproducible procedure to induce a lumbar radiculopathy. Second, our electrophyiologic study was an in vitro study and the drugs were administered directly to the DRG neurons. Adrenoceptors are said to exist systemically, not just in DRG. Accordingly, a specific method for delivering drugs to DRG neurons is needed to examine the effects of α2-antagonists in humans. Third, the concentration of NE that reaches DRG neurons in our preparation is probably much lower than that in the perfusion solution yet is likely to be higher than that in the plasma of humans [12]. Fourth, we used collagenase to dissociate the excised DRG. Thus, in this model, communication with neighboring glial and DRG cells no longer is intact. However, in isolating the effects of single DRG neurons, this model provides certain advantages. To resolve these limitations, patch clamp recording in vivo should be performed to evaluate NE-induced activity. Fifth, we selected DRG neurons less than 40 μm in diameter for electrophysiologic studies. Although small neurons may be related to the transmission of the nociceptive signals including pain sensation, the function of the DRG neurons used in the current study was not clear.
Our observations confirm L5 root constriction results in behavioral signs of mechanical allodynia and thermal hyperalgesia. These pain behaviors were maintained for up to 28 days after surgery. Radiculopathy is induced by inflammation and mechanical compression. Epidural application of autologous nucleus pulposus has been used for an inflammatory radiculopathy model [45]. However, mechanical compression has been provided by suturing the nerve root or inserting a stainless steel rod in the spinal canal [14, 16, 48]. In the root constriction model, nerve deformation can be observed during surgery and a nylon suture which does not induce inflammation was used to ligate the L5 root. Accordingly, the responses to a root constriction model are presumed to be evoked by mechanical compression. These results are consistent with those of previous reports of lumbar root constriction models [14, 23, 30].
The current electrophysiologic analysis showed NE application induced further excitability of DRG neurons in the root constriction group using a whole-cell patch clamp recording technique, which resulted in larger dV/dt max and greater maximum spike count. Compared with the sham neurons, the root constriction neurons exhibited lower threshold current, more depolarized RMP, and greater maximum spike count, thus suggesting root constriction causes increased excitability of DRG neurons. These changes have been observed in different animal models of neuropathic pain including axotomy [1, 22, 49, 51], CCI [40, 44], spinal nerve ligation [27], and chronic compression of the DRG [26, 54]. In previous electrophysiologic studies, extracellular recording techniques have been used to evaluate the effects of NE on DRG neurons. Xie et al. found the spontaneous discharge rates of C-fibers after CCI were greater after application of NE [50]. Stimulation of the postganglionic sympathetic efferents reportedly augments sensory afferent discharge [11, 25, 29, 50]. The increase in dV/dt max indicates an increase in inward current, which may involve sodium ions. Honma et al. reported NE inhibited inward currents through voltage-dependent calcium channels and outward potassium currents in cultured DRG neurons in the CCI model [15]. Petersen et al. reported application of NE causes membrane depolarization in the responses of DRG after CCI [33]. These studies support the notion that excitation of DRG neurons is controlled by potassium channels. In our study, however, the RMP and APD50, which are parameters related to potassium channels, were not influenced by NE. Although this discrepancy may be the result of the differences in animal models and cell preparation, further studies are needed to clarify the current properties for NE-induced hyperexcitability of DRG neurons in the root constriction model.
We also observed that these excitatory effects of NE were inhibited by pretreatment with an α2-antagonist but not an α1-antagonist. These findings suggest the NE-induced hyperexcitability of DRG neurons was modulated by α2-adrenoceptors. Chen et al. [7] and Xie et al. [50] reported the response to NE was blocked by α2-antagonist yohimbine. Zhang et al. reported that clonidine, an α2-agonist, enhanced the spontaneous activity of C- and Aδ-fibers [53]. The expression of adrenoceptors in DRG neurons are altered after nerve injury. Birder and Perl reported the number of L4 and L5 DRG neurons expressing α2A-adrenoceptors increased sharply after sciatic nerve transection [5]. Using molecular biology techniques, they confirmed α2A-adrenoceptor mRNA levels increased in DRG neurons of rats with SNL or axotomy [8, 38, 39]. Accordingly, DRG neurons in the root constriction model appeared to have an increased expression of α2-adrenoceptors and responded more sensitively to the application of NE.
Our observations suggest NE enhanced the excitability of DRG neurons presumably through mediation of α2-adrenoceptors. These findings indicate that NE may play a role in the development and persistence of lumbar radicular pain, leading to excitation of DRG neurons by activating α2-adrenoceptors. However, intrathecal administration of NE has reversible antinociceptive effects by inhibiting Aδ- and C-fiber-mediated sensory transmission to substantia gelatinosa neurons in the spinal dorsal horn through the activation of α2-adrenoceptors [17]. The presence of adrenoceptors in DRG and spinal dorsal horn neurons has been observed [42], thus suggesting a role for NE at presynaptic and postsynaptic sites in the modulation. Therefore, NE may have a different role in modulation of pain sensation depending on its release site. Our observations suggest the sympathetic nervous system plays an important role in generating radicular pain and inhibiting the input through adrenoceptors and an α2-antagonist could be a promising therapeutic agent for radicular pain. However, a more comprehensive understanding of the sympathetic nervous system is needed for treating lumbar radicular pain in humans.
Acknowledgments
We thank Takehito Iwase for performing the behavioral studies and Dr. Takashi Kirita, Dr. Satoshi Mizuno, and Dr. Yoshinori Terashima for technical assistance related to the electrophysiologic experiments.
Footnotes
One or more of the authors (TT, NT, and TY) have received funding from a research grant for the Grant-in-Aid for Science Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant Number of 19390398) and the JOA-Subsidized Science Project Research 2007-8.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Sapporo Medical University, Sapporo, Japan.
References
- 1.Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J Neurophysiol. 2001;85:630–643. doi: 10.1152/jn.2001.85.2.630. [DOI] [PubMed] [Google Scholar]
- 2.Atlas SJ, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, Singer DE. The Maine Lumbar Spine Study, Part II. 1-year outcomes of surgical and nonsurgical management of sciatica. Spine (Phila Pa 1976) 1996;21:1777–1786. doi: 10.1097/00007632-199608010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Atlas SJ, Keller RB, Chang Y, Deyo RA, Singer DE. Surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine (Phila Pa 1976) 2001;26:1179–1187. doi: 10.1097/00007632-200105150-00017. [DOI] [PubMed] [Google Scholar]
- 4.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J Physiol. 1999;515(Pt 2):533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boas RA. Sympathetic nerve blocks: in search of a role. Reg Anesth Pain Med. 1998;23:292–305. doi: 10.1016/s1098-7339(98)90058-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Michaelis M, Janig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721–3730. doi: 10.1152/jn.1996.76.6.3721. [DOI] [PubMed] [Google Scholar]
- 8.Cho HJ, Kim DS, Lee NH, Kim JK, Lee KM, Han KS, Kang YN, Kim KJ. Changes in the alpha 2-adrenergic receptor subtypes gene expression in rat dorsal root ganglion in an experimental model of neuropathic pain. Neuroreport. 1997;8:3119–3122. doi: 10.1097/00001756-199709290-00022. [DOI] [PubMed] [Google Scholar]
- 9.Chung K, Chung JM. Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: evidence of regenerative collateral sprouting. Brain Res. 2001;895:204–212. doi: 10.1016/S0006-8993(01)02092-3. [DOI] [PubMed] [Google Scholar]
- 10.Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosci Lett. 1993;162:85–88. doi: 10.1016/0304-3940(93)90566-4. [DOI] [PubMed] [Google Scholar]
- 11.Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS. Plasma catecholamines and essential hypertension: an analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- 13.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine (Phila PA 1976) 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Honma Y, Yamakage M, Ninomiya T. Effects of adrenergic stimulus on the activities of Ca2 + and K + channels of dorsal root ganglion neurons in a neuropathic pain model. Brain Res. 1999;832:195–206. doi: 10.1016/S0006-8993(99)01499-7. [DOI] [PubMed] [Google Scholar]
- 16.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain. 1998;77:15–23. doi: 10.1016/S0304-3959(98)00067-0. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98:682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain. 2007;130:66–75. doi: 10.1016/j.pain.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6:829–836. doi: 10.1016/j.jpain.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Chung JM. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci Lett. 1991;134:131–134. doi: 10.1016/0304-3940(91)90524-W. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim YI, Na HS, Kim SH, Han HC, Yoon YW, Sung B, Nam HJ, Shin SL, Hong SK. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience. 1998;86:301–309. doi: 10.1016/S0306-4522(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 23.Kirita T, Takebayashi T, Mizuno S, Takeuchi H, Kobayashi T, Fukao M, Yamashita T, Tohse N. Electrophysiologic changes in dorsal root ganglion neurons and behavioral changes in a lumbar radiculopathy model. Spine. 2007;32:E65–E72. doi: 10.1097/01.brs.0000252202.85377.96. [DOI] [PubMed] [Google Scholar]
- 24.Lee DH, Katner J, Iyengar S, Lodge D. The effect of lumbar sympathectomy on increased tactile sensitivity in spinal nerve ligated rats. Neurosci Lett. 2001;298:99–102. doi: 10.1016/S0304-3940(00)01726-2. [DOI] [PubMed] [Google Scholar]
- 25.Leem JW, Gwak YS, Nam TS, Paik KS. Involvement of alpha2-adrenoceptors in mediating sympathetic excitation of injured dorsal root ganglion neurons in rats with spinal nerve ligation. Neurosci Lett. 1997;234:39–42. doi: 10.1016/S0304-3940(97)00658-7. [DOI] [PubMed] [Google Scholar]
- 26.Ma C, Greenquist KW, Lamotte RH. Inflammatory mediators enhance the excitability of chronically compressed dorsal root ganglion neurons. J Neurophysiol. 2006;95:2098–2107. doi: 10.1152/jn.00748.2005. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 28.McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis M, Devor M, Janig W. Sympathetic modulation of activity in rat dorsal root ganglion neurons changes over time following peripheral nerve injury. J Neurophysiol. 1996;76:753–763. doi: 10.1152/jn.1996.76.2.753. [DOI] [PubMed] [Google Scholar]
- 30.Mizuno S, Takebayashi T, Kirita T, Tanimoto K, Tohse N, Yamashita T. The effects of the sympathetic nerves on lumbar radicular pain: a behavioural and immunohistochemical study. J Bone Joint Surg Br. 2007;89:1666–1672. doi: 10.1302/0301-620X.89B12.19258. [DOI] [PubMed] [Google Scholar]
- 31.Murata Y, Olmarker K, Takahashi I, Takahashi K, Rydevik B. Effects of lumbar sympathectomy on pain behavioral changes caused by nucleus pulposus-induced spinal nerve damage in rats. Eur Spine J. 2006;15:634–640. doi: 10.1007/s00586-005-1020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng PW, Castano ED. Survey of chronic pain practice by anesthesiologists in Canada. Can J Anaesth. 2005;52:383–389. doi: 10.1007/BF03016281. [DOI] [PubMed] [Google Scholar]
- 33.Petersen M, Zhang J, Zhang JM, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- 34.Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–244. doi: 10.1016/S0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- 35.Ramer MS, French GD, Bisby MA. Wallerian degeneration is required for both neuropathic pain and sympathetic sprouting into the DRG. Pain. 1997;72:71–78. doi: 10.1016/S0304-3959(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 36.Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons of different size. J Physiol. 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiguchi M, Kobayashi H, Sekiguchi Y, Konno S, Kikuchi S. Sympathectomy reduces mechanical allodynia, tumor necrosis factor-alpha expression, and dorsal root ganglion apoptosis following nerve root crush injury. Spine. 2008;33:1163–1169. doi: 10.1097/BRS.0b013e31817144fc. [DOI] [PubMed] [Google Scholar]
- 38.Shi TS, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution and regulation of alpha(2)-adrenoceptors in rat dorsal root ganglia. Pain. 2000;84:319–330. doi: 10.1016/S0304-3959(99)00224-9. [DOI] [PubMed] [Google Scholar]
- 39.Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/A:1007090105840. [DOI] [PubMed] [Google Scholar]
- 40.Song XJ, Zhang JM, Hu SJ, LaMotte RH. Somata of nerve-injured sensory neurons exhibit enhanced responses to inflammatory mediators. Pain. 2003;104:701–709. doi: 10.1016/S0304-3959(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 41.Stafford MA, Peng P, Hill DA. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99:461–473. doi: 10.1093/bja/aem238. [DOI] [PubMed] [Google Scholar]
- 42.Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Study RE, Kral MG. Spontaneous action potential activity in isolated dorsal root ganglion neurons from rats with a painful neuropathy. Pain. 1996;65:235–242. doi: 10.1016/0304-3959(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 45.Takebayashi T, Cavanaugh JM, Cuneyt Ozaktay A, Kallakuri S, Chen C. Effect of nucleus pulposus on the neural activity of dorsal root ganglion. Spine (Phila Pa 1976) 2001;26:940–945. doi: 10.1097/00007632-200104150-00018. [DOI] [PubMed] [Google Scholar]
- 46.Tran KM, Frank SM, Raja SN, El-Rahmany HK, Kim LJ, Vu B. Lumbar sympathetic block for sympathetically maintained pain: changes in cutaneous temperatures and pain perception. Anesth Analg. 2000;90:1396–1401. doi: 10.1097/00000539-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 47.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe K, Konno S, Sekiguchi M, Sasaki N, Honda T, Kikuchi S. Increase of 200-kDa neurofilament-immunoreactive afferents in the substantia gelatinosa in allodynic rats induced by compression of the dorsal root ganglion. Spine (Phila Pa 1976) 2007;32:1265–1271. doi: 10.1097/BRS.0b013e318059aef8. [DOI] [PubMed] [Google Scholar]
- 49.Xie W, Strong JA, Li H, Zhang JM. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97:492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Zhang J, Petersen M, LaMotte RH. Functional changes in dorsal root ganglion cells after chronic nerve constriction in the rat. J Neurophysiol. 1995;73:1811–1820. doi: 10.1152/jn.1995.73.5.1811. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JM, Donnelly DF, Song XJ, Lamotte RH. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J Neurophysiol. 1997;78:2790–2794. doi: 10.1152/jn.1997.78.5.2790. [DOI] [PubMed] [Google Scholar]
- 52.Zhang JM, Li H, Munir MA. Decreasing sympathetic sprouting in pathologic sensory ganglia: a new mechanism for treating neuropathic pain using lidocaine. Pain. 2004;109:143–149. doi: 10.1016/j.pain.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JM, Song XJ, LaMotte RH. An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain. 1997;72:51–57. doi: 10.1016/S0304-3959(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]