Abstract
Background
Many patients with rheumatoid arthritis develop superior migration of the humeral head because of massive cuff tears, causing loss of active motion. Reverse shoulder arthroplasty could potentially restore biomechanical balance but a high incidence of glenoid failure has been reported. These studies do not, however, typically include many patients with rheumatoid arthritis (RA) and it is unclear whether the failure rates are similar.
Questions/purposes
We therefore (1) evaluated pain relief and shoulder function after reverse arthroplasty in RA; (2) compared results between primary and revision procedures; (3) determined the incidence of scapular notching; and (4) determined the complication rate.
Methods
We identified 29 patients with RA who had 33 reverse arthroplasties from among 412 patients having the surgery. Six patients were lost to followup. Twenty three patients (27 shoulders) were evaluated after a minimum followup of 18 months (mean, 56 months; range, 18–143 months), including 18 primary and nine revision arthroplasties. All patients were evaluated preoperatively and 23 shoulders postoperatively by an independent physiotherapist and four were assessed postoperatively by phone. Level of pain, range of motion, and Constant-Murley score were recorded and new radiographs taken.
Results
Visual Analog Scale score for pain decreased from 8.0 to 1.0. Constant-Murley score increased from 13 to 52. Primary procedures had higher scores compared with revisions. Three patients had revision surgery. Notching occurred in 52% of shoulders but no loosening was seen.
Conclusions
Reverse arthroplasty in rheumatoid arthritis improved shoulder function with a low incidence of complications. We believe it should be considered in elderly patients with rheumatoid arthritis with pain and poor active range of motion resulting from massive cuff tears.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Standard shoulder arthroplasty, hemi- or total, improves function and relieve pain in patients with rheumatoid arthritis [3, 6, 21]. However, patients with an intact rotator cuff have experienced better pain relief and greater improvements in ROM [21].
The reverse shoulder arthroplasty was designed to treat cuff tear arthropathy, ie, a condition with massive rotator cuff tears and destroyed joint surfaces [2, 9, 10]. In patients with cuff tear arthropathy and resulting pain who are unable to raise their arm above shoulder level (pseudoparalysis), reverse arthroplasty can restore function and reduce pain [2, 4, 7, 9, 18, 20, 22–24]. The combination of destroyed joint surfaces and cuff deficiency can be seen in many pathologic conditions, including cuff tear arthropathy [4, 7, 22], Milwaukee shoulder [1], inflammatory diseases such as rheumatoid arthritis [12, 13, 15, 22], and massive degenerative rotator cuff tears [9, 17, 22].
Lehtinen et al. [12] followed 74 patients with rheumatoid arthritis for 15 years and concluded that progressive upward migration of the humeral head is an inevitable consequence of the disease and indicates progressive rotator cuff failure. Therefore, patients with rheumatoid arthritis develop a condition similar to cuff tear arthropathy and could have better pain relief and ROM with a reverse arthroplasty compared with anatomic arthroplasty. Three studies [15, 16, 25] have reported outcomes from a total of 30 patients who have undergone this procedure. Significant improvement in range of movement and painrelief were observed but a high incidence of glenoid loosening or radiographic lucencies raised concerns for the longevity of the glenoid component. Degree of painrelief and improvement in shoulder mobility after primary reverse arthroplasty in cuff tear arthropathy have been better compared with those after revision of failed anatomic to a reverse arthroplasty [4, 12]. However, no studies have specifically reported revision of failed anatomic arthroplasties to reverse arthroplasty in patients with rheumatoid arthritis.
We therefore: (1) analyzed the clinical results (pain relief and shoulder function) after reverse arthroplasty; (2) compared results between primary and revision arthroplasties; (3) determined the incidence of scapular notching; and (4) determined the rate of complications in a series of patients with rheumatoid arthritis.
Patients and Methods
Between June 1995 and May 2008, we performed 412 reverse shoulder arthroplasties (Delta III or Delta Xtend; DePuy, Leeds, UK). The indication for performing the reverse arthroplasty was a combination of arthritic changes in the glenohumeral joint and rotator cuff insufficiency in all patients. We identified 29 patients (33 shoulders) with rheumatoid arthritis and massive rotator cuff tears. There were 22 women and seven men with a mean age of 68 years (range, 45–80 years) at the time of surgery. There were 24 primary and nine revision procedures (revision of failed anatomic arthroplasty). Twenty-five of the shoulders had a Delta III reverse arthroplasty, whereas eight had the more recent Delta Xtend. Six patients were lost to followup. One patient did not want to participate in the study and one patient had a recent stroke and therefore could not be evaluated. Four patients had died. Thus, 27 shoulders were retrospectively evaluated; 19 patients (23 shoulders) had a complete physical examination in the clinic, whereas four were assessed by phone. The minimum followup was 18 months (mean, 56 months; range, 18–143 months). There were 18 primary and nine revision procedures (eight hemiarthroplasty and one total shoulder arthroplasty). The mean time from the primary arthroplasty to revision was 5 years (range, 2–16 years) and the indication for revision was massive rotator cuff tear with pain and pseudoparalysis in all cases.
Preoperatively, all patients were evaluated by a physiotherapist and ROM, pain (subjectively measured on a 10-graded visual analog scale [VAS], in which 10 is the worst possible pain), and Constant-Murley score [5] were recorded. Rotation was measured according to Constant-Murley (maximum, 10 points). Plain radiographs and CT scans were performed in all patients before surgery and interpreted by one of the authors (AE). All patients had massive irreparable cuff tears visible on CT scans. Radiographs were classified according to Levigne et al. [13, 15] as: concentric type without glenoid erosion (C1) and with glenoid erosion (C2), ascendant type without erosion (A1) and with erosion (A2), and destructive type without erosion (D1) and with erosion (D2). Preoperative classification of the 18 primary cases showed no patients with C1, four with C2, seven with A1, three with A2, three with D1, and one with D2.
The Delta total shoulder is a reverse ball and socket arthroplasty. The glenoid component of the Delta reverse arthroplasty consists of two parts. A “metaglene” component (small round plate with a central peg) is attached to the glenoid surface with up to four screws. A large hemisphere (36 or 42 mm) can then be attached to this plate. The center of rotation is medialized and located at the bone-implant interface. The humeral component is modular and available for cemented or cementless fixation. A plastic polyethylene insert can be attached to the humeral component. Various heights and depths (standard or retentive) are available for optimal soft tissue balancing.
All surgeries were performed by a senior surgeon specialized in shoulder surgery (AE). All patients were operated on in the beach chair position. Antibiotic prophylaxis (cloxacillin; Stragen Nordic, Stenløse, Denmark) was given for 24 hours in all primary cases and for 3 days in revisions. The first dose of antibiotics was given 30 minutes before surgery. In case of allergy, clindamycin (Stragen Nordic) was used. In primary cases, a superior deltoid split was used, but in revisions or if a structural bone graft was needed on the glenoid side, a deltopectoral approach was preferred. If the deltoid split was used, the remaining rotator cuff was not detached. If the deltopectoral incision was used, the subscapularis, if present, was cut and not reattached at the end of surgery. The metaglene was placed in a low position and in all cases; we aimed to use an inferior screw at least 36 mm in length, preferably 42 mm. The superior screw was aimed at the base of the coracoid process and placed bicortically. Four screws were used in all metaglene components. All humeral components were cemented and the standard position was neutral rotation, ie, no retro- or anteversion. No retentive polyethylene inserts were used. Stability was subjectively assessed during surgery and was considered adequate if three criteria were met: no laxity between components when the arm was pulled downward, only slight opening (2–3 mm) between the glenosphere and polyethylene insert when the arm was placed in extension and external rotation, and no or minimal opening between components laterally when the arm was placed in maximum adduction. The resected humeral head was used as the bone graft to reconstruct the glenoid in four shoulders with severe bone loss (Fig. 1). Two patients with superior defects (A2) and one patient with severe central erosion (C2) had a structural bone graft on the glenoid side. In one patient, pieces of the head were impacted in a central contained defect on the glenoid surface. The three structural grafts were temporarily fixed with Kirschner wires, whereas the definite fixation was achieved using the same screws that fixed the metaglene. In one patient, bone cement was used to augment fixation of the inferior screw and to fill a small defect on the glenoid.
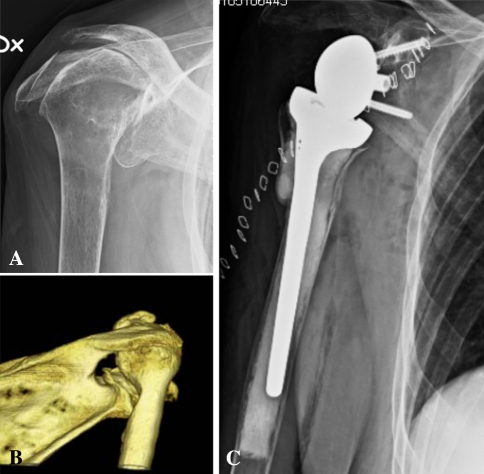
Fig. 1A–C.
Preoperative and postoperative radiographs of a patient with (A) superior migration of the humeral head and (B) medial erosion of the glenoid (Type A2) are shown. (C) The glenoid was reconstructed with an allograft.
A standard rehabilitation protocol was followed. If the superior deltoid split was used, the patient had a sling for 2 weeks but passive ROM was started the day after surgery. After 2 to 3 weeks, assisted active ROM and the use of the operated arm for activities of daily living were allowed. After 4 weeks, free active ROM was started. If a deltopectoral approach was used, the patients were allowed immediate active ROM as much as pain allowed. Regardless of the type of incision used, the patients were told not to use the operated arm when they pushed themselves upward from sitting to a standing position for the first 6 weeks after surgery.
Our routine followup for all reverse arthroplasties is clinical and radiographic evaluation at 1, 2, 5, and 10 years. For this study, the 23 patients available for followup were asked to have their operated shoulder clinically assessed by the same physiotherapist (RN) as for the preoperative assessment at an additional visit and to have plain radiographs taken to maximize the duration of followup. The active ROM was recorded as well as pain, activities of daily living, and Constant-Murley score. Patients were also asked what shoulder function they had using Single Shoulder Value (SSV) [8], in which 100 is a normal shoulder. All postoperative radiographs were taken under fluoroscopic control to provide a true AP film for assessment of the degree of notching. If recent radiographs had been taken (less than 1 year old), these were used for the radiographic evaluation. The radiographs were analyzed by one investigator (AE) looking for signs of loosening or scapular notching [14, 17, 19]. All 23 patients available for followup had radiographs of good quality with a mean followup of 45 months (range, 6–143 months). Any complication that had occurred during the followup period was recorded.
Four patients did not want to come for an extra visit and were assessed by telephone by a physiotherapist (RN) and an orthopaedic surgeon (AE). Function was evaluated by the SSV and the subjective questions in the Constant-Murley score, pain by the VAS, and ROM by asking them how they could move the arm in different directions. One investigator (AE) evaluated all radiographs at last followup [14, 17, 19]. In all cases, this evaluation agreed with the assessment of the degree of notching and potential signs of loosening given in the routine report performed by a trained radiologist in our Department of Radiology.
We determined differences between pre- and postoperative pain, ROM, and Constant-Murley score using the nonparametric Wilcoxon signed rank test. We used the Mann-Whitney test for comparing pain and Constant-Murley score between primary and revision cases at followup. SPSS Version 7.5 (SPSS Inc, Chicago, IL) was used for all analyses.
Results
Overall, pain relief and improved ROM were seen in this series of patients (Table 1). Preoperative pain on the VAS scale was reduced (p < 0.001) from a mean of 8.0 before surgery to a mean of 1.0 at last followup. Eighteen patients were pain-free. Active flexion improved (p < 0.001) from 33° to 115° and abduction from 26° to 103°, whereas Constant-Murley score increased (p < 0.001) from 13 to 52. Functional external rotation improved (p < 0.001), whereas no change (p = 0.15) in internal rotation was observed. Five patients had slightly worse internal rotation at followup compared with before surgery.
Table 1.
Results of reverse arthroplasty in patients with rheumatoid arthritis (N = 23, except for pain, N = 27) expressed as mean values ± SD (range)*
| Variable | Preoperative | Postoperative | p Value |
|---|---|---|---|
| Pain (Visual Analog Scale) | 8.0 ± 2.4 (4.9–10) | 1.0 ± 2.1 (0–9) | < 0.001 |
| Flexion | 33° ± 29° (0°–110°) | 115° ± 32° (0°–160°) | < 0.001 |
| Abduction | 26° ± 23° (0°–80°) | 103° ± 37° (20°–180°) | < 0.001 |
| External rotation | 0.6 ± 1.2 | 5.8 ± 3.4 | < 0.001 |
| Internal rotation | 2.1 ± 2.2 | 2.9 ± 2.4 | NS |
| Constant score | 13 ± 8.1 (2–34) | 52 ± 15 (15–77) | < 0.001 |
* Functional rotation assessed according to points in Constant-Murley score (maximum 10 points) and expressed as mean value ± SD; NS = nonsignificant.
Primary arthroplasties had better (p = 0.03) function than revision cases. The mean Constant-Murley score was 57 ± 13 compared with 43 ± 15. There was a nonsignificant tendency (p = 0.15) toward a higher SSV in the primary arthroplasty group (85 ± 16 versus 64 ± 26 in revision cases). There was no difference (p = 0.6) in pain relief at latest followup (postoperative VAS: 0.8 ± 2 in primary procedures versus 1.3 ± 2 in revisions).
Notching of various degrees was seen in 52% of the 23 shoulders with good-quality radiographs. There was no notch (Nerot 0) in 11 patients, a slight notch (Nerot 1–2) in one patient, a moderate notch (Nerot 3) in seven patients, and a notch above the inferior screw in four patients (Nerot 4). All patients with a notch above the inferior screw were pain-free.
No loosening of the glenoid or humeral component was seen and the overall complication rate was 15%. There were three complications (11%) that needed revision and one preoperative rim fracture of the glenoid that occurred during reaming of the glenoid surface. These patients were operated on early in the series (more than 9 years ago) and all with the older version of the Delta reverse arthroplasty (Delta III). The clinical findings for these four patients are included with those for all patients. The three revisions were further analyzed. One patient started to have instability 5 years after surgery as a result of wear of the polyethylene insert and underwent revision. The worn insert was replaced with a new insert plus a lengthener and the shoulder has been stable since revision. The Constant-Murley score at followup was 47. One of the patients who underwent revision developed a deep infection within the first year. A successful two-step revision was performed and the Constant-Murley score at followup was 48. The third patient who underwent revision reported severe pain for 6 months at the time of followup (9 years after surgery). CT scans and plain radiographs showed no loosening and the Constant-Murley score was 15. Exploration revealed breakage of the central screw connecting the metaglene with the glenosphere. The metaglene was well fixed but had to be revised because of the broken screw (Fig. 2).
Fig. 2.
Glenosphere and metaglene removed from a patient in whom the central screw had broken, creating severe pain.
Discussion
Many patients with rheumatoid arthritis develop rotator cuff deficiency similar to cuff tear arthropathy [12]. Reverse arthroplasty could be the best strategy for these patients because this procedure can restore function in rotator cuff-deficient shoulders. Published series of reverse shoulder arthroplasty in patients with rheumatoid arthritis are small, and high failure rates on the glenoid side have been reported; furthermore, there are no series describing the revision of failed anatomic arthroplasties [15, 16, 25]. Therefore, we analyzed clinical results, including a comparison between primary and revision procedures, incidence of notching, and rate of complications, in a series of reverse shoulder arthroplasties in patients with rheumatoid arthritis.
We acknowledge limitations to our study. First, it is retrospective, and the relatively small number of patients and lack of control group makes it difficult to formulate strong conclusions. However, it is difficult to collect a large series of patients with rheumatoid arthritis undergoing a reverse shoulder arthroplasty, because most patients with rheumatoid arthritis needing an arthroplasty have a functional cuff and can be treated with a standard arthroplasty. Therefore, this report provides the largest study of reverse shoulder arthroplasty in patients with rheumatoid arthritis to date. Second, it is a mixture of primary and revision cases and the patients had various degrees of bony deficiencies on the glenoid side. However, a detailed analysis of clinical results in the different subgroups was not performed as a result of the small patient numbers. Third, the mean followup is 5 years and complications including loosening and wear are likely to increase over time.
In the present study of 27 shoulders with rheumatoid arthritis, two-thirds of the patients were pain-free at followup and all but two could raise their arm to shoulder level or above. The Constant-Murley score increased to 57 in primary cases. These findings are similar to those of Rittmeister and Kershbaumer [16], who described seven patients with rheumatoid arthritis (eight shoulders) with a postoperative Constant-Murley score of 63 after a mean followup of 54 months. Woodruff et al. [25] reported on 13 shoulders with a postoperative score of 59 after a mean followup of 87 months. However, our scores are slightly inferior to some that have been reported in patients with cuff tear arthropathy with Constant-Murley scores of up to 60 to 65 (Table 2) [4, 7, 11, 18, 20, 22–24].
Table 2.
Published results of reverse total shoulder arthroplasty in shoulders with cuff tear arthropathy and after revision of failed anatomic arthroplasties
| Study | Shoulders (number) | Followup (mean, months) | Primary cuff tear arthropathy (mean, CS) | Revision arthroplasty (mean, CS) | Notching (%) | Complications (%) |
|---|---|---|---|---|---|---|
| Boileau et al. [4] | 45 | 40 | 66 | 46 | 68 | 24 |
| Frankle et al. [7] | 60 | 33 | 68* | — | — | 17 |
| Seebauer et al. [18] | 57 | 18 | 67 | 25 | 10 | |
| Sirveaux et al. [20] | 80 | 44 | 66 | 64 | 5 (loosening) | |
| Wall et al. [23] | 191 | 40 | 65 | 52 | 51 | 19 |
| Werner et al. [24] | 58 | 38 | 64† | 55† | 96 | 50 |
* American Shoulder & Elbow Score; †Relative Constant-Murley score (%); CS = Constant-Murley score.
Reverse arthroplasty for the revision of failed anatomic arthroplasties has been reported to provide inferior functional scores compared with primary reverse arthroplasty in cuff tear arthropathy (Table 2) [4, 22, 23]. This is in accordance with the Constant-Murley score of 43 in our patients with rheumatoid arthritis who received revision reverse arthroplasty. However, in our study, pain relief was similar in primary and revision cases, and pain is usually the main indication for performing the surgery. Thus, the lower Constant-Murley score in patients who underwent revisions resulted from less improvement in ROM and subjective functional variables compared with patients who underwent primary procedures. In contrast to our finding, Boileau et al. [4] reported less pain relief in patients undergoing revision compared with patients with primary cuff tear arthropathy. The reason for this difference is unclear. However, in our series, all surgeries were revised because of progressive cuff failure, whereas the reasons for revisions in the report by Boileau et al. were multifactorial [4].
Radiographic analysis showed scapular notching in 52% of the 27 shoulders. Levigne et al. [15] reported a notch in four patients (50%). However, the other two previous series of patients with rheumatoid arthritis did not report on notching. The clinical effect of scapular notching remains unclear. A negative impact on Constant-Murley score has been reported [19, 20], whereas other series have not found any correlation between the degree of scapular notch and level of pain or shoulder function measured according to Constant-Murley [14, 23]. Longer followup is needed to determine whether the notching process leads to component loosening. The finding in our series that all four patients with a notch above the inferior screw were pain-free is encouraging.
We found no glenoid or humeral loosening but an overall complication rate of 15%, and three of the four complications needed revision surgery. This is in accordance with the literature describing reverse arthroplasties in other etiologies, in which Wall et al. [23] reported a complication rate of 19% in a series of 191 reverse arthroplasties. Dislocation and infection were the most common complications. In a series of 58 primary procedures in patients with massive cuff tears, Frankle et al. [7] found a complication rate of 17%. However, complication rates as high as 50% and revision rates of 33% have been reported [24]. Earlier studies on reverse arthroplasties in patients with rheumatoid arthritis have reported a high incidence of glenoid failure and radiolucencies on the glenoid side [16, 25]. Both of these reports raised concerns regarding the ability to achieve good glenoid fixation in patients with rheumatoid arthritis. In our series, all glenoid components were fixed with four screws for the duration of the study or until revision was performed for other reasons. This could have contributed to the absence of glenoid loosening or radiolucencies. Furthermore, we found that revision of a failed reverse arthroplasty can be successful. In two of the three revised patients, the outcome at 6 and 8 years after revision was satisfactory. The third patient, who underwent a recent revision for the broken screw, cannot be evaluated yet.
In conclusion, we observed a substantial improvement in Constant-Murley score after reverse arthroplasty in patients with rheumatoid arthritis. Our clinical results and the incidence of complications after a primary reverse arthroplasty in patients with rheumatoid arthritis were similar to those seen in patients with cuff tear arthropathy. Even in revisions, improvement was seen and pain relief was as good as that recorded after primary procedures. However, in patients with rheumatoid arthritis, bone loss on the glenoid side can be severe and surgery therefore challenging. No loosening of components was seen. However, the mean followup was only 56 months and further studies are needed to determine long-term survival of the implant in this patient group. Age must therefore be taken into consideration when considering a reverse arthroplasty [11]. If an elderly patient with rheumatoid arthritis has pain and poor active ROM (pseudoparalysis) as a result of arthritic changes and a massive cuff tear, a reverse arthroplasty should be considered.
Acknowledgment
We thank D. Burrage, PhD, for editorial assistance.
Footnotes
One of the authors (AE) received royalties from DePuy Inc.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Antoniou J, Tsai A, Baker A, Schumacher R, Williams G, Ianotti JP. Milwaukee shoulder: correlating possible etiologic variables. Clin Orthop Relat Res. 2003;407:79–85. doi: 10.1097/00003086-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Baulot E, Chabernaud D, Grammont P. Results of Grammont’s inverted prosthesis in arthritis associated with major cuff destruction. A propos of 16 cases [in French] Acta Ortop Belg. 1995;61:112–119. [PubMed] [Google Scholar]
- 3.Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease. J Bone Joint Surg Br. 2009;91:1197–1200. doi: 10.1302/0301-620X.91B9.22035. [DOI] [PubMed] [Google Scholar]
- 4.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 6.Deshmukh AV, Koris M, Zurakowski D, Thornhill T. Total shoulder arthroplasty: long term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14:471–479. doi: 10.1016/j.jse.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2005;87:1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 9.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 10.Grammont PM, Trouilloud P, Laffay JP, Deries X. Design and manufacture of a new shoulder prosthesis [in French] Rheumatologie. 1987;39:407–418. [Google Scholar]
- 11.Guery J, Favard L, Sirveaux F, Oudet D, Molé D, Walsh G. Reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 12.Lehtinen JT, Belt EA, Lybäck CO, Kauppi MJ, Kaarela K, Kautiainen HJ, Lehto MUK. Subacromial space in the rheumatoid shoulder: a radiographic 15-year follow-up study of 148 shoulders. J Shoulder Elbow Surg. 2000;9:183–187. doi: 10.1016/S1058-2746(00)90053-3. [DOI] [PubMed] [Google Scholar]
- 13.Levigne C. Radiographic classifications and evolutions of the rheumatoid shoulder [in French] Rev Rhum (Ed Fr) 2002;69(Suppl 3):108–112. doi: 10.1016/S1169-8330(02)00413-1. [DOI] [Google Scholar]
- 14.Levigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2008;17:925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Levigne CH, Boileau P, Favard L, Molé D, Sirvaux F, Walch G. Reverse arthroplasty in rheumatoid arthritis. In: Walch G, Boileau P, Molé D, Favard L, Lévigne C, Sirveaux F, eds. Reverse Arthroplasty. Montpellier, Canada: Sauramps Medical; 2006.
- 16.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 17.Roberts CC, Ekelund AL, Renfree KJ, Liu PT, Chew FS. Radiologic assessment of reverse arthroplasty. Radiographics. 2007;27:223–235. doi: 10.1148/rg.271065076. [DOI] [PubMed] [Google Scholar]
- 18.Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in German] Operative Orthopädie und Traumatologie. 2005;1:1–24. doi: 10.1007/s00064-005-1119-1. [DOI] [PubMed] [Google Scholar]
- 19.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 20.Sirveaux F, Favard L, Oudet D, Huguet D, Walsh G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 21.Sperling JW, Cofield RH, Schleck CD, Harmsen S. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16:683–690. doi: 10.1016/j.jse.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 22.Walch G, Boileau P, Molé D, Favard L, Lévigne C, Sirveaux F, editors. Reverse Shoulder Arthroplasty. Montpellier, Canada: Sauramps Medical; 2006. [Google Scholar]
- 23.Wall B, Nové-Josserand L, O′Connor P, Edwards TB, Walsh G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 24.Werner CML, Steinman PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff MJ, Cohen AP, Bradley JG. Arthroplasty of the shoulder in rheumatoid arthritis with rotator cuff dysfunction. Int Orthop. 2003;27:7–10. doi: 10.1007/s00264-002-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]