Abstract
Background
Early failure due to glenoid loosening with anatomic total shoulder arthroplasty in patients with severe rotator cuff deficiency led to the development of the reverse ball-and-socket shoulder prosthesis. The literature reports improved short-term pain and function scores following modern reverse total shoulder arthroplasty (RTSA) in patients with cuff tear arthropathy (CTA).
Questions/purposes
We therefore sought to confirm previously reported short-term improvements in pain, function scores, and range of motion, in patients treated with RTSA for CTA and to identify clinical complications and radiographic notching.
Methods
We retrospectively reviewed 67 patients who underwent 71 primary RTSAs for CTA. The average age was 74 years (range, 54–92 years). All were preoperatively and postoperatively assessed using Constant-Murley and American Shoulder and Elbow Society (ASES) scores. We identified complications and examined radiographs for notching. The minimum followup was 12 months (average, 24 months; range, 12–58 months).
Results
Average Constant-Murley scores improved from 28 preoperatively to 62 postoperatively. Average ASES scores improved from 26 to 76. Subjective Shoulder Value (SSV) improved from 23 to 77. Active forward flexion improved from 61° preoperatively (range, 0°–137°) to 121° postoperatively (range, 52°–170°). Active external rotation was not affected. Thirty-five of the 71 shoulders (49%) showed radiographic notching. The overall complication rate was 23%. No patient required reoperation. One patient required closed reduction of a perioperative dislocation.
Conclusions
RTSA for CTA results in functional improvement, with a low complication rate. However, the longevity of the device is currently unknown.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Rotator CTA is a difficult clinical problem, and the etiology is not completely understood. First described in 1857, it was considered a localized form of rheumatic arthritis [1]. Neer et al. [20] coined the term “cuff tear arthropathy” in 1983 to describe the clinical scenario of a massive rotator cuff tear associated with glenohumeral joint degeneration. It is often associated with anterosuperior subluxation of the humeral head, which allows articulation of the humeral head with the acromion. Clinically, weakness or pseudoparalysis of forward elevation and abduction are seen, and patients complain of pain with motion. Attritional rupture of the long head of the biceps tendon is common. A bald humeral head can often be seen intraoperatively.
In 1990, Hamada et al. [15] described the progression of glenohumeral joint degeneration associated with rotator cuff tears. In this classification, only types IV and V included CTA (Table 1). Visotsky et al. [27] further subclassified these two groups based on whether the humeral head is centered on the glenoid and its stability (Table 2).
Table 1.
Hamada classification for massive rotator cuff tears
| Hamada grade | Radiographic findings |
|---|---|
| 1 | Acromiohumeral interval at least 6 mm |
| 2 | Acromiohumeral interval less than 6 mm |
| 3 | Acetabularization of the acromion |
| 4 | Arthrosis of the glenohumeral joint |
| 5 | Humeral head collapse |
Table 2.
Visotsky classifications for cuff tear arthroplasty
| Visotsky classification | Position and stability of humeral head |
|---|---|
| IA | Centered and stable |
| IB | Centered but medialized |
| IIA | Not centered with some limited stability |
| IIB | Not centered and unstable |
Prior to the advent of modern Grammont-type RTSA [3, 9, 10], surgical options for CTA included open or arthroscopic débridement, resection arthroplasty, arthrodesis, and humeral head arthroplasty (HHA) with or without an extended coverage head. Anatomic total shoulder arthroplasty in CTA led to eccentric loading of the glenoid component (rocking horse phenomenon) and early failure via glenoid loosening [8]; it has since been abandoned for CTA. Although HHA was once the preferred option, it has unpredictable functional outcomes and is unable to restore stability. HHA remains a treatment option, but is usually reserved for types IA and IIA, as described by Visotsky et al. [27]. HHA reportedly has “89% successful results with ‘limited goal criteria’” [27].
RTSA, utilizing a deltopectoral or superolateral approach, results in substantial improvement in pain and function when used for the treatment of CTA [3, 6, 7, 9, 11–13, 21–25, 29, 30]. It has been successfully used in Europe for over 20 years. Our PubMed search found over 100 articles on RTSA, a procedure that the FDA first approved for use in the United States in late 2003. Thus, a great deal of interest has been generated for RTSA in a short time.
The literature reports improved pain and function scores with RTSA for multiple indications [9, 11–13, 20, 22–25, 31]. Many of these series include revisions for failed arthroplasties. Inclusion of other diagnoses, including acute proximal humeral fracture, tuberosity malunion, and pseudo-paralysis due to rotator cuff deficiency without arthrosis, further complicates interpretation of the existing data. The results of RTSA as a salvage procedure may be less favorable than as an initial arthroplasty. Wall et al. [29] showed RTSA resulted in smaller functional gains and higher complication rates in patients with posttraumatic arthritis or prior failed shoulder arthroplasty than in patients having RTSA for other indications.
Therefore, we sought to confirm previously reported improvements in pain, functional scores, and range of motion in patients treated with RTSA for CTA. We identified clinical complications and radiographic notching.
Patients and Methods
We enrolled all patients undergoing RTSA in a prospective database. Between December 2004 and February 2009, the senior author (JMW) performed 277 RTSAs in 265 patients. Of these, 184 shoulders in 174 patients were available for clinical and radiographic evaluation with minimum 12-month followup. We excluded 93 RTSAs in 91 patients with less than 12 months followup. Additionally, we excluded 113 shoulders in 107 patients with diagnoses other than CTA. This left 71 shoulders in 67 patients diagnosed preoperatively with CTA and with at least 12 months followup. No patient had previous ipsilateral shoulder arthroplasty. We did not offer RTSA to any patient with a nonfunctioning deltoid or known infection. Patients with rotator cuff tears without glenohumeral joint involvement (Hamada I through III) did not meet the criteria for diagnosis of CTA. We did not exclude patients with previous rotator cuff repairs and/or débridements. Of the patients in the study, 20 were men and 47 women. We performed 56 procedures on the right shoulder and 15 on the left. Four patients underwent bilateral RTSA. The average age at the time of surgery was 74.2 years (range, 54–92). The minimum followup was 12 months (average, 24 months; range, 12–58 months). No patients were lost to followup. We did not recall any patients specifically for this study; all data were obtained from medical records and radiographs. We clinically evaluated all patients prior to RTSA. Patients gave informed consent to participate. The William Beaumont Hospital Human Investigations Committee approved this study, HIC # 2006-088.
Clinical examination included range of motion and strength testing, neurovascular examination of the involved extremity, and Visual Analog Scale (VAS) pain rating on a scale of 0 (none) to 10 (maximal) [17]. We noted the presence or absence of a preoperative external rotation lag sign, but these were not recorded in six patients preoperatively. All other preoperative and postoperative data were recorded and available for this analysis. Patients were also evaluated both preoperatively and postoperatively using Constant-Murley scores [4], ASES scores [18], and SSV [10]. Preoperatively, we obtained radiographs that consisted of an AP Grashey view [13], scapular Y view, and axillary view of the shoulder, as well as AP and lateral full-length humerus films.
All clinical measurements and radiographic assessments were performed by an independent observer. We performed the procedure in all patients using a limited 5 cm to 10 cm skin incision for the deltopectoral approach. The subscapularis was intact in 70 of 71 shoulders, and was tenotomized and repaired following placement of the prosthesis using heavy nonabsorbable sutures. In 56 patients, we released a portion of the pectoralis major insertion to aid in exposure, which we then repaired at the conclusion of the case. In cases of major soft-tissue contracture, we elevated a small portion of the anterior deltoid insertion. The long head of the biceps tendon was tenodesed in 28 of 71 patients. In the remaining 43 patients, the tendon had a pre-existing attritional rupture. The osteotomy was made in neutral version for patients with tearing of the teres minor and a preoperative external rotation lag sign [28]. We made the osteotomy in 20° of retroversion for patients with an intact teres minor and a preoperative negative external rotation lag sign. We routinely performed a 360° release of adhesions around the subscapularis. We placed the guide inferiorly on the glenoid so that the base plate was fully supported by bone and flush with the inferior glenoid margin. We placed the guide wire in approximately 10° to 15° inferior tilt, as this position minimized the risk of scapular notching [25]. Seven patients required bone graft for glenoid bony deficiency. We placed two superior and inferior locking 4.5 mm screws through the holes in the baseplate with bone purchase into the base of the coracoid and inferior scapular border. We inserted the glenosphere with its inferior border slightly overhanging the inferior glenoid margin. Polyethylene insert thickness was chosen based on soft-tissue tension during trial reduction. In judging proper soft-tissue tension, our goal was minimal shuck (1–2 mm) with tension on conjoint tendon, which was taught, but not so tight as to cause bowstringing of the tendon. Our goals for passive intraoperative range of motion included 130° forward elevation and 45° external rotations, both in neutral flexion and extension. We also attempted to manually dislocate the humerus superiorly off the glenosphere with the arm in slight abduction and neutral rotation. It required a moderate laterally directed force to do this.
We routinely used two drains. In addition to RTSA, five patients underwent combined latissimus dorsi and teres major transfers, and one had transfer of the latissimus dorsi only.
Patients were placed in an abduction sling for 4 weeks. The initiation of progressive range of motion with low intensity supervised physical therapy began 2 weeks after surgery, progressive from active assist range of motion to full active range of motion. Passive external rotation was initially avoided to protect the repair of the subscapularis tendon. We instructed patients not to lift anything heavier than a gallon of milk during the recovery period.
Postoperatively, we evaluated patients clinically and radiographically at 2 weeks, 3 months, 6 months, 1 year, and then yearly thereafter. Constant-Murley scores and ASES scores were recorded at yearly visits. Patients rated their shoulder using the SSV and VAS both preoperatively and postoperatively. We recorded the postoperative Constant-Murley score, ASES score, SSV, VAS pain level, and range of motion. We defined major complications as those which prolonged the hospital stay or required reoperation; we considered all other complications to be minor [26].
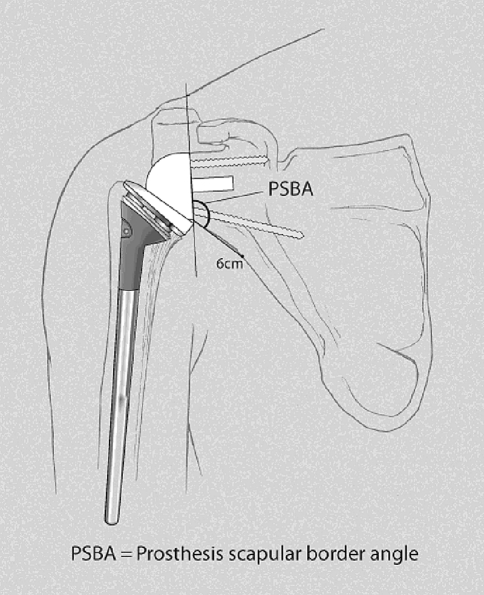
Postoperatively, we performed a standard three-view shoulder series at each visit. An independent observer, not involved in any of these surgical cases (BMN), evaluated all radiographs for signs of fracture or dislocation, scapular notching according to Sirveaux et al. [24] (Table 3), heterotopic ossification or spur formation, and prosthetic failure due to loosening, dissociation, wear, migration, or breakage. We measured the prosthesis-scapular border angle (PSBA) at a distance of 6 cm from the inferior margin of the baseplate (Fig. 1). There were no missing radiographs.
Table 3.
Sirveaux classification of scapular notching
| Sirveaux notching grade | Extent of involvement |
|---|---|
| 1 | Notch limited to scapular pillar |
| 2 | Extends to inferior screw |
| 3 | Extends beyond inferior screw |
| 4 | Notch reaches baseplate |
Fig. 1.
A diagram of measurement of prosthesis-scapular border angle (PSBA) is shown.
Results
The mean ASES score, SSV, Constant-Murley score, and VAS were all substantially improved (Table 4). Range of motion improved in active forward elevation, but active external rotation was not substantially improved (Table 4). Preoperatively, 24 patients had an external rotation lag sign, while 41 had intact external rotation; we did not record the preoperative external rotation lag sign in six patients. Average postoperative active external rotation was 8° in the group with preoperative external rotation lag signs, and 17° in the group with intact preoperative external rotation (Table 5).
Table 4.
Clinical results of RTSA for CTA
| Variable | Preoperative average value | Preoperative range | Postoperative average value | Postoperative range |
|---|---|---|---|---|
| Active forward elevation (degrees) | 61.2 | 0 to 137 | 121.3 | 52 to 170 |
| Active external rotation (degrees) | 13.8 | −35 to 60 | 14.6 | −44 to 60 |
| ASES score | 26.0 | 0 to 63 | 76.1 | 21 to 100 |
| SSV | 23 | 0 to 75 | 76.9 | 35 to 100 |
| Constant-Murley score | 27.5 | 5 to 58 | 61.8 | 30 to 87 |
| VAS pain | 7 | 0 to 10 | 1.4 | 0 to 8 |
RTSA - reverse total shoulder arthroplasty; CTA - cuff tear arthropathy.
Table 5.
Clinical results according to preoperative external rotation lag sign
| Variable | Preoperative external rotation lag sign* | Preoperative intact active external rotation* |
|---|---|---|
| Active forward elevation (degrees) | 120 (62 to 150) | 120.4 (52 to 170) |
| Active external rotation (degrees) | 8.1 (−15 to 55) | 17.1 (−44 to 60) |
| ASES score | 72.7 (40 to 98.3) | 76 (21 to 100) |
| SSV | 76.5 (50 to 100) | 75.2 (35 to 100) |
| Constant-Murley score | 59.4 (40 to 75) | 61.1 (30 to 73) |
| VAS pain | 1.3 (0 to 6) | 1.7 (0 to 8) |
* Measurements – average (range); ASES - American Shoulder and Elbow Society; SSV - Subjective Shoulder Value; VAS - Visual Analog Scale.
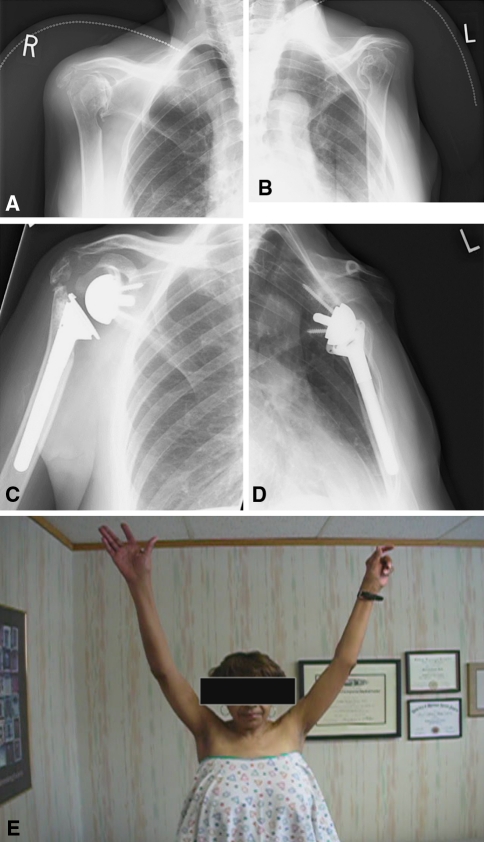
We observed the following grades of scapular notching: 36 shoulders were Grade 0; 11 were Grade 1; 15 were Grade 2; seven were Grade 3; one was Grade 4. Notching occurred in the early postoperative period and tended to be nonprogressive. We did not include the one shoulder with superior migration of the glenoid component in the notching analysis. The PSBA averaged 135° (range, 110°–159°). Twenty-six patients had spur formation or heterotopic ossification at the inferior glenoid neck, and nine (13%) had lucency behind the baseplate. Surgery typically improved positioning (Fig. 2A–D) and improved forward elevation (Fig. 2E).
Fig. 2A–E.
Preoperative AP views of the (A) right and (B) left shoulders in a 69-year-old woman demonstrate bilateral cuff tear arthropathy with glenohumeral joint degeneration and severe narrowing of the acromiohumeral distance, allowing articulation of the humeral head with the acromion. Acetabularization of the acromion is seen. A right acromial insufficiency fracture is noted preoperatively. Postoperative AP views demonstrate reverse total shoulder prostheses in good position. Postoperative radiographs of the patient following bilateral RTSA for bilateral show improved alignment of the (C) right and (D) left shoulder. (E) After bilateral RTSA, the patient demonstrates improved forward elevation despite a right acromial fracture.
We identified 16 complications in 14 patients, including of 10 perioperative complications and six late complications. Perioperative complications included two patients with deep venous thrombosis that lead to pulmonary embolism, two patients with broken hardware (one broken retained drill bit and one broken anterior screw head), and one patient each with: periprosthetic humerus fracture, retinal artery thrombosis, postoperative pneumonia, and musculocutaneous mononeuropathy. An additional patient had an intraoperative anterior glenoid rim fracture beneath the baseplate, but did not prevent the placement of the glenoid implant, and healed without any alteration to the postoperative course. None of these patients required further operation. One patient sustained a perioperative dislocation, underwent closed reduction the same day of surgery, and has remained stable. Late complications included one patient with superior glenosphere migration, one with an acromial fracture at 5 weeks, one with a acute coracoid process fracture, and one who had nonunited insufficiency fractures of the scapular spine and distal clavicle at 32 months. Another patient had a self-reduced dislocation at 33 months, followed by recurrent subluxations, but was satisfied. No patient required further surgery. The patient with the coracoid fracture was treated with a sling for 6 weeks, and the shoulder remains stable at latest followup. Overall, there were four major and 12 minor complications.
Discussion
Relatively high rates of glenoid loosening of anatomic total shoulder arthroplasty in patients with severe CTA led to the development of the RTSA. The literature suggests pain and function is improved following modern reverse total shoulder arthroplasty in patients with CTA, although the long-term durability is unknown. Our purpose was to confirm previously reported short-term improvements in pain and function scores, as well as range of motion, in patients treated with RTSA for CTA. We also reported radiographic notching and clinical complications.
We note several limitations associated with our study. First, the minimum followup of 12 months (average, 24 months) is too short to determine the longevity of the implant, or evaluate the long-term implications of radiographic findings, such as notching. The longevity of the implant remains to be determined, as it has only been FDA approved for use in the United States for 6 years. Further study with long-term followup is needed. Further, the implications of notching are not yet well understood; although concern exists regarding possible glenoid loosening in patients with scapular notching, that association is not present in the literature. Second, we did not compare results of patients treated with RTSA for CTA to those treated with RTSA for other reasons. Since CTA is the most common indication for RTSA, we believe it helpful to evaluate the outcomes of a series of patients with one diagnosis. To compare these results with the results of RTSA for other etiologies would be difficult due to the heterogeneous nature of the other diagnoses. However, this does make it difficult to compare these results to those of other authors, since those series report groups of patients with varying diagnoses. Third, we did not use fluoroscopy for postoperative radiographic analysis, thus the frequency of lucent lines may be underestimated. No symptomatic loosening has yet to occur at latest followup.
Our series, limited to RTSA for CTA, experienced substantial improvements in mean Constant-Murley scores, ASES scores, SSV, VAS, and forward elevation. These findings confirm those from studies reporting RTSA for all indications (Table 6) [9, 12, 14, 19, 24, 25, 31]. The exclusion of other diagnoses, such as pseudoparalysis without glenohumeral arthrosis, acute fracture, malunion, and revision arthroplasties, may explain our outcomes. The results of RTSA depend upon indication; functional scores of RTSA in revision settings are inferior to primary RTSA with more complications [13, 25, 29, 30]. Werner et al. [30] suggested RTSA with a Grammont-type prosthesis can restore forward elevation and abduction, but not external rotation. Our patients had no change in mean external rotation after surgery. In the six patients who underwent tendon transfer in addition to RTSA, external rotation decreased from an average of 13.5° preoperatively to 9.8° postoperatively. This does not appear to support the recommendation of Gerber et al. [9] for latissimus dorsi transfer in patients with profound external rotation weakness, indicated by a hornblower’s sign [24] or external rotation lag sign [16].
Table 6.
Current study results and published literature results
| Author | Number of shoulders | Average duration of followup (months) | Active forward elevation (degrees) | Active external rotation (degrees) | ASES score | Constant-Murley score | SSV | VAS pain |
|---|---|---|---|---|---|---|---|---|
| Nolan et al. [current study] | 71 | 24 | 121 | 15 | 76 | 62 | 77 | 1 |
| Boileau et al. [2] | 45 | 40 | 121 | 11 | 65 | 59 | * | * |
| Frankle et al. [7] | 60 | 33 | 105 | 41 | 68 | * | * | 2 |
| Rittmeister and Kerschbaumer [21] | 8 | 54 | * | * | * | 63 | * | * |
| Sirveaux et al. [25] | 80 | 44 | 138 | 11 | * | 66 | * | * |
| Wall et al. [29] | 240 | 40 | 137 | 6 | * | 60 | * | * |
| Werner et al. [30] | 58 | 38 | 100 | 12 | * | 64 | 56 | * |
* Not reported.
ASES - American Shoulder and Elbow Society; SSV - Subjective Shoulder Value; VAS - Visual Analog Scale.
Our rate of definitive glenoid loosening was low (1.4%, 1/71). Altogether, nine patients had evidence of lucency behind the base plate, so the total percent of at-risk glenoids was 13%. This compares favorably with the 16% rate of radiographic loosening reported in the French multicenter studies [14, 25]. Our shorter followup time may account for this difference. Notching rates of 50% to 96% have been reported [21, 25, 30]. Higher grades of notching negatively correlate with Constant-Murley scores [23]. Simovitch et al. [23] calculated a notching index based on the prosthesis-scapular neck angle (PSNA) and the superior-inferior placement of the base plate on the glenoid. We have modified the PSNA to account for the anatomic variability in the scapular neck. We call this modification the prosthesis-scapular border angle (PSBA); the angle between the base plate and a line drawn from the inferior aspect of the base plate to a point 6 cm away on the lateral scapular border. We believe this is a more appropriate measurement since most notching occurs between 1 cm and 6 cm away from the base plate, rather than within 1 cm, and the scapular border is more anatomically consistent than the scapular neck. Our patients averaged 135° for the prosthesis-scapular border angle, yet our notching rate was not higher. This supports the findings of Simovitch et al. [23], which suggests inferior tilt is not the primary determinant of notching.
We included all perioperative systemic complications, such as pulmonary embolism, in our analysis. However, most investigators only reported on complications directly related to the shoulder. Despite our more liberal inclusion criteria for reporting a complication, our complication rate of 23% is within the range of previous reports (Table 6). Many of ours were systemic complications and not directly implant-related. If the complications not directly related to the shoulder are removed, our complication rate is 17%. The age and medical comorbidities of CTA patients may affect the number of medical complications. No patient in this study required reoperation, and only one required formal closed reduction in the operating room for a perioperative dislocation. Two shoulders in our series suffered a dislocation, resulting in an instability rate of 2.8%, similar to those previously reported (Table 6). Instability continues to be a concern with RTSA, and is one reason that complete deltoid paralysis is considered a contraindication to RTSA. Additionally, a two-fold increased risk of instability was seen in patients with a preoperative subscapularis rupture [6]. Therefore, it is important to determine the status of the subscapularis tendon preoperatively and, if present, repair it after implantation.
Authors have reported revision rates of 4.2% to 13% following RTSA [3, 5, 25]. There were no revisions in our series. It is possible that the revision rate is less due to the exclusion of patients with a previous arthroplasty and our relatively short followup.
In conclusion, RTSA for CTA results in major improvements in function, and a low complication rate. Scapular notching does occur, and its relationship to pain and function scores, as well as implant longevity, requires further study.
Acknowledgments
We thank Mamtha Balasubramaniam, Senior Biostatistician, for help in statistical analysis.
Footnotes
One or more of the authors (JMW) is a paid consultant, and he or she has or may receive payments or benefits from a commercial entity related to this work (Zimmer Inc., Warsaw, IN). This study was funded by a grant from the William Beaumont Hospital Research Institute.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Contributor Information
Betsy M. Nolan, Email: shoulderandelbowdoc@gmail.com.
J. Michael Wiater, Email: jmwiater@aol.com.
References
- 1.Adams R. A Treatise on Rheumatic Gout, or Chronic Rheumatic Arthritis of All the Joints. London, England: J Churchill; 1857:91–157
- 2.Boileau P, Chuinard C, Roussanne Y, Neyton L, Trojani C. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16:671–682. doi: 10.1016/j.jse.2007.02.127. [DOI] [PubMed] [Google Scholar]
- 3.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Constant CR, Murley AHG. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 5.Ecklund KJ, Lee TQ, Tibone J, Gupta R. Rotator cuff tear arthropathy. J Am Acad Orthop Surg. 2007;15:340–349. doi: 10.5435/00124635-200706000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18:892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum 2-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–1705. doi: 10.2106/JBJS.D.02813. [DOI] [PubMed] [Google Scholar]
- 8.Franklin JL, Barrett WP, Jackins SE, Matsen FA., 3rd Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3:39–46. doi: 10.1016/S0883-5403(88)80051-2. [DOI] [PubMed] [Google Scholar]
- 9.Gerber C, Pennington SD, Lingenfelter EJ, Sukthankar A. Reverse Delta-III total shoulder replacement combined with latissimus dorsi transfer: A preliminary report. J Bone Joint Surg Am. 2007;89:940–947. doi: 10.2106/JBJS.F.00955. [DOI] [PubMed] [Google Scholar]
- 10.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 11.Grammont P, Trouillard P, Laffay JP, Deries X. Study and realization of a new shoulder prosthesis [in French] Rhumatologie. 1987;39:407–418. [Google Scholar]
- 12.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 13.Grashey, R. Atlas of Common Radiographs [in German]. Munchen, Germany: Lehman; 1923:131–138.
- 14.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survival analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: A long-term observation. Clin Orthop Relat Res. 1990;254:92–96. [PubMed] [Google Scholar]
- 16.Hertel R, Ballmer FT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg. 1996;5:307–313. doi: 10.1016/S1058-2746(96)80058-9. [DOI] [PubMed] [Google Scholar]
- 17.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/S0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 18.Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: Reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11:587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 19.Mole D, Favard L. Excentered scapulohumeral osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:37–94. doi: 10.1016/s0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 20.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 21.Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10:17–22. doi: 10.1067/mse.2001.110515. [DOI] [PubMed] [Google Scholar]
- 22.Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg. 2007;89:934–939. doi: 10.2106/JBJS.F.01075. [DOI] [PubMed] [Google Scholar]
- 23.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 24.Sirveaux F. The prosthesis of Grammant in the treatment of arthropathies of the shoulder with cuff tear: A multicenter study of 42 cases. Faculte′ de me′decine de Nancy, the`se del’universite′ de Nancy I 245, 1997.
- 25.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 26.Swanson KC, Valle AG, Salvati EA, Sculco TP, Bottner F. Perioperative morbidity after single-stage bilateral total hip arthroplasty: A matched control study. Clin Orthop Relat Res. 2006;451:140–145. doi: 10.1097/01.blo.0000223992.34153.5d. [DOI] [PubMed] [Google Scholar]
- 27.Visotsky JL, Basamania C, Seebauer L, Rockwood CA, Jensen KL. Cuff tear arthropathy: Pathogenesis, classification and algorithm for treatment. J Bone Joint Surg Am. 2004;86:35–40. [PubMed] [Google Scholar]
- 28.Walch G, Boulahia A, Calderone S, Robinson AH. The ‘dropping’ and ‘hornblower’s’ signs in evaluation of rotator-cuff tears. J Bone Joint Surg Br. 1998;80:624–628. doi: 10.1302/0301-620X.80B4.8651. [DOI] [PubMed] [Google Scholar]
- 29.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: A review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 30.Werner CM, Steinman PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 31.Wiater JM, Fabing MH. Shoulder arthroplasty: prosthetic options and indications. JAAOS. 2009;17:415–425. doi: 10.5435/00124635-200907000-00002. [DOI] [PubMed] [Google Scholar]