Abstract
Background
Scapular notching, prosthetic instability, limited shoulder rotation and loss of shoulder contour are associated with conventional medialized design reverse shoulder arthroplasty. Prosthetic (ie, metallic) lateralization increases torque at the baseplate-glenoid interface potentially leading to failure.
Questions/purposes
We asked whether bony lateralization of reverse shoulder arthroplasty would avoid the problems caused by humeral medialization without increasing torque or shear force applied to the glenoid component.
Patients and Methods
We prospectively followed 42 patients with rotator cuff deficiency treated with bony increased-offset reverse shoulder arthroplasty. A cylinder of autologous cancellous bone graft, harvested from the humeral head, was placed between the reamed glenoid surface and baseplate. Graft and baseplate fixation was achieved using a lengthened central peg (25 mm) and four screws. Patients underwent clinical, radiographic, and CT assessment at a minimum of 2 years after surgery.
Results
The humeral graft incorporated completely in 98% of cases (41 of 42) and partially in one. At a mean of 28 months postoperatively, no graft resorption, glenoid loosening, or postoperative instability was observed. Inferior scapular notching occurred in 19% (eight of 42). The absolute Constant-Murley score improved from 31 to 67. Thirty-six patients (86%) were able to internally rotate sufficiently to reach their back over the sacrum.
Conclusions
Grafting of the glenoid surface during reverse shoulder arthroplasty effectively creates a long-necked scapula, providing the benefits of lateralization. Bony increased-offset reverse shoulder arthroplasty is associated with low rates of inferior scapular notching, improved shoulder rotation, no prosthetic instability and improved shoulder contour. In contrast to metallic lateralization, bony lateralization has the advantage of maintaining the prosthetic center of rotation at the prosthesis-bone interface, thus minimizing torque on the glenoid component.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Reverse shoulder arthroplasty (RSA) restores function and active elevation in patients with pseudoparalysis related to rotator cuff deficiency [3]. The two basic biomechanical principles of the Grammont RSA are a medialization of the glenohumeral center of rotation and a lowering of the humerus [6]. These principles reduce torque on the glenoid component and increase the deltoid lever arm, overcoming weak or absent rotator cuff musculature [5, 34]. However, a number of problems and complications, attributed to the medialized design, have been reported in the literature [5, 6, 13, 16, 21, 22, 27, 29, 37, 40, 41]. Inferomedial impingement of the humeral insert against the pillar of the scapula during adduction and rotation of the arm is responsible for bone erosion and polyethylene wear (known as “inferior scapular notching”) and has been observed in 50% to 96% of postoperative radiographs [6, 7, 27, 29, 30, 32, 36, 41]. Anterior scapular impingement may restrict internal rotation, while posterior impingement restricts external rotation. Limited postoperative shoulder rotation after RSA is related to the limited excursion of the cup around the medialized glenosphere, as well as mechanical impingement of the tuberosities against the coracoid process in internal rotation and the scapular spine in external rotation [5, 13]. Impingement of the greater tuberosity upon the acromion may also limit abduction and forward elevation. Prosthetic instability is also a consequence of humeral medialization (because of poor soft tissue tension and glenohumeral impingement) and has been observed in 3% to 6% of cases at followup [5, 13, 24, 26]. Finally, humeral medialization may raise cosmetic concerns, as some patients dislike the loss of their normal shoulder contour after RSA [5, 6].
To address these problems, several authors have proposed a change in the design of Grammont’s prosthesis, promoting an increased-offset RSA [12, 35]. Such prosthetic lateralization, achieved by increasing the offset of the glenosphere and/or baseplate (metallic lateralization), has the disadvantage of increasing torque or shear force applied to the glenoid component and potentially increasing the risk of glenoid loosening [5, 18]. We adopted a novel approach to address the problematic issues encountered with standard medialized RSA: we presumed it would be possible to lateralize the prosthesis by placing an autogenous bone graft harvested from the humeral head on a specifically designed baseplate with a long central peg. This novel surgical procedure, which keeps the center of rotation at the glenoid bone-prosthesis interface once the bone graft has healed, is called the bony increased-offset reversed shoulder arthroplasty (BIO-RSA) (Fig. 1).
Fig. 1A–C.
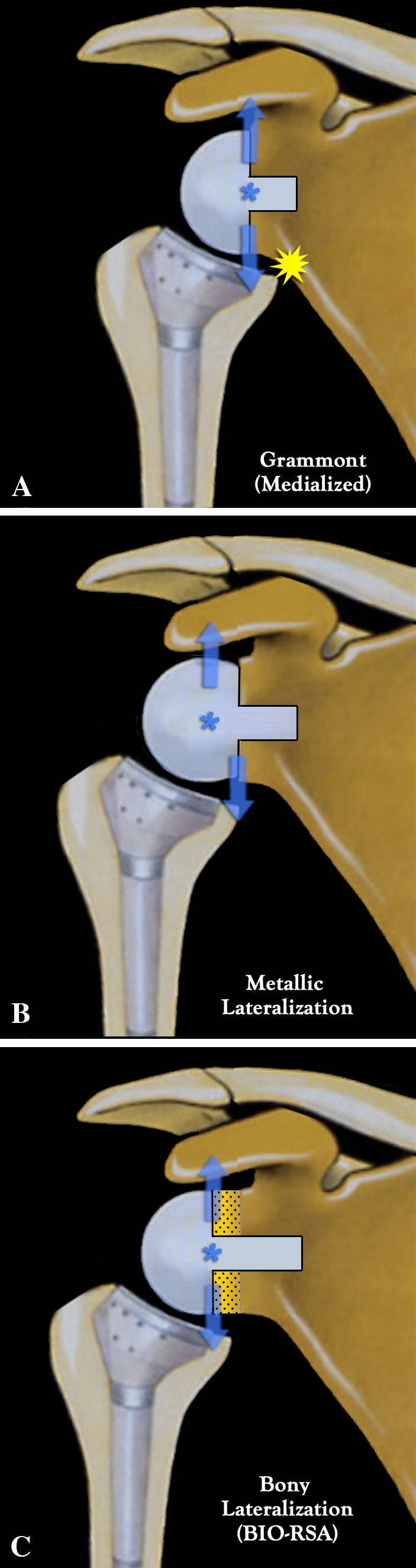
Diagrams show medialization versus lateralization in RSA. (A) Medialized (Grammont) RSA (hemisphere) places the center of rotation at the bone-prosthesis interface. Deltoid force applied to the center of rotation does not develop any torque because there is no lever arm, but there is a risk of scapular notching. (B) Metallic lateralized RSA (two-thirds of a sphere) reduces the risk of scapular notching but at the price of creating a lever arm because a lateralized center of rotation produces shear forces detrimental to glenoid fixation. (C) BIO-RSA reduces the risk of scapular notching (due to the lateralization), while maximizing glenoid fixation (because the center of rotation remains at the bone-prosthesis interface and there is no lever arm).
Our aim was to verify whether this novel surgical method would provide the benefits of lateralization without its potential drawbacks. Our hypotheses were (1) a cancellous bone graft, harvested from the humeral head during RSA, would heal to the native glenoid and thus would keep the center of rotation at the glenoid-baseplate interface and (2) bony lateralization of the RSA would be associated with lower rates of scapular notching and instability while allowing greater shoulder mobility in rotation.
Patients and Materials
Between March 2006 and March 2008, we treated 45 patients with cuff-deficient shoulders with RSA combined with glenoid bone grafting, using a structural bone graft harvested from the humeral head and a modified long-peg glenoid baseplate (BIO-RSA). Since our main goal was to determine the effect of bony lateralization with a humeral graft, we excluded patients who were treated with this procedure but who had severe glenoid bone deficiency in either the vertical plane (stage E3 of Favard et al. [11]) or horizontal plane (Type B2 and C of Walch et al. [39]); in these cases, glenoid bone grafting restored the normal glenohumeral joint line position but did not achieve lateralization. Patients who underwent the procedure using a bone graft other than autologous humeral head, such as iliac crest bone graft or allograft, were also excluded. Such patients had humeral deficiency precluding bone graft harvesting. Patients with humeral head necrosis and those requiring revision of failed hemi- or total shoulder arthroplasties are typical examples of this group.
Before surgery, all patients had exhausted nonoperative management, consisting of physical therapy for a minimum of 6 months and/or intra-articular corticosteroid injections. In 27 patients, the preoperative diagnosis was cuff tear arthritis; 12 patients had failed previous rotator cuff surgery with a pseudoparalyzed shoulder (eight failed rotator cuff repairs and four biceps tenodesis); and three had sequelae of proximal humeral fractures. No patient was lost to followup, but three patients died from unrelated conditions before the 2-year followup, leaving 42 patients available for complete functional, radiographic, and CT assessment. Thus, 42 shoulders in 42 patients represent the basis of this study. There were 28 women and 14 men, and the dominant side was affected in 32 cases. The average age of the patients at the time of surgery was 72 years (range, 52–86 years). The minimum followup was 24 months (mean, 28 months; range, 24–40 months).
The study protocol required the implantation of the same reverse geometry prosthesis in all patients. The Aequalis® Reversed Shoulder Prosthesis (Tornier Inc, Houston, TX) is a reverse ball-and-socket prosthesis designed according to Grammont’s principles [15, 16]. To accommodate the bone graft and facilitate stable fixation in the native glenoid, a specific baseplate with a lengthened central peg (25 mm) was designed and implanted in all cases. The circular baseplate is 29 mm in diameter, has a rough surface, and is hydroxyapatite coated (Fig. 2).
Fig. 2.
A photograph shows a standard baseplate with a 15-mm-long central peg (left) and a BIO-RSA baseplate with a lengthened, 25-mm-long peg (right) used for primary fixation of the bone graft in the native glenoid vault.
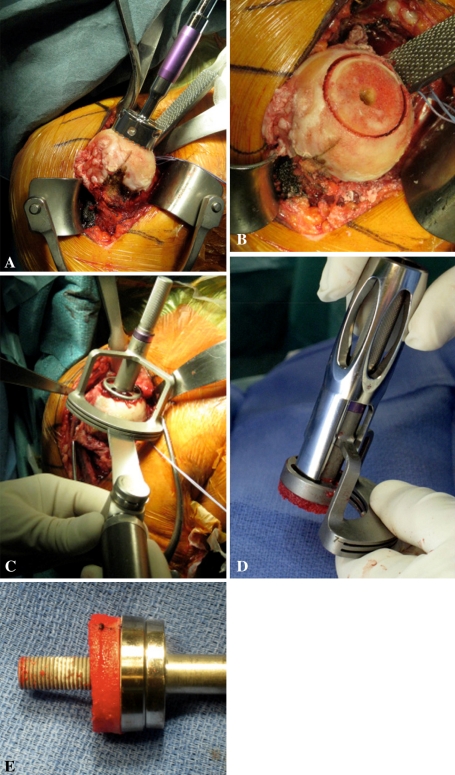
All operations were performed by the senior author (PB) or under his direction. The procedure was carried out under general anesthesia with an interscalene block in the beach chair position. Although feasible through an anterosuperior deltoid splitting approach, a deltopectoral approach was used. Any remaining subscapularis tendon was detached from the lesser tuberosity. If still present, the long head of the biceps tendon underwent tenodesis. The procedure started with the humeral osteotomy and graft harvesting. A 155° inclined humeral guide was placed at the summit of the humeral head and set parallel to the forearm axis (0°–30°, according to the transepicondylar axis) and a 2.5-mm threaded guidewire inserted in its axis. A 29-mm reamer, guided along the threaded guidewire, was used to flatten the humeral head until the subchondral bone was reached. A bell saw, also guided along the threaded guidewire, was used to create a cylinder of cancellous bone, 29 mm in diameter, corresponding to the dimensions of the baseplate (Fig. 3A–B). A cannulated drill, guided along the threaded guidewire, was used to bore a central hole, 8 mm in diameter. A cutting guide was inserted to harvest the desired thickness of bone graft (10 mm or 7 mm) (Fig. 3C). The disc of pure cancellous bone graft (Fig. 3D) was inserted along the lengthened central peg (Fig. 3E) and placed in a wet sponge on the back table.
Fig. 3A–E.
Photographs illustrate the cancellous bone graft being harvested from the humerus. (A) A bell saw is used to create a cylinder of cancellous bone. (B) The cylinder of cancellous bone is shown. (C) A cutting guide is inserted to harvest the desired thickness of bone graft. The disc of cancellous bone graft is (D) retrieved from the cutting guide and (E) inserted along the lengthened central peg of the baseplate.
The next step was the glenoid preparation. Achieving adequate glenoid exposure is essential to this procedure. A 29-mm circular glenoid guide was placed flush with the inferior border of the glenoid and a 2.5-mm threaded wire was inserted into the glenoid vault with either 0° or 10° of inferior tilt (in case of superior orientation of the native glenoid). The exposure and reaming of the glenoid are facilitated by the fact that only one small (29-mm) reamer is used to abrade the glenoid surface and there is no need to introduce large (36- and 42-mm) reamers inside the joint. The reamer flattened the glenoid surface until the subchondral plate was reached; this represents an approximate 5 mm deep reaming. The goal of the glenoid reaming was to reach the cancellous bleeding bone, while avoiding superior tilt of the baseplate. In the superior portion of the glenoid, where the bone is more dense and cortical, small (2.5-mm) drill holes were made using a threaded pin to obtain a complete bleeding bone surface (Fig. 4A) [1, 38]. The central hole was then drilled to 8 mm. The baseplate, with the harvested disc of bone graft, was then impacted into the center hole (Fig. 4B–C). Fixation was obtained using two 4.5-mm AO (anterior and posterior) convergent compressive screws and two 4.5-mm divergent locking screws (one inferior in the pillar and one superior in the base of the coracoid). The glenosphere was fixed to the baseplate via a Morse taper and countersunk set screw. The glenosphere diameter was chosen depending on the size of the humerus (patient). A 36-mm-diameter glenosphere was usually used in women, while the 42 mm was preferred in men. The disc of cancellous bone graft thickness was adjusted according to the size of the sphere. A 10-mm graft was used for a 36-mm sphere and a 7-mm graft for a 42-mm sphere since it is already more lateralized than the 36 mm.
Fig. 4A–C.
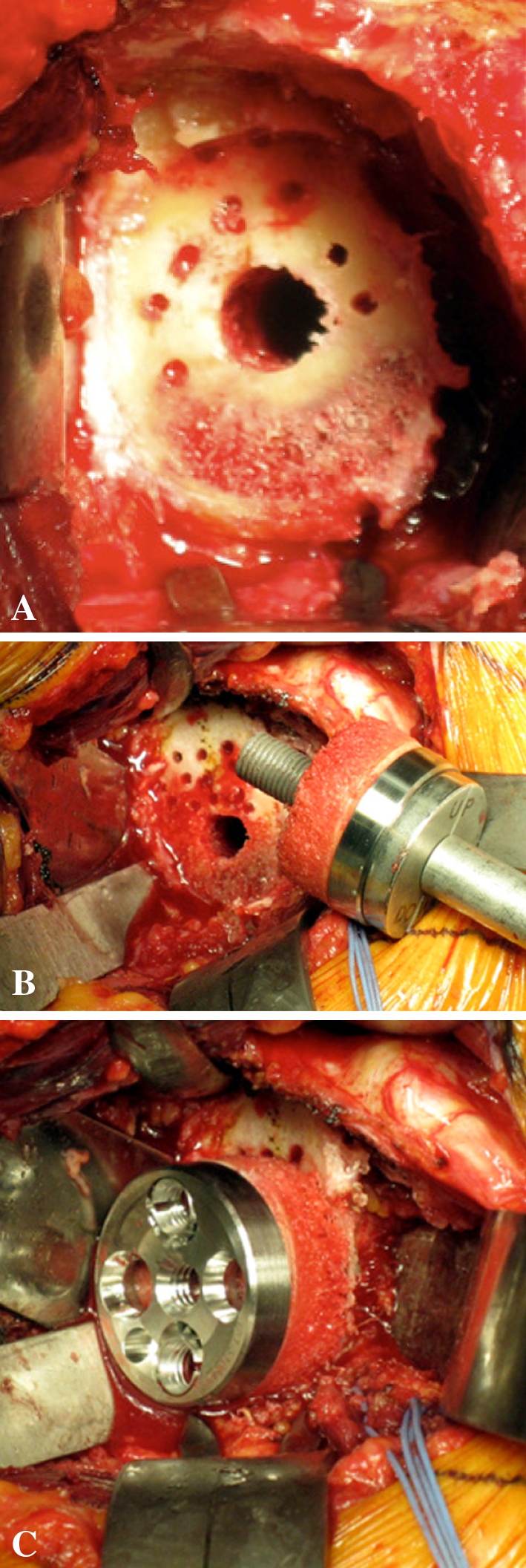
Photographs illustrate glenoid preparation. (A) The glenoid surface must be reamed until the subchondral plate is reached (this represents an approximate 5-mm deep reaming) and with some inferior tilt. In addition to glenoid reaming, small drill holes (2.5 mm) are made at the periphery of the glenoid to obtain a complete bleeding bone surface and the central hole is overdrilled with an 8-mm drill bit. (B) The baseplate, with the disc of cancellous bone graft inserted along the lengthened central peg. (C) It is impacted until it reaches the cancellous glenoid surface and then fixed with four (two compressive and two locking) screws.
The humeral step was performed using the standard surgical technique described for implantation of Aequalis® reversed prosthesis [19]. A bone hook was used to translate the humerus anteriorly. In primary cases, a polyethylene thicker than 6 mm was not required, as the prosthetic lateralization enhances stability and soft tissue tension.
Patients were discharged 1 or 2 days after surgery. A sling was worn during the first 4 weeks. The rehabilitation protocol used for the BIO-RSA was no different from that for a standard RSA. Self-directed rehabilitation with pendulum exercises started immediately (five times a day, 5 minutes each session). The patient was encouraged to immediately use his or her hand for activities of daily living, such as eating, drinking, holding a newspaper or a book, typewriting, dressing, etc. After 4 weeks, formal rehabilitation with a physiotherapist started. Aquatherapy in a swimming pool was recommended. No heavy lifting was allowed until 12 weeks to ensure solid bony union of the graft was obtained. Return to all types of activities, including gardening or leisure sports, was permitted after 3 to 6 months.
All patients were prospectively evaluated at 3, 6, and 12 months and yearly thereafter. The Constant-Murley functional score was measured preoperatively and at each review [8, 10, 28]. The ROM measured consisted of forward elevation in the scapular plane, external rotation, and internal rotation. Strength was measured using a handheld dynamometer with the arm elevated 90° in the scapular plane. Episodes of instability (prosthetic subluxation or dislocation) during the followup period were recorded. Patients were asked to estimate the value of their shoulder as a percentage of an entirely normal shoulder preoperatively and at last followup [14].
Postoperative radiographs and CT scans with minimum 2 years of followup were performed for all 42 patients. Two subspecialty-trained shoulder surgeons (GM, YR) not associated with the surgery reviewed all radiographs and CT scans. We made no attempt to determine the reliability of the observations; when differences in assessments were noted, the two observers reached a consensus. Postoperative plain radiographic assessment was performed using fluoroscopy and magnification control, according to the recommendations of Lévigne et al. [21], to align the central radiographic beam and be tangent to the posterior surface of the baseplate. Analysis of CT scans was performed using the Osirix® software (Pixmeo, Geneva, Switzerland). Radiographs and CT scans were examined by both surgeons for (1) bone graft healing (absence of lucent line observed between humeral bone graft and native glenoid), (2) bone graft resorption or lysis (bone graft disappearance), (3) glenoid component partial fixation (lucent lines under glenoid baseplate and around the central peg or screws), (4) glenoid component loosening (lucent lines greater than 1 mm under the glenoid or around peg/screws, hardware migration, shift, or breakage), (5) inferior scapular notching (graded according to the classification system of Sirveaux et al. [32]), and (6) inferior glenoid osteophytes or spurs at the level of the pillar. Sirveaux et al. [32] described four grades of notching: in Grade 1, the defect involves only the scapular pillar and does not extend as far as the inferior most screw; in Grade 2, the notch contacts the inferior-most baseplate screw; in Grade 3, the notch extends beyond the inferior most screw; and in Grade 4, the notch extends as far as the central peg of the baseplate. CT scans were also evaluated for anterior or posterior scapular notching using the axial slices. Any radiographic or CT abnormality identified by either surgeon was considered present and recorded.
The distribution of data was analyzed with the d’Agostino-Pearson test. The pre- and postoperative patient scores and measurements of shoulder mobility were analyzed for differences between means; paired observations were compared using a paired t test and unpaired observations were compared with the Mann-Whitney test. The chi square test and Fisher’s exact test for small numbers were used to compare categorical data. Statistical analysis was performed with MedCalc software 11.0 (MedCalc Software, Mariakerke, Belgium).
Results
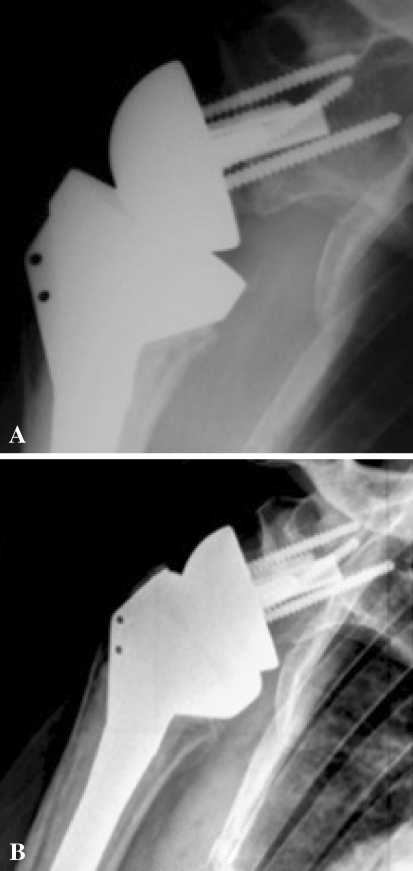
Radiographs and CT scans showed the disc of cancellous bone graft healed to the native glenoid in 98% (41 of 42) of cases (no lucent line observed between humeral bone graft and native glenoid) (Fig. 5). One patient had a partial radiolucent line between the graft and the native scapula, located only above the central peg, but no radiolucent line was observed at the inferior part of this graft. No graft resorption or lysis under the baseplate was observed in any patients.
Fig. 5A–B.
(A) An AP radiograph performed 3 months after surgery demonstrates complete bone graft healing. (B) No bone graft resorption or lysis and no scapular notching are observed at 36 months’ followup. Note the low and inferiorly tilted positioning of the glenoid implant in addition to its lateralization.
There were no patients with glenoid component loosening at latest followup. No lucent lines around the peg or screws and no screw breakage were observed in any case. We found no radiographic evidence of any humeral-side component loosening or failure.
Inferior scapular notching was observed in 19% (eight of 42) of the latest radiographs and CT scans. The notch was classified as Grade 1 in five cases, Grade 2 in two cases, and Grade 3 in one case. An inferior osteophyte (spur) located at the level of the pillar was observed in 17 cases. On CT scans, a posterior notch was observed in three cases, isolated in two cases, and associated with an inferior notch in one. Anterior scapular notching was not encountered.
Each parameter of the Constant-Murley score increased (Table 1). The gain in active mobility was 60° for anterior active elevation, 10° for active external rotation, and 1.3 points for internal rotation (Fig. 6). One patient had negative external rotation with the arm at the side because of absent infraspinatus and teres minor. Mean active external rotation in abduction was 53° (range, 10°–90°). Thirty-six patients (86%) were able to internally rotate at least to the sacrum (Fig. 7).
Table 1.
Functional results
| Parameter | Preoperative | Postoperative | Gain | p Value (test) |
|---|---|---|---|---|
| Active mobility | ||||
| AAE (°) | 86 ± 35 (20–160) | 146 ± 21 (80–170) | +60 (−20–130) | < 0.0001 (MWT) |
| AER (°) | 12.8 ± 21.4 (−40–50) | 22.9 ± 16.4 (−10–70) | +10 (−20–60) | < 0.01 (t test) |
| AIR (level) | 4.4 ± 2.2 (0–10) | 5.7 ± 2.5 (0–10) | +1.3 (−10–10) | < 0.031 (MWT) |
| Constant-Murley score | ||||
| Pain (of 15 points) | 5.5 ± 3.4 (0–15) | 12.7 ± 2.8 (5–15) | +7 (−10–15) | < 0.0001 (MWT) |
| Activities (of 20 points) | 7 ± 3.3 (5–12) | 16.5 ± 2.9 (10–20) | +9.5 (−2–18) | < 0.0001 (t test) |
| ROM (of 40 points) | 17 ± 9.6 (4–38) | 30.4 ± 5.8 (14–40) | +13 (−8–30) | < 0.0001 (MWT) |
| Strength (of 25 points) | 1.8 ± 4.9 (0–10) | 7 ± 3.8 (0–18) | +5 (−3–18) | < 0.0001 (MWT) |
| Absolute score (of 100 points) | 31 ± 13 (8–60) | 66.6 ± 11 (31–88) | +35 (−15–64) | < 0.0001 (MWT) |
| Adjusted score (%) | 44 ± 18.4 (13–87) | 93.4 ± 15.6 (48–136) | 49 (−22–100) | < 0.0001 (MWT) |
| Subjective shoulder value (/100%) | 34 ± 16 (10–80) | 75 ± 14 (30–95) | +41 (−15–70) | < 0.0001 (MWT) |
Values are expressed as mean ± SD, with range in parentheses; AAE = active anterior elevation; AER = active external rotation; AIR = active internal rotation; MWT = Mann-Whitney test.
Fig. 6A–C.
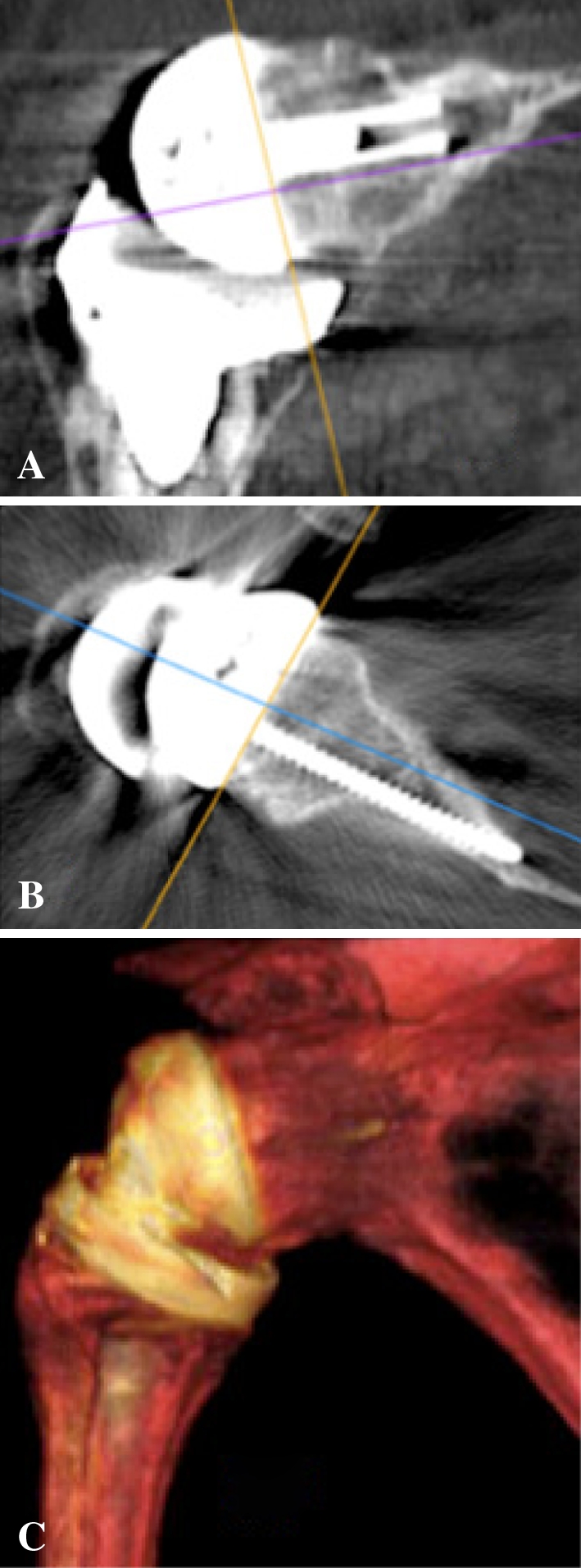
CT scans at 38 months’ followup show bone graft healing in both (A) coronal and (B) axial planes. (C) A three-dimensional reconstruction demonstrates the lengthened scapular neck obtained after bone graft healing. The bony lateralization offers the advantage of keeping the humeral cup away from the pillar of the scapula, thus decreasing the risk of impingement while keeping the center of rotation within the bone.
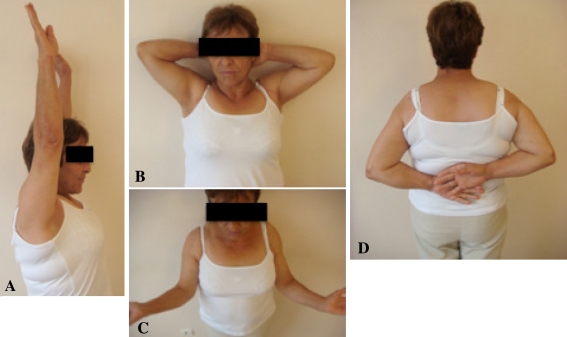
Fig. 7A–D.
Photographs of a 70-year-old patient 29 months after BIO-RSA performed on the right shoulder show (A) active anterior elevation of 170°, (B) elevation and external rotation, (C) external rotation of 45°, and (D) internal rotation to L1. Notice the shoulder contour which is similar to the contralateral side.
No patient had a dislocation or subluxation. None was revised or reoperated during the period of followup. One patient had a temporary postoperative brachial plexus palsy with complete recovery at 6 months.
Discussion
Inferior scapular notching, prosthetic instability, limited postoperative shoulder rotation, and loss of shoulder contour have all been attributed to humeral medialization after RSA [5–7, 11, 15, 16, 23, 30, 32, 33]. We report our experience with bony lateralization of RSA, an option we propose to address the shortcomings of humeral medialization. The principle of BIO-RSA is, once the bone graft has healed to the native scapula, the articular center of rotation is maintained at the bone-prosthesis interface. Baseplate fixation is maximized while scapular notching is minimized, retaining advantages of both medialization and lateralization. Our aim was to verify whether this novel surgical method would provide the benefits of lateralization without its potential drawbacks.
Our study has a number of limitations. No control group was used and only short-term results are reported. A concern exists that bone graft resorption may occur with longer follow-up. This has motivated us to closely follow all patients, both with sequential radiographs (at 3, 6, and 12 months and yearly) and CT scans performed at a minimum of 24 months postoperatively. To date, all autografts have clearly incorporated on plain radiographs and CT scans (Figs. 5, 6). We believe the methodology used to assess graft incorporation was a strength of our study. Our results cannot be compared with those of structural allografts, which are known to fail with time, or with those of bone grafting in nonconstrained anatomic shoulder prostheses, where a detrimental shear force is generated during abduction and may be responsible for graft failure or resorption [2, 20, 25]. Due to the fact that autologous graft has been used in our technique and that the graft is placed under compressive forces, there is no reason to believe resorption or lysis will occur with extended follow-up.
Our study confirms our two hypotheses: (1) a cancellous bone graft, harvested from the humeral head during RSA, does heal to the native glenoid with no radiographic evidence of baseplate failure observed at 28 months’ followup and (2) bony lateralization of RSA achieves lower rates of scapular notching when compared to the standard medialized Grammont design. Active elevation and shoulder rotation are preserved. Shoulder contour is improved and no prosthetic instability has been observed. The functional results are equivalent to or even better than those reported with the standard medialized Grammont RSA for cuff tear arthritis (Table 2) [7, 12, 13, 19, 24, 33, 37, 40].
Table 2.
Comparison of the results of BIO-RSA with those of Grammont (medialized) RSA
| Parameter | Grammont RSA (medialized) (Molé and Favard [24]) | BIO-RSA (lateralized) (Boileau et al.) |
|---|---|---|
| Number of cases | 484 | 42 |
| Etiology | CTA, MRCT | CTA, MRCT, FS |
| Followup (months) | 52 | 38 |
| Constant-Murley (points) | 62 | 66 |
| Active anterior elevation (°) | 130 | 146 |
| Active external rotation (°) | 13 | 23 |
| External rotation in abduction (°) | 42 | 53 |
| Active internal rotation > S1 (%) | 26 | 86 |
| Scapular notching (%) | 68 | 19 |
| Prosthetic instability (%) | 3.4 | 0 |
| Glenoid loosening (%) | 3.6 | 0 |
BIO-RSA = bony increased-offset reversed shoulder arthroplasty; RSA = reverse shoulder arthroplasty; CTA = cuff tear arthritis; MRCT = massive rotator cuff tear; FS = fracture sequelae.
Metallic lateralization, increasing the offset of the glenosphere and/or baseplate, is an alternative approach but has the disadvantage of increasing torque or shear force application to the glenoid component [5, 18]. Historically, clinical experience (in the 1970s and 1980s) with lateralized offset prostheses has been disastrous, with a high rate of glenoid loosening and screw breakage witnessed, leading to abandonment of the design [5, 14, 35]. Recently, metallic prosthetic lateralization has been revisited by different authors [5, 18]. Frankle et al. [12] in their initial series of increased-offset RSAs demonstrated the beneficial effects of lateralization in reducing scapular notching. A 12% rate of glenoid loosening was reported after a mean of 21 months, all requiring revision. This rate was higher than the 2% to 5% reported at medium- to long-term followup with the Grammont design [6, 7, 32, 36, 37, 40, 41]. The greater risk of baseplate failure after increased-offset reversed prostheses has been anticipated by biomechanical studies [18]. Harman et al. [18] found, during eccentric loading, the motion of a +7-mm increased-offset baseplate was four times greater than that observed with the Grammont medialized prosthesis. The results of both clinical and biomechanical studies led Frankle et al. to modify their initial lateral offset design, using 5-mm locking screws to increase baseplate stability and enhance glenoid component fixation [18]. Cuff et al. [9] reported encouraging early clinical results using such a design. Our belief is, for long-term prosthetic survival, the biomechanical principle of bony lateralization is preferable to that of metallic prosthetic lateralization.
Although the BIO-RSA does not entirely solve the problem of scapular notching, our 19% rate of inferior scapular notching is lower than that reported in the literature with a standard medialized RSA [5–7, 11, 13, 15, 16, 23, 30–33], even accounting for the fact that more sensitive detection techniques have been used (CT scanning). It may be argued the prevalence and severity of inferior scapular notching increase over time. Experience to date with our current technique is that notches are typically evident on imaging studies at 6 months postoperatively and do not show progression, at least at a minimum followup of 2 years [13, 21]. A potential argument is that inferior placement of the glenosphere prevents scapular notching with standard RSA [17, 27]. However, recent studies have suggested that this technique alone is not sufficient to avoid notching [24, 40]. Based on our experience, low positioning of the glenosphere (flush to the inferior glenoid rim) and inferior tilt (with the help of asymmetrical reaming and/or the use of asymmetrical bone graft), together with lateralization, are the optimal configuration to reduce the risk of inferior scapular notching and to provide favorable compressive forces on the glenoid bone graft [4, 15, 23].
In conclusion, BIO-RSA improves the results obtained with conventional medialized design RSA. Bony lateralization of the center of rotation in RSA reduces the rate of inferior scapular notching without increasing the risk of glenoid component failure. Healing of the humeral bone graft on the native glenoid is consistently observed, effectively creating a scapula with a long neck. In addition, BIO-RSA, like any lateralized RSA, has the potential to improve shoulder mobility (because of greater clearance for the humeral cup around the glenoid sphere), shoulder stability (because of improved tension of the deltoid and remaining cuff), and shoulder contour. In contrast to metallic increased-offset RSA, BIO-RSA offers the advantage of maintaining the joint center of rotation at the prosthesis-glenoid interface, thereby minimizing torque on the glenoid component. Although ongoing clinical and radiographic assessment is mandated, our results to date encourage us to continue to perform this procedure enthusiastically in primary RSA with preserved humeral bone stock. The procedure can also be performed in case of glenoid bone loss to restore the glenoid bone stock.
Footnotes
One author (PB) declares a commercial association (Tornier) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abrassart S, Stern R, Hoffmeyer P. Arterial supply of the glenoid: an anatomic study. J Shoulder Elbow Surg. 2006;15:232–238. doi: 10.1016/j.jse.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Antuna S, Sperling JW, Cofield RH. Reimplantation of a glenoid component after component removal and allograft bone grafting: a report of 3 cases. J Shoulder Elbow Surg. 2002;11:637–641. doi: 10.1067/mse.2002.126100. [DOI] [PubMed] [Google Scholar]
- 3.Baulot E, Chabernaud D, Grammont PM. Results of Grammont’s inverted prosthesis in omarthritis associated with major cuff destruction: apropos of 16 cases [in French] Acta Orthop Belg. 1995;61(Suppl 1):112–119. [PubMed] [Google Scholar]
- 4.Boileau P, Chuinard C, Roussanne Y, Bicknell RT, Rochet N, Trojani C. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res. 2008;466:584–593. doi: 10.1007/s11999-008-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. doi: 10.3928/0147-7447-20020201-16. [DOI] [PubMed] [Google Scholar]
- 8.Constant CR, Murley AH. A clinical method for functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 9.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90:1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 10.Ellman H, Hanker G, Bayer M. Repair of the rotator cuff: end-result study of factors influencing reconstruction. J Bone Joint Surg Am. 1986;68:1136–1144. [PubMed] [Google Scholar]
- 11.Favard L, Lautmann S, Sirveaux F. Hemi arthroplasty versus reverse arthroplasty in the treatment of osteoarthritis with massive rotator cuff tear. In: Walch G, Boileau P, Mole D, editors. 2000 Shoulder Prostheses: Two to Ten year Follow-up. Montpellier, France: Sauramps Medical; 2001. pp. 261–268. [Google Scholar]
- 12.Frankle M, Levy JC, Pupello D, Siegal S, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency: a minimum two-year follow-up study of sixty patients: surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1):178–190. doi: 10.2106/JBJS.F.00123. [DOI] [PubMed] [Google Scholar]
- 13.Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2009;17:284–295. doi: 10.5435/00124635-200905000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the Constant score. J Shoulder Elbow Surg. 2007;16:717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 15.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 16.Guéry J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. doi: 10.2106/JBJS.E.00851. [DOI] [PubMed] [Google Scholar]
- 17.Gutiérrez S, Luo ZP, Levy J, Frankle MA. Arc of motion and socket depth in reverse shoulder implants. Clin Biomech (Bristol, Avon) 2009;24:473–479. doi: 10.1016/j.clinbiomech.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14(1 Suppl S):162S–167S. doi: 10.1016/j.jse.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Hatzidakis AM, Norris TM, Boileau P. Reverse shoulder arthroplasty: indications, techniques, and results. Tech Shoulder Elbow Surg. 2005;6:135–149. doi: 10.1097/01.bte.0000169730.36840.4b. [DOI] [Google Scholar]
- 20.Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. doi: 10.1302/0301-620X.83B8.11709. [DOI] [PubMed] [Google Scholar]
- 21.Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. In: Walch G, Boileau P, Molé D, Favard L, Lévigne C, Sirveaux F, editors. Reverse shoulder arthroplasty. Montpellier, France: Sauramps Medical; 2006. pp. 353–372. [Google Scholar]
- 22.Matsen FA, 3rd, Boileau P, Walch G, Gerber C, Bicknell RT. The reverse total shoulder arthroplasty. Instr Course Lect. 2008;57:167–174. [PubMed] [Google Scholar]
- 23.Middernacht B, Roo PJ, Maele G, Wilde LF. Consequences of scapular anatomy for reversed total shoulder arthroplasty. Clin Orthop Relat Res. 2008;466:1410–1418. doi: 10.1007/s11999-008-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molé D, Favard L. Excentred scapulohumeral osteoarthritis [in French] Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 Suppl):37–94. doi: 10.1016/s0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 25.Neyton L, Boileau P, Nove-Josserand L, Edwards TB, Walch G. Glenoid bone grafting with a reverse design prosthesis. J Shoulder Elbow Surg. 2007;16(3 Suppl):S71–S78. doi: 10.1016/j.jse.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Nové-Josserand L, Walch G. Instability after reverse shoulder arthroplasty. In: Walch G, Boileau P, Molé D, Favard L, Lévigne C, Sirveaux F, editors. Reverse shoulder arthroplasty. Montpellier, France: Sauramps Medical; 2006. pp. 81–101. [Google Scholar]
- 27.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14:524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Richards RR, An KN, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, Iannotti JP, Mow VC, Sidles JA, Zuckerman JD. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 29.Seebauer L. Total reverse shoulder arthroplasty: European lessons and future trends. Am J Orthop. 2007;36(Suppl 1):22–28. [PubMed] [Google Scholar]
- 30.Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy. Oper Orthop Traumatol. 2005;17:1–24. doi: 10.1007/s00064-005-1119-1. [DOI] [PubMed] [Google Scholar]
- 31.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. doi: 10.2106/JBJS.F.00226. [DOI] [PubMed] [Google Scholar]
- 32.Sirveaux F, Favard L, Oudet D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive cuff rupture of the cuff: results of a multicenter study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 33.Sirveaux F, Favard L, Oudet D, Huguet D, Lautman S. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive and non repairable cuff rupture. In: Walch G, Boileau P, Mole D, editors. 2000 Shoulder Prostheses: Two to Ten Year Follow-up. Montpellier, France: Sauramps Medical; 2001. pp. 247–252. [Google Scholar]
- 34.Terrier A, Reist A, Merlini F, Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J Bone Joint Surg Br. 2008;90:751–756. doi: 10.1302/0301-620X.90B6.19708. [DOI] [PubMed] [Google Scholar]
- 35.Valenti P, Sauzieres P, Cogswell L, O’Toole G, Katz D. The reverse shoulder prosthesis: surgical technique. Tech Hand Up Extrem Surg. 2008;12:46–55. doi: 10.1097/BTH.0b013e3181572b14. [DOI] [PubMed] [Google Scholar]
- 36.Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: long term results (> 5 years) In: Walch G, Boileau P, Mole D, editors. 2000 Shoulder Prostheses: Two to Ten Year Follow-up. Montpellier, France: Sauramps Medical; 2001. pp. 253–259. [Google Scholar]
- 37.Vanhove B, Beugnies A. Grammont’s reverse shoulder prosthesis for rotator cuff arthropathy: a retrospective study of 32 cases. Acta Orthop Belg. 2004;70:219–225. [PubMed] [Google Scholar]
- 38.Schroeder HP, Kuiper SD, Botte MJ. Osseous anatomy of the scapula. Clin Orthop Relat Res. 2001;383:131–139. doi: 10.1097/00003086-200102000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14:756–760. doi: 10.1016/S0883-5403(99)90232-2. [DOI] [PubMed] [Google Scholar]
- 40.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 41.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]