Abstract
Multivesicular bodies (MVBs) are endosomes that have internalized portions of the limiting membrane into the compartment, thereby forming intralumenal vesicles. This vesicle formation is unusual in that it is directed away from the cytoplasm, which requires a unique mechanism unlike any mechanism described for other vesicle formation events. The best contenders for the machinery that drives MVB vesicle formation are the ESCRT protein complexes. However, increasing evidence suggests that lipids may play a key role in this membrane-deformation process. This review attempts to combine the seemingly contradictory findings into a MVB vesicle formation model that is based on a lipid-driven and ESCRT-regulated mechanism.
Introduction
Multivesicular bodies are late endosomal structures formed when portions of the limiting membrane invaginate and bud into the lumen of the compartment to form intralumenal vesicles (ILVs). During this process, a subset of transmembrane proteins and lipids are sorted into these vesicles. Upon fusion with lysosomes/vacuoles, the ILVs are exposed to the hydrolytic lumen of the compartment, causing the degradation of both ILV cargo proteins and lipids [1,2]. Therefore, the MVB pathway represents a major transmembrane protein and lipid turnover system in eukaryotic cells. For the majority of membrane proteins studied, the sorting into lumenal vesicles of MVBs is effected by ubiquitination, the addition of the small protein ubiquitin to lysine residues of target proteins. These ubiquitin tags are recognized by a group of protein complexes collectively called ESCRTs (Endosomal Sorting Complex Required for Transport), which bind to ubiquitinated cargoes and ensure their proper sorting into the forming ILVs [3–5]. Four distinct ESCRT protein complexes have been identified, each orchestrating a discrete step in MVB vesicle formation. ESCRT-0, together with flat clathrin coats, forms a protein network on endosomal membranes, capturing ubiquitinated cargo proteins and initiating their sorting into the MVB pathway [6–9]. ESCRT-I binds to both ESCRT-0 and to ubiqutinated cargo proteins, suggesting that it functions as an additional cargo sorting system [10–13]. ESCRT-I also interacts with ESCRT-II [14,15], which is important for downstream ESCRT-II functions, specifically in initiating ESCRT-III recruitment and assembly [16–18]. ESCRT-III is comprised of several subunits, a subset of which forms linear polymers that have been implicated in cargo trapping, membrane deformation and vesicle abscission [19–24]. The final step of MVB vesicle formation requires the Vps4 complex. This ATPase dissociates ESCRT-III, which is essential for the recycling of the ESCRT machinery for subsequent rounds of sorting and may also be involved in the scission of the forming vesicle [21,25–27].
Several recent reviews have highlighted structural elements of the ESCRT machinery and how they function in MVB sorting, cytokinesis, viral budding and autophagy [5,28–32]. This review will focus on a major, unresolved issue within the MVB field: the physical mechanism of MVB vesicle formation. For simplicity, this review will use yeast nomenclature when referring to ESCRT factors.
Different mechanisms for ILV formation?
Several models have been proposed that attempt to explain how endosomal membranes deform away from the cytoplasm and detach in order to form ILVs [3,33–36]. These different models are based on seemingly contradictory observations from both in vivo and in vitro experiments. For example, studies in yeast have established an essential role for the ESCRT machinery in ILV formation [37,38]. Mammalian cells, however, have been shown to maintain the ability to form ILVs in the absence of key ESCRT components [39]. Furthermore, melanosomes and exosomes (endosomal structures with MVB-like morphology) have been shown to form independently of ESCRT function [40,41]. These observations led to the conclusion that higher eukaryotes utilize the established ESCRT system as understood in yeast, and possibly additional ESCRT-independent pathways to form ILVs. These ‘unconventional’ pathways seem to be driven by the presence of certain lipids, such as lysobisphosphatidic acid (LBPA) and ceramides. These lipids might organize into specialized endosomal regions that, simply because of the local lipid composition, bend inward and ultimately form vesicles. In vitro studies supported this notion by demonstrating that only a pH gradient across the membrane is required to form ILVs in LBPA-containing liposomes [42]. The observation that lipids are sufficient for ILV formation poses two important questions: (1) do cells indeed employ different mechanisms to form ILVs and (2) if this is not the case, what is the contribution of the ESCRTs to ILV formation?
A problem with testing any model involving ILV formation is the lack of the proper tools for studying membrane-based systems. Although we are able to analyze the overall lipid and protein composition of an organelle, current methods are incapable of discerning the composition of small, specialized regions of organelle membranes in vivo. Furthermore, in vitro systems that attempt to recapitulate membrane-based reactions are difficult to control and are likely to produce artifacts. Thus, it is difficult to determine the contributions of proteins in membrane-based cellular functions, a quandary not unique to the MVB field. This problem is further compounded by the fact that most in vivo observations of membrane morphology changes (tubulation, vesiculation, fusion) can be mimicked in vitro in the absence of proteins, simply with liposomes containing the proper lipid composition. Proteins, therefore, seem to be inessential in inducing many membrane topology changes; they seem to function mainly in ensuring specificity and efficiency of membrane-based reactions. Similarly, the ESCRTs may not drive MVB vesicle formation but could serve to ensure proper composition of the vesicles (i.e. containing the proper cargo) in addition to coordinating the overall maturation of the endosome. In support of this model, a study in mammalian cells found that the absence of the ESCRT machinery did not block the formation of MVB vesicles, but resulted in impaired cargo sorting into ILVs and variations in ILV number and size [39]. Other studies have questioned the necessity of key ESCRT factors in ILV formation. In yeast, for example, overexpression of ESCRT-II partially suppresses the MVB defect of an ESCRT-I mutant strain, demonstrating that ESCRT-I is not an essential factor in MVB cargo sorting and vesicle formation [16]. In mammalian cells, ESCRT-II, but not ESCRT-I, has been shown to be dispensable for the downregulation of MVB cargoes, arguing that ESCRT-II is a non-essential MVB factor [43]. Finally, genomic analyses of protozoan parasites indicate that these organisms express homologues of the ESCRT-III subunits and Vps4, but none of the genes encoding ESCRT-I or ESCRT-II [44].
ILV formation is lipid-driven
Based on the published data, it seems plausible that all observed cases of ILV formation rely principally on lipid-driven mechanisms, and that the ESCRTs, in the case of MVB sorting, function in regulating the lipid-based reaction and coupling it to cargo sorting. These two mechanistic facets yield MVB vesicles that are uniform in size and contain the appropriate membrane proteins destined for degradation. ESCRT-independent ILV budding reactions, such as those observed during maturation of melanosomes, might be the consequence of specialized cargo proteins that self-organize, together with the appropriate lipids, in order to form vesicles.
A recent publication seems to challenge this lipid-driven mechanism of ILV formation. This in vitro study used giant unilamellar vesicles (GUVs) and purified proteins in order to identify the essential factors involved in ILV production [45]. These experiments found no particular lipid to have an essential role in ILV formation. On the contrary, recombinant ESCRT-I and ESCRT-II together were implicated in driving membrane deformation, while ESCRT-III was shown to be important for abscission of the forming vesicles. This GUV-based assay, however, was not able to recapitulate cargo sorting into ILVs. Furthermore, the ILVs formed in the assay were not uniform, and roughly ten-fold larger than MVB vesicles found in vivo. These discrepancies between the in vitro and in vivo systems are indeed significant and could indicate the lack of an important factor in the in vitro assay, such as a specialized lipid domain within the GUV membrane.
Though the in vitro assay of Wollert et al. was unable to fully reconstitute MVB vesicle formation [45], the experiments provided important insights into the mechanism of vesicle budding. A key observation from the in vitro system involved the position of the ESCRT factors with respect to the forming ILVs. All of the ESCRTs were shown to remain at the bud neck and did not enter the forming vesicle. This suggests that the ESCRTs, unlike COP and clathrin coats, are not physically inducing the deformation of the membrane; rather, they likely function in stabilizing the neck that forms as a consequence of a lipid-driven, inward budding reaction. This stabilization role of the ESCRTs might support vesicle formation by capturing intermediates and thus inhibiting the reversal of the membrane budding reaction. The localization of the ESCRTs to the bud neck is consistent with the in vivo observation of ESCRT recycling (as opposed to entrapment of ESCRTs inside the ILVs) and explains how the large ESCRT protein complexes, which are in the size range of the final vesicle (~25 nm in yeast), are able to support vesicle formation.
ESCRT-III and Vps4: the membrane fission machinery
Homologues of Vps4 and ESCRT-III subunits function in archaea to orchestrate the membrane fission reaction that completes cell division [46–48]. Similarly mammalian ESCRTs function in the final steps of cytokinesis [49–51]. Furthermore, many enveloped viruses require these late-acting ESCRT factors to detach newly formed virus particle from the host membrane (reviewed in [29]. These observations strongly suggest a role for ESCRT-III and Vps4 in the final step of MVB vesicle formation: scission of the vesicle neck.
ESCRT-III subunits have been shown to assemble into large filamentous structures in vitro [19,21,23]. In vivo, artificially high levels of the ESCRT-III subunit Snf7 cause the formation of curved polymeric spirals on membranes [20]. These Snf7 spirals are able to induce negative curvature in the underlying membrane, giving rise to membrane tubules that are extruded away from the cytoplasm. This effect of ESCRT-III polymers on membranes can result in ILV formation, as was shown in another GUV-based in vitro assay [24]. Contrary to this idea, a recent in vitro study contends that ESCRT-III is not involved in deformation of the membrane, but exclusively in the scission of the bud neck [45]. This argument was based on the observation that, when added to the GUVs, ESCRT-III was recruited to the ESCRT-I/ESCRT-II-containing necks of forming vesicles. This recruitment resulted in the scission of vesicles from the limiting membrane. Studies in yeast, however, have shown that ESCRT-III facilitates the localization of the deubiqutinating enzyme Doa4 to MVBs, where this enzyme removes the ubiquitin tag from cargo prior to sorting into forming ILVs [52–54]. These data indicate that ESCRT-III acts earlier in the vesicle formation process, at a point when cargo is still accessible for Doa4. Thus, the in vitro assay may have revealed only the essential function of ESCRT-III in ILV formation: facilitating vesicle release. Because cargo sorting was not recapitulated in the in vitro assay, additional ESCRT-III functions with regard to vesicle composition might have been missed. Similarly, the in vitro study might have missed some of the subtler functions of the ATPase Vps4. In the in vitro assay, Vps4 seemed to act solely in the disassembly and recycling of ESCRT-III following completion of ILV formation [45]. However, electron microscopy studies in yeast revealed that deletions of Vps4 co-factors that are known to negatively impact Vps4 activity resulted in enlarged MVB vesicles, suggesting that Vps4 plays an active role in the formation of ILVs [38]. This observation could be explained by a model in which the disassembly function of Vps4 drives the constriction of ESCRT-III polymers, ultimately resulting in the scission of the bud neck. Alternatively, Vps4 might be involved during ESCRT-III assembly, assisting the formation of the proper ESCRT-III structure.
A model for ILV formation
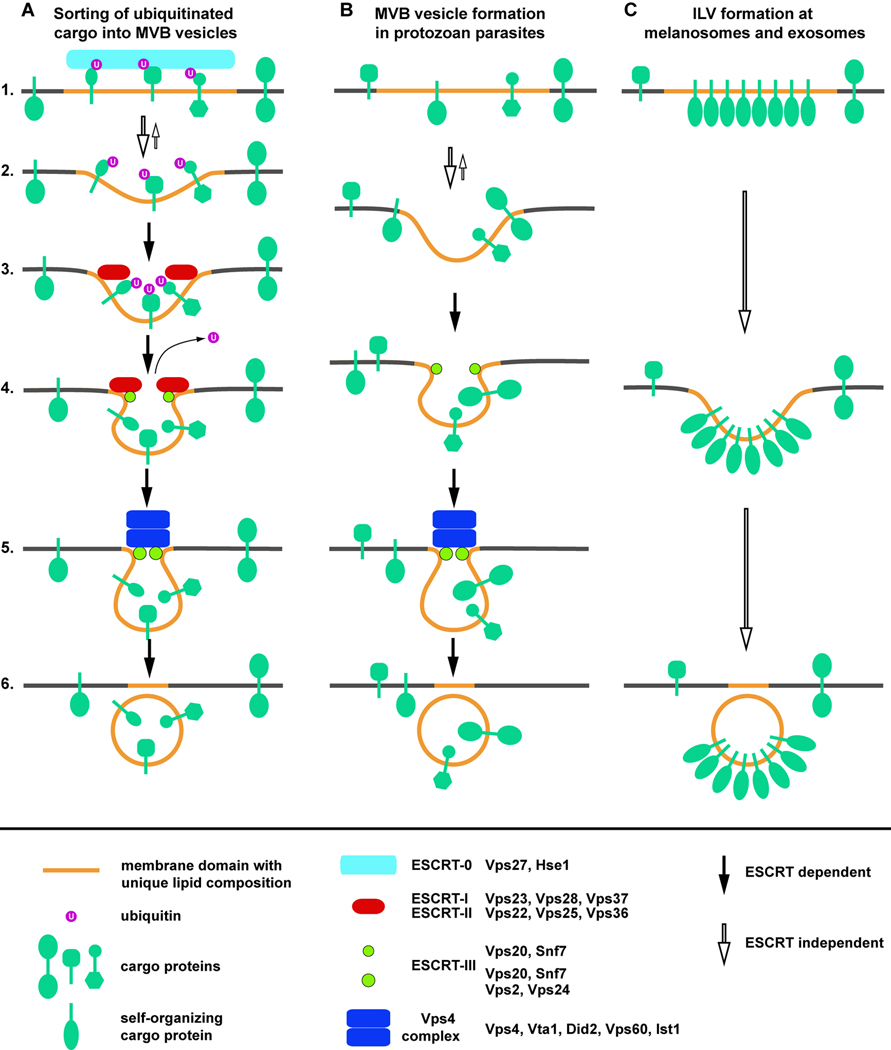
Taking into account all the published data, a model for ILV formation emerges that combines lipid-driven membrane deformation with ESCRT-mediated specificity and efficiency. In this model, endosomal membrane constituents spontaneously form specialized lipid domains (or lipid rafts) with unique local composition. Studies on endosomal membranes have suggested that late endosomes contain lipid rafts rich in cholesterol and sphingomyelin [55], and might also contain LBPA and phosphatidylinositol-3-phosphate (PI-3P). MVB vesicles have been shown to be enriched in cholesterol and LBPA, consistent with the idea that ILVs are formed from these lipid rafts [56,57]. The model predicts that ESCRT-0 binds to the lipid rafts and sorts ubiquitinated membrane proteins into these domains (Figure 1A, step 1). Then, driven by the unique lipid composition and possibly the pH gradient across the membrane, the lipid-cargo domain deforms and invaginates (Figure 1A, step 2). The presence of certain lipids (including PI-3P), ubiquitinated cargoes and the curved membrane topology recruits ESCRT-I and ESCRT-II, which assemble into a network that stabilizes the membrane neck and prevents the reversal of the membrane deformation (Figure 1A, step 3). ESCRT-II present in this network will recruit Vps20 and Snf7, subunits of the ESCRT-III complex that will polymerize and recruit Doa4 for cargo deubiquitination (Figure 1A, step 4). Together with the remaining two subunits, Vps2 and Vps24, the forming ESCRT-III polymer further stabilizes and narrows the neck of the forming vesicle. Recruitment of Vps4 results in the fully assembled ESCRT-III – Vps4 scission machinery (Figure 1A, step 5) that releases the vesicle from the limiting membrane. Furthermore, Vps4 disassembles the ESCRT-III polymer and recycles the subunits for further rounds of vesicle formation (Figure 1A, step 6).
Figure 1.
Models for ESCRT-dependent and independent mechanisms of ILV formation. The deformation of the membrane is mainly driven by the formation of a membrane domain with a unique lipid composition and is supported by the pH gradient across the endosomal membrane. (A) ESCRT-0 sorts ubiquitinated cargo proteins into the lipid domain. The neck of the forming vesicle is first stabilized by ESCRT-I and ESCRT-II. The neck is further narrowed by the formation of ESCRT-III. The recruitment of the Vps4 complex to ESCRT-III initiates the scission of the vesicle neck and the disassembly of the ESCRT-III complex. (B) Protozoan parasites lack ESCRT-I and ESCRT-II and thus exhibit a simplified MVB vesicle formation that may not include sorting of ubiquitinated cargoes. (C) Melanosomes and exosomes exhibit ESCRT-independent ILV formation that is dependent on self-organizing lipid and cargo domains.
A simplified version of this system is found in protozoan parasites that lack all ESCRT factors except ESCRT-III and Vps4 (Figure 1B). In these organisms, ESCRT-III assembles without the upstream ESCRT machinery on the neck of spontaneously forming vesicles. ESCRT-III stabilizes the neck and, together with Vps4, drives abscission of the vesicle. However, because cargo sorting does not occur, the vesicles contain a random set of endosomal membrane proteins. This system, with its lack of specificity, seems to be sufficient in an organism that, as a parasite, is not energy limited.
ESCRT-independent vesicle formation is found in melanosomes, which are specialized endosomal/lysosomal structures present in pigment cells that exhibit MVB-like morphology. The melanosomal protein Pmel17 contains a lumenal domain that allows for its localization to ILVs in the absence of the ESCRT machinery. This model of ILV formation predicts that Pmel17 self-organizes with the proper lipid to form a specialized domain within pre-melanosomal membranes. These domains invaginate and pinch-off, driven by the properties of both the lipids and Pmel17 (Figure 1C).
Finally, cells mutated for key ESCRT factors are still able to form ILVs, simply because the lipid domains present in the endosomal membrane are able to spontaneously deform and detach from the limiting membrane. However, this vesicle formation is inefficient, due to the reversibility of membrane deformation, and is not coupled to cargo sorting. Furthermore, the ILVs resulting from this spontaneous reaction are not uniform in composition and size.
Concluding remarks
In short, the question ‘Which ESCRT components are necessary for ILV formation?’ is not the right question, as the ESCRTs themselves are not essential for the deformation of the membrane or the fission of the vesicle neck. However, the ESCRTs regulate ILV formation by ensuring the specificity and efficiency of this reaction. Most importantly, the ESCRTs link ILV formation with protein sorting and thus allow proteins to enter ILVs without the need for a specialized lipid interaction domain. Future ESCRT studies will need to account for an additional member of the ESCRT machinery, the endosomal membrane and its sub-domains.
Acknowledgements
The research conducted in the Babst laboratory on the ESCRT proteins is supported by NIH Grant R01 GM074171.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodman PG, Futter CE. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408–414. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies BA, Lee JR, Oestreich AJ, Katzmann DJ. Membrane protein targeting to the MVB/lysosome. Chem Rev. 2009;109:1575–1586. doi: 10.1021/cr800473s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanson PI, Shim S, Merrill SA. Cell biology of the ESCRT machinery. Curr Opin Cell Biol. 2009;21:568–574. doi: 10.1016/j.ceb.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 5.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raiborg C, Bache KG, Mehlum A, Stang E, Stenmark H. Hrs recruits clathrin to early endosomes. Embo J. 2001;20:5008–5021. doi: 10.1093/emboj/20.17.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 8.Mayers JR, Fyfe I, Schuh AL, Chapman ER, Edwardson JM, Audhya A. ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J Biol Chem. 2010 doi: 10.1074/jbc.M110.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren X, Kloer DP, Kim YC, Ghirlando R, Saidi LF, Hummer G, Hurley JH. Hybrid structural model of the complete human ESCRT-0 complex. Structure. 2009;17:406–416. doi: 10.1016/j.str.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 11.Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol. 2003;162:413–423. doi: 10.1083/jcb.200302136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilodeau PS, Winistorfer SC, Kearney WR, Robertson AD, Piper RC. Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J Cell Biol. 2003;163:237–243. doi: 10.1083/jcb.200305007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci U S A. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo H, Gill DJ, Sun J, Perisic O, Veprintsev DB, Vallis Y, Emr SD, Williams RL. ESCRT-I Core and ESCRT-II GLUE Domain Structures Reveal Role for GLUE in Linking to ESCRT-I and Membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Gill DJ, Teo H, Sun J, Perisic O, Veprintsev DB, Emr SD, Williams RL. Structural insight into the ESCRT-I/-II link and its role in MVBStructural insight into the ESCRT-I/-II link and its role in MVB. EMBO J. 2007;26:600–612. doi: 10.1038/sj.emboj.7601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 17.Teo H, Perisic O, Gonzalez B, Williams RL. ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Im YJ, Wollert T, Boura E, Hurley JH. Structure and function of the ESCRT-II-III interface in multivesicular body biogenesis. Dev Cell. 2009;17:234–243. doi: 10.1016/j.devcel.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghazi-Tabatabai S, Saksena S, Short JM, Pobbati AV, Veprintsev DB, Crowther RA, Emr SD, Egelman EH, Williams RL. Structure and disassembly of filaments formed by the ESCRT-III subunit Vps24. Structure. 2008;16:1345–1356. doi: 10.1016/j.str.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 20. Hanson PI, Roth R, Lin Y, Heuser JE. Plasma membrane deformation by circular arrays of ESCRT-III protein filaments. J Cell Biol. 2008;180:389–402. doi: 10.1083/jcb.200707031. ** This study demonstrates the ability of ESCRT-III subunits to polymerize and deform the underlying membrane in vivo.
- 21.Lata S, Schoehn G, Jain A, Pires R, Piehler J, Gottlinger HG, Weissenhorn W. Helical structures of ESCRT-III are disassembled by VPS4. Science. 2008;321:1354–1357. doi: 10.1126/science.1161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. * This study presents the first model of the sequential action of ESCRT-I, ESCRT-II and ESCRT-III.
- 23.Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieffer C, Skalicky JJ, Morita E, De Domenico I, Ward DM, Kaplan J, Sundquist WI. Two distinct modes of ESCRT-III recognition are required for VPS4 functions in lysosomal protein targeting and HIV-1 budding. Dev Cell. 2008;15:62–73. doi: 10.1016/j.devcel.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Shim S, Merrill SA, Hanson PI. Novel interactions of ESCRT-III with LIP5 and VPS4 and their implications for ESCRT-III disassembly. Mol Biol Cell. 2008;19:2661–2672. doi: 10.1091/mbc.E07-12-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. Embo J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf D, Isaacs AM. The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem Soc Trans. 2010;38:1469–1473. doi: 10.1042/BST0381469. [DOI] [PubMed] [Google Scholar]
- 29.Carlton JG, Martin-Serrano J. The ESCRT machinery: new functions in viral and cellular biology. Biochem Soc Trans. 2009;37:195–199. doi: 10.1042/BST0370195. [DOI] [PubMed] [Google Scholar]
- 30.Pincetic A, Leis J. The Mechanism of Budding of Retroviruses From Cell Membranes. Adv Virol. 2009;2009:6239691–6239699. doi: 10.1155/2009/623969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roxrud I, Stenmark H, Malerod L. ESCRT & Co. Biol Cell. 2010;102:293–318. doi: 10.1042/BC20090161. [DOI] [PubMed] [Google Scholar]
- 32.Saksena S, Emr SD. ESCRTs and human disease. Biochem Soc Trans. 2009;37:167–172. doi: 10.1042/BST0370167. [DOI] [PubMed] [Google Scholar]
- 33.Falguieres T, Luyet PP, Gruenberg J. Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp Cell Res. 2009;315:1567–1573. doi: 10.1016/j.yexcr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabrikant G, Lata S, Riches JD, Briggs JA, Weissenhorn W, Kozlov MM. Computational model of membrane fission catalyzed by ESCRT-III. PLoS Comput Biol. 2009;5:e1000575. doi: 10.1371/journal.pcbi.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 38.Nickerson DP, West M, Henry R, Odorizzi G. Regulators of vps4 ATPase activity at endosomes differentially influence the size and rate of formation of intralumenal vesicles. Mol Biol Cell. 2010;21:1023–1032. doi: 10.1091/mbc.E09-09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. * This study observes the formation of MVB vesicles in the absence of key ESCRT factors.
- 40.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 41. Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10:343–354. doi: 10.1016/j.devcel.2006.01.012. * This study identifies the first ubiquitin-independent signal to sort cargo into endosomal ILVs.
- 42. Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. ** This in vitro study demonstrates that liposomes with the proper lipid composition can form ILVs.
- 43.Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- 44.Yang M, Coppens I, Wormsley S, Baevova P, Hoppe HC, Joiner KA. The Plasmodium falciparum Vps4 homolog mediates multivesicular body formation. J Cell Sci. 2004;117:3831–3838. doi: 10.1242/jcs.01237. [DOI] [PubMed] [Google Scholar]
- 45. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. ** This in vitro study analyzes the function of the ESCRT protein complexes in ILV formation.
- 46.Lindas AC, Karlsson EA, Lindgren MT, Ettema TJ, Bernander RT. A unique cell division machinery in the Archaea. Proc Natl Acad Sci U S A. 2008;105:18942–18946. doi: 10.1073/pnas.0809467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samson RY, Obita T, Freund SM, Williams RL, Bell SD. A role for the ESCRT system in cell division in archaea. Science. 2008;322:1710–1713. doi: 10.1126/science.1165322. * This study shows that in archaea homologues of the ESCRTs function in cell division.
- 48.Samson RY, Obita T, Hodgson B, Shaw MK, Chong PL, Williams RL, Bell SD. Molecular and Structural Basis of ESCRT-III Recruitment to Membranes during Archaeal Cell Division. Mol Cell. 2011;41:186–196. doi: 10.1016/j.molcel.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. ** This study demonstrates a role for the ESCRTs in mammalian cytokinesis.
- 50.Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morita E, Colf LA, Karren MA, Sandrin V, Rodesch CK, Sundquist WI. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proc Natl Acad Sci U S A. 2010;107:12889–12894. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter C, West M, Odorizzi G. Dual mechanisms specify Doa4-mediated deubiquitination at multivesicular bodies. EMBO J. 2007;26:2454–2464. doi: 10.1038/sj.emboj.7601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikko E, Andre B. Split-ubiquitin two-hybrid assay to analyze protein-protein interactions at the endosome: application to Saccharomyces cerevisiae Bro1 interacting with ESCRT complexes, the Doa4 ubiquitin hydrolase, and the Rsp5 ubiquitin ligase. Eukaryot Cell. 2007;6:1266–1277. doi: 10.1128/EC.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amerik A, Sindhi N, Hochstrasser M. A conserved late endosome-targeting signal required for Doa4 deubiquitylating enzyme function. J Cell Biol. 2006;175:825–835. doi: 10.1083/jcb.200605134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sobo K, Chevallier J, Parton RG, Gruenberg J, van der Goot FG. Diversity of raft-like domains in late endosomes. PLoS One. 2007;2:e391. doi: 10.1371/journal.pone.0000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 57.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]