Abstract
This study tested the hypothesis that activation of β2-adrenoceptors on DCs influences NOD2 signaling along with its cross-talk with Toll-like receptor-2 resulting in altered Th cell priming ability. Th17 cells are a newly discovered lineage of CD4+ T cells involved in defense against extracellular bacteria and also implicated in autoimmune disorders. Initiation and polarization of the adaptive immune response is controlled by innate immune recognition mediated by DCs. Previous studies demonstrated that adrenergic receptors modulate cytokine production by DCs and affect their Th cell priming ability. We show that the β2-adrenoceptor agonist salbutamol enhanced IL-6 production in murine bone marrow-derived DCs stimulated with the nucleotide-binding oligomerization domain 2 ligand muramyl dipeptide. However, when the Toll-like receptor-2 ligand Pam3CysSK4 was added, salbutamol inhibited IL-12 but did not alter IL-6 and IL-23 expression. Gene expression analysis showed that salbutamol inhibited the p40 subunit as well as IL-12p35, while IL-23p19 and IL-6 were stimulated. Therefore, β2-adrenoceptors modulated cytokine production resulting in a Th17 cell priming cytokine pattern. Indeed, when antigen-pulsed DCs stimulated by muramyl dipeptide or Pam3CysSK4+muramyl dipeptide in the presence of salbutamol were used for in vivo immunization, the resulting Th17/Th1 cell ratio was increased as evaluated by IL-17 and IFN-γ production. In addition, intradermal injection of norepinephrine along with Pam3CysSK4+muramyl dipeptide increased the Th17 response to an immunogenic protein and this effect was reversed by a β2-adrenoceptor antagonist. Thus, β2-adrenoceptors may be involved in the regulation of defense against extracellular bacteria and the pathogenesis of inflammatory diseases.
Keywords: Pattern recognition receptor, Th17, adrenergic receptor, cytokine, dendritic cell
1. Introduction
Dendritic cells (DCs) are professional antigen presenting cells (APCs) involved in the initiation and polarization of the adaptive immune response. The priming of Th cell subsets is orchestrated by cytokines produced by DCs that sense pathogen-associated molecular patterns (PAMPs) and local microenvironmental factors. Th17 cells are a recently discovered lineage of effector CD4+ T cells characterized by the production of IL-17, IL-21 and IL-22 [1]. Th17 cells provide defense against extracellular bacteria but are also implicated in autoimmune disorders. In mice, the development of a Th17 immune response depends on the presence of the proinflammatory cytokines IL-6 and TGF-β1 and is suppressed by the Th1-type cytokines IFN-γ and IL-12 and by the Th2-type cytokine IL-4 [2]. Furthermore, IL-23 was shown to be important for Th17 expansion [3]. Previous studies have demonstrated that adrenergic receptors may modulate cytokine production in DCs and affect their Th cell priming ability [4]. In particular, activation of β2-adrenergic receptors (β2-ARs) in DCs stimulated by Toll-like receptor (TLR) agonists hampered IL-12 and stimulated IL-10 production resulting in reduced migration and Th1 priming [5, 6]. More recently, we demonstrated that β-ARs in mouse skin may modulate the innate and adaptive immune response to certain, but not all, PAMPs suggesting that the physiological role of the skin adrenergic system might be that of limiting the immune response to specific pathogens [7]. We observed that inhibition of β-ARs in the skin increased the inflammatory cytokine response to peptidoglycan (PGN). When a protein antigen was injected after PGN administration and β-AR blockade, the consequent adaptive memory response was shifted toward the Th1-type. This was validated by increased interferon-γ (IFN-γ) production in cell suspensions from draining lymph nodes and by the delayed-type hypersensitivity response to a protein antigen [7]. However, the increased production of IFN-γ was not associated with a corresponding decrease of the Th2 cytokine IL-4, indicating that the Th1 shift did not depend on polarization of naïve Th cells toward the Th2-type. As IFN-γ may suppress Th17 cell formation [2], we hypothesized that the increased Th1 priming observed after β-ARs blocking was at the expense of Th17 cells. In our murine model, β-ARs in the skin could influence the response to the TLR-2 agonist PGN but not to the TLR-4 agonist lipopolysaccharide (LPS). Unlike LPS that signals only via TLR-4 [8], there are numerous PGN recognition molecules that are distinct from TLR2. These include CD14, the nucleotide oligomerization domain (NOD)-containing proteins, a family of peptidoglycan recognition proteins and PGN-lytic enzymes [9]. NOD2 is a cytosolic receptor, which induces innate immune responses by recognizing the PGN derivative muramyl dipeptide (MDP). It has been observed that TLR-2 and NOD2 co-stimulation was associated with a dose-dependent inhibition of IL-12 expression and stimulation of IL-6 and IL-10 resulting in negative regulation of the TLR-2-mediated Th1 response [10]. Moreover, it has been recently shown that NOD2 is involved in human Th17 differentiation [11].
In the present study, we investigated whether β-AR activation could influence NOD2 signaling along with its cross-talk with TLR-2 and the resulting Th cell priming ability by murine DCs. We found that the β2-AR agonist salbutamol modulates cytokine production in DCs stimulated with MDP or simultaneously by both TLR-2 and NOD2 ligands, resulting in a cytokine pattern suggestive for Th17 priming. Indeed, in mice immunized with antigen-pulsed DCs stimulated by MDP or by the TLR-2 ligand Pam3CysSK4 (PAM)+MDP in the presence of salbutamol, the resulting IL-17/IFN-γ production ratio was increased. These results were confirmed by the examination of cytokine production ratios by draining lymph node cells after direct injection in mice of the β-AR agonist norepinephrine (NE) along with PAM+MDP. Thus, β2-ARs represent an important mechanism by which the adaptive immune response to certain pathogens is regulated. Remarkably, β2-ARs might be involved in the defense against extracellular bacteria and in the pathogenesis of inflammatory diseases by modulating Th17 polarization.
2. Materials and Methods
2.1 Mice
C57BL/6 inbred mice were purchased from Harlan, Udine, Italy. All the mice used in these experiments were female, 2–3 months old and were maintained under a standard 12 hours photoperiod, at 21 ± 1 °C, with food and water ad libitum. All the experiments performed were authorized by the local veterinary committee.
2.2 Bone marrow-derived DCs
Bone marrow cells from C57BL/6 mice were cultured in 10 cm petri dishes at a concentration of 2.5×106 cells/10 ml at 37°C, 5% CO2 in complete medium: RPMI 1640 (Gibco, Karlsruhe, Germany) containing 25 mM HEPES (Bioconcept, Allschwil, Switzerland), 10% FCS (Bioconcept), 2 mM L-glutamine (Bioconcept), 50 μM 2-mercaptoethanol (Bioconcept), 100 U/ml Penicillin (Bioconcept), 100 μg/ml streptomycin (Bioconcept) and 20 ng/ml granulocyte/macrophage-colony–stimulating factor (GM-CSF, ReliaTech Gmbh, Braunschweig, Germany). At day 2, 10 ml of fresh medium was added to each plate. At days 4 and 7, half of the culture medium was replaced with fresh medium. At day 9 cells were collected and selected by CD11c positivity using magnetic microbeads in a magnetic cell sorting system (Miltenyi Biotech, Bergisch Gladbach, Germany). The purity of CD11c+ cells obtained was routinely >95% as assessed by flow cytometry.
2.3 In vitro experiments
DCs were incubated at 106 cells/ml in complete medium and stimulated by either 2 μg/ml MDP (NeoMPS, Strasbourg, France), 10 μg/ml PAM (N-palmitoyl-2-(2,3-bis (palmitoyloxy)-(2R, S)-propyl)-(R)-cysteinyl-seryl-(lysyl)3-lysine, EMC microcollections Gmbh, Tuebingen, Germany) or a combination of PAM+MDP. In some wells 1 μM salbutamol (Fluka, Buchs, Switzerland), a specific β2-adrenergic receptor agonist, was added. Cells were collected 3 hours later for gene expression analysis, and supernatants 6 hours later for cytokine protein quantification by ELISA.
2.4 ELISA
To quantitate protein production in culture supernatants, IL-6, IL-12, IL-17, IL-23 and IFN-γ Ready-Set-Go! Kits (eBioscience, San Diego, CA, USA) were used following the manufacturer’s instructions. Optical density was determined at 450 nm in a MRX microplate reader (DynexTechnologies Inc., Chantilly, UK).
2.5 Real-time RT-PCR
Total RNA was extracted from DCs using the RNAqueous kit (Ambion, Foster City, CA, USA) following the manufacturer’s instructions. Genomic DNA was removed using the Turbo DNA-free kit (Ambion) and cDNA was synthesized from 1 μg of total RNA using random hexamer primers (Pharmacia Biotech, Uppsala, Sweden) and Superscript II (Invitrogen, Basel, Switzerland). The PCR reaction was performed using the SensiMix DNA kit (Quantance, London, UK) and pre-developed TaqMan probes (Applied Biosystem, Foster City, CA, USA). Amplification of 18S rRNA was performed for each sample as an endogenous control for the amount and quality of total RNA added to each reaction. Data were analyzed using the ΔΔCt methods and results were expressed as fold difference relative to the amount of target mRNA in unstimulated control cells.
2.6 Immunization with DCs and Th cell polarization
DCs were incubated at 106 cells/ml in a Petri dish with 10 μg/ml PAM and/or 2 μg/ml MDP in the presence of endotoxin-free keyhole limpet hemocyanin (KLH, Calbiochem, Nottingham, UK) and in the presence or absence of 1 μM salbutamol. After 3 hours, cells were collected, washed and counted. One million cells were injected intradermally (i.d.) in the right hind footpad of each mouse. The animals were sacrificed 5 days later and cells from the draining popliteal lymph nodes were incubated in complete medium at 5×105 cells/250 μl/well at 37°C for 48 hours. Culture supernatants were then collected and the concentration of IFN-γ and IL-17 were quantified by ELISA.
2.7 Direct in vivo experiments
Mice were shaved on the back and injected i.d. with either 50 μg PAM, 50 μg MDP or both in the presence or absence of 2 μg norepinephrine (NE, Calbiochem) ± 5 μg of the β2-AR antagonist ICI 118,551 hydrochloride (Tocris, Bristol, UK). Three hours later, mice were injected in the same skin site with 50 μg of KLH. After 7 days, single cell suspensions were prepared from the draining inguinal lymph node and 5×105 cells were incubated with 0, 10, 100 μg/ml of KLH for 48 hours. Supernatants were harvested and IL-17 and IFN-γ concentration was determined by ELISA.
2.8 Statistical analysis
The statistical significance of differences between experimental groups was assessed by analysis of variance (ANOVA) performed with the computer-assisted software JMP.
3. Results
3.1 β-adrenergic effect on cytokine production in DCs
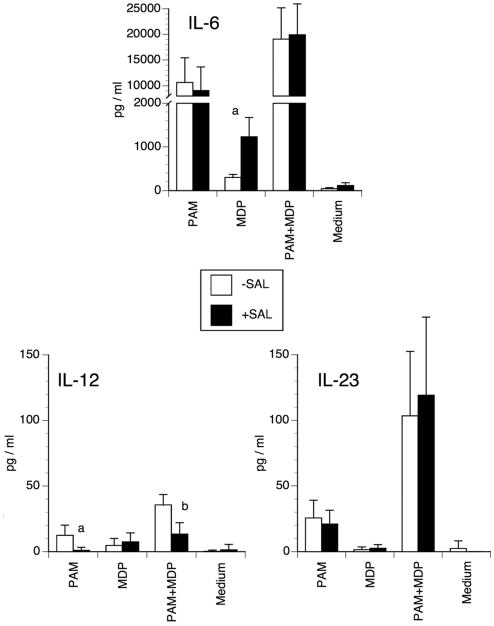
We examined the effect of salbutamol, a specific β2-AR agonist, on IL-6, IL-12 and IL-23 production by DCs stimulated by TLR-2 and/or NOD2 ligands. These cytokines are known to be implicated in the Th1/Th17 cell polarization. We used the lipopeptide PAM as a TLR-2 agonist and the NOD2 ligand MDP. Cytokine concentrations were measured in the supernatants after 6 hours of culture. Salbutamol significantly increased IL-6 production when DCs were activated by MDP suggesting a possible involvement of β2-AR in the modulation of DC antigen presentation for an IL-17 immune response (Fig. 1). A minor, albeit significant, increase of IL-6 was also evident when salbutamol was added to DCs cultured in medium alone. This is in agreement with a recent report showing that β2-ARs may slightly stimulate IL-6 in murine macrophages [12]. Although PAM− or PAM+MDP-induced IL-6 production was much higher than in MDP-stimulated cells, salbutamol did not affect it. With regard to IL-12 production, the potency of the stimuli was PAM+MDP ≫ PAM > MDP, with the latter giving values similar to those of non-stimulated DCs. Salbutamol inhibited the production of IL-12 in DCs activated by PAM or PAM+MDP, i.e. when IL-12 was effectively stimulated. In both the PAM and PAM+MDP groups, IL-23 production was high but salbutamol did not affect it. Again, in the MDP group, the concentration of IL-23 was similar to that found in the supernatant of non-stimulated DCs.
Figure 1. Effect of salbutamol on IL-6, IL-12 and IL-23 protein production.
Murine DCs were incubated with 10 μg/ml PAM or 2 μg/ml MDP alone or in combination in the presence or absence of 1 μM salbutamol. Six hours later, the supernatants were collected and cytokine concentrations were determined by ELISA. The bars represent the mean ± SD of 5 independent experiments (a: p<0.001; b: p<0.005).
3.2 β-adrenergic effect on cytokine gene expression in DCs
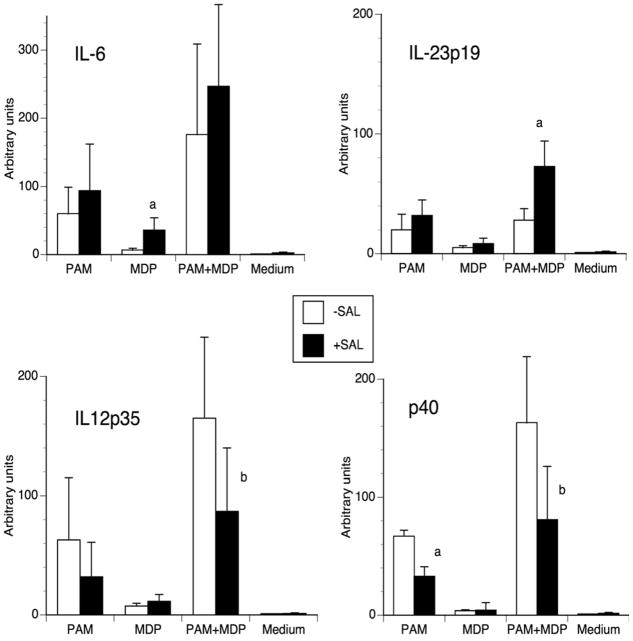
To investigate whether the effects reported above were reflected in cytokine gene expression, we evaluated by real-time RT-PCR the relative amount of mRNA. IL-12 and IL-23 share the p40 subunit [13]; therefore, we measured mRNA levels for IL-12/IL-23 shared subunit p40, IL-12 p35, IL-23 p19 as well as that of IL-6. As expected, salbutamol increased IL-6 mRNA in DCs stimulated with MDP (Fig. 2). In the other groups the expression of IL-6 was higher but salbutamol did not affect it, correlating nicely with the protein concentration results. With regard to IL-12 and IL-23, salbutamol inhibited both IL-12 p35 and IL-12/IL-23 p40 mRNA and, conversely, increased the expression of IL-23 p19 in DCs activated by PAM+MDP. No significant effect was seen in the other groups, which had a much lower expression of both IL-12 and IL-23. Thus, in the PAM+MDP group salbutamol inhibited the expression of both IL-12 heterodimers and stimulated IL-23 p19 expression. This differential effect at the gene expression level may explain the outcome observed on relative protein production, notably no difference in IL-23 production and inhibition of IL-12. These results, along with the cytokine production results, suggest that salbutamol may be implicated in supporting Th17 development.
Figure 2. Effect of salbutamol on IL-6, IL-12 and IL-23 gene expression.
Murine DCs were incubated with 10 μg/ml PAM or 2 μg/ml MDP alone or in combination in the presence or absence of 1 μM salbutamol. Three hours later, cells were harvested and the specific mRNA expression was semi-quantitated by real-time RT-PCR. The bars represent the mean ± SD of the arbitrary units (n-fold increase over unstimulated DCs) as obtained in 6 independent experiments (a: p<0.03; b: p<0.05).
3.3 Immunization with DCs
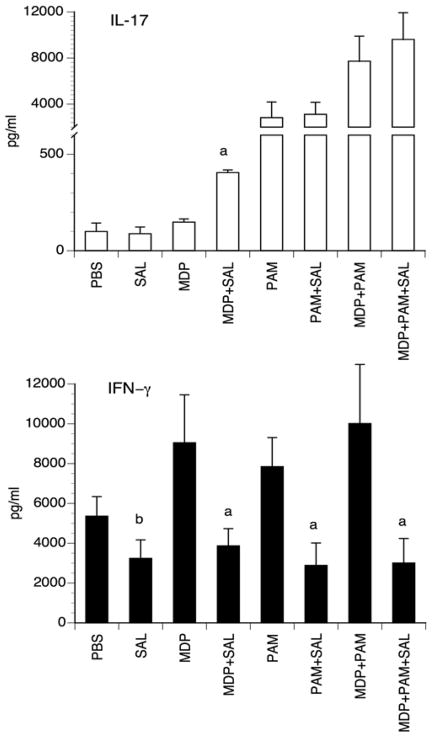
To examine the possibility that exposure of DCs to salbutamol might enhance Th17 type cells priming, we set-up experiments in vivo in which mice were immunized with DCs pulsed with endotoxin–free KLH and pre-incubated for 3h with MDP, PAM or PAM+MDP in the presence or absence of salbutamol. Five days after injections, spontaneous IL-17 and IFN-γ production was assessed in supernatants of cultures of draining lymph node cell suspensions. The data shows that in mice injected with MDP-activated DCs pulsed with KLH, salbutamol enhanced IL-17 production in the draining lymph node cells while inhibiting IFN-γ production Fig 3. In the other groups, Th1 priming was consistently reduced by β2-AR stimulation as assessed by a significantly reduced IFN-γ production (Fig. 3). Th17 cell differentiation, as evaluated by IL-17 production, was most manifest in the PAM or PAM+MDP groups compared to the MDP group but treatment of DCs with salbutamol did not influence it. Taken together, these results indicate that β2-AR agonists in the presence of the NOD2 ligand results in a DC-expressed cytokine pattern that preferentially instructs an IL-17 immune response or, in other terms, that appears to shift the Th balance towards the Th17-type at the expense of the Th1-type. Nevertheless, in PAM and PAM+MDP groups salbutamol influences the IL-17/IFN-γ ratio in favor of IL-17 suggesting also a shift towards a Th17-type immune response.
Figure 3. IL-17 and IFN-γ production by lymph node cells from mice immunized with DCs preincubated with PAMPs ± salbutamol.
Mice were injected i.d. with KLH-pulsed DCs preincubated for 3 h with 10 μg/ml PAM or 2 μg/ml MDP alone or in combination in the presence or absence of 1 μM salbutamol. Five days later, cell cultures from the draining lymph nodes were set-up and cytokine concentrations were measured in the supernatants after 48 hours. The bars represent the mean ± SD of 3 independent experiments with 3 mice per group. (a: p<0.001; b: p< 0.03).
3.4 Direct effect in vivo
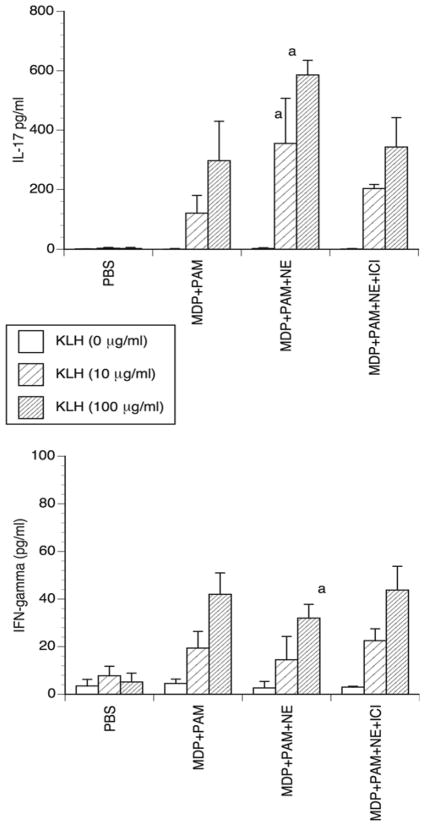
The immunization model, although informative and potentially interesting for immunotherapy, is of limited physiological significance. Hence, we asked whether the direct injection of PAM + MDP along with a β-AR agonist before immunization with KLH at the same site could result in an increased IL-17 immune response. Mice were injected i.d. with PAM+MDP in the presence or absence of the sympathetic neurotransmitter norepinephrine (NE) followed 3 hours later by KLH injection into the same skin site. This injection schedule proved capable of revealing a β-adrenergic influence on the innate and adaptive recall memory response in our previous studies [7]. Here we investigated the primary response and, therefore, 7 days after treatment the draining lymph nodes were removed to obtain cell suspensions to be re-stimulated in vitro with KLH. Preconditioning mouse skin with PAM+MDP before KLH injection resulted in a robust IL-17-type immune response as shown by the much higher IL-17 production compared to IFN-γ Fig 4. This finding is consonant with previous results obtained in vitro with human cells, which showed that MDP is able to orchestrate Th17 immunity [11]. Addition of NE during the skin preconditioning further increased the production of IL-17 while slightly, yet significantly, decreasing that of IFN-γ. These effects were reversed by addition of the specific β2-AR antagonist ICI 118,551. Thus, we demonstrated that in vivo, in the presence of NOD2 and TLR-2 ligands, i.e. in a situation mimicking bacterial infections, NE is able to increase the IL-17 immune response via β2-ARs. The weak effect on IFN-γ could depend either on the fact that the IFN-γ response was very low or on the NE activation of other skin adrenoceptors, which we have recently shown to enhance expression of Th-1-type cytokines [7].
Figure 4. IL-17 and IFN-γ production by lymph node cells from mice injected with PAM+MDP ± NE followed by KLH immunization.
Mice were injected i.d with 50 μg/mouse PAM plus 2 μg/mouse MDP in the presence or absence of 2 μg/mouse NE and of 5 μg/mouse ICI 118,551. Seven days later, cell cultures from the draining lymph nodes were set-up and the cells were restimulated with graded concentrations of KLH for 48 hours. The concentrations of IL-17 and IFN-γ were then determined in the supernatants by ELISA. The bars represent the mean ± SD of 3 independent experiments with 3 mice per group. (a: p < 0.01).
4. Discussion
In this study we show that β2-AR activation may modulate Th cell priming in favor of an IL-17 immune response. We found that the β2-AR agonist salbutamol enhanced IL-6 protein and gene expression in murine DCs stimulated with the NOD2 agonist MDP. IL-12 mRNA and protein were inhibited by salbutamol in PAM and PAM+MDP treated cells. This result confirms the ability of β2-AR agonists to inhibit IL-12 production in DCs stimulated by various PAMPs as previously reported by us and others [5, 12, 14] and suggests an inhibitory role of β2-AR agonist in the development of a Th1 immune response. Surprisingly, PAM-, MDP- and PAM+MDP-induced IL-23 was not influenced at the protein level by β2-ARs activation. These results were confirmed at the mRNA level for PAM and MDP treated cells but not for PAM+MDP. This discrepancy between mRNA and protein level is easily explained by the structure of IL-23 protein, which is composed by two subunits, IL-12/IL-23 p40 and IL-23 p19. We found that IL-12/IL-23 p40 subunit expression is downregulated by salbutamol while IL-23 p19 is upregulated, explaining the resulting absence of influence of salbutamol at the protein level. These results suggest that in the presence of PAMPs stimulating NOD2 or both TLR-2 and NOD2, activation of β2-ARs in DCs results in a cytokine pattern characterized respectively by induction of IL-6 or inhibition of IL-12 without affecting IL-23. These cytokine patterns are favorable for Th17 priming. In fact, both IL-6 and IL-23 have been shown to be central for Th17 cell generation [15, 16] while IL-12 is known to inhibit it. The in vivo immunization experiments correlated nicely with the in vitro experiment. In this test we found that in lymph node cells from mice immunized by injection with KLH-pulsed DCs treated with MDP and salbutamol, IL-17 production was significantly enhanced while IFN-γ was inhibited. In PAM and PAM+MDP groups IL-17 production was not affected by salbutamol treatment while IFN-γ was inhibited. Therefore, in both groups the resulting IL-17/IFN-γ ratio was skewed towards IL-17. These results were further confirmed by direct i.d. injection of NE and PAM+MDP in the presence or absence of the β2-ARs antagonist ICI 118,551. The evidence that β2-ARs may facilitate a cytokine pattern favorable to Th17 cell priming was unanticipated. This effect seems to depend on the ability of β2-ARs to inhibit IL-12 while sparing and/or stimulating IL-23. The combination of this effect with the β2-AR stimulation of IL-6, occurring in the presence of NOD2 ligand, may well explain the final outcome, i.e. the shift in favor of Th17 cells at the expense of Th1 cells as shown by the in vivo immunization and in vitro experiments. Our results confirm and further expand recently published data, which show that epinephrine- and LPS-treated DCs enhance IL-17 and IL-4 but not IFN-γ production by CD4+ T cells in vitro [17]. Future experiments using mouse models in which β2-AR is selectively knocked out in specific cells such as keratinocytes, Langerhans cells or dermal dendritic cells would be useful in investigating the in vivo contribution of each cell type to our model.
These findings have both basic and clinical relevance. Indeed, besides being important effectors against extracellular bacteria and fungi [18], IL-17 and Th17 cells have also been implicated in the pathogenesis of autoimmune and inflammatory disorders [19]. Thus, contrary to the concept that β2-AR agonists are immunosuppressive agents able to counteract autoimmune and allergic diseases [20], the findings reported here suggest that in combination with TLR-2 and/or NOD2 activation, stimulation of β2-ARs may enhance an IL-17-type immune response. Thus, administration of β2-AR agonists might conceivably be useful in augmenting IL-17 type immunity for therapeutic purposes in some situations, such as in the setting of an infection where a microorganism product is stimulating TLR and/or NOD2 activation. It should be noted that NOD2 is the intracellular pattern recognition receptor (PRR) that senses MDP derived from both gram-positive and gram-negative bacteria [11]. On the other hand, β2-AR agonists are widely used in the management of asthma with some evidence of adverse effect on disease control. Interestingly, IL-17 has been reported to exert a dual role in experimental asthma [21]; it is needed during antigen sensitization to establish allergic asthma, while in sensitized mice it attenuates the allergic response by inhibiting DC function and chemokine synthesis. IL-17 has been found to be increased in skin inflammatory disorders such as psoriasis [22] and in rheumatoid arthritis [23]. Indeed, although IFN-γ has been reported to be highly expressed in psoriasis and may well participate in the pathophysiology of that disorder, Th17 cells together with IL-17 and IL-22 appear to play a key role in its pathogenesis [24]. In this regard, it may be of interest that CD4+ and CD8+ T cells that express both IL-17 and IFN-γ have been described and it has been demonstrated that in psoriatic lesions CD8+ T cells have been found that produce Th17-related cytokines (including IL-17) as well as IFN-γ [25,26]. Our data suggest that stress-induced release of adrenergic agents by the sympathetic nervous system with consequent stimulation of Th17-type immunity may explain stress induced exacerbation of psoriasis.
Highlights.
Salbutamol (β2-AR agonist) enhanced IL-6 production following NOD2 activation of DCs
Salbutamol inhibited IL-12 expression in TLR-2-activated DCs
Thus, β2-AR signaling alters DC cytokine expression to favor Th17 cell development
An enhanced Th17 response induced by β2-AR stimulation was observed in vivo
Acknowledgments
The authors thank Mrs. Elisabeth Hertens and Mrs. Paola Galli for excellent technical assistance. The Swiss National Science Foundation grant no. 310000-107524/1 and NIH grant 5R01 AR042429 supported this study.
Abbreviation
- AR
adrenergic receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seiffert K, Hosoi J, Torii H, Ozawa H, Ding W, Campton K, et al. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans cells. J Immunol. 2002;168:6128–35. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 5.Maestroni GJ. Short exposure of maturing, bone marrow-derived dendritic cells to norepinephrine: impact on kinetics of cytokine production and Th development. J Neuroimmunol. 2002;129:106–14. doi: 10.1016/s0165-5728(02)00188-1. [DOI] [PubMed] [Google Scholar]
- 6.Maestroni GJ, Mazzola P. Langerhans cells beta 2-adrenoceptors: role in migration, cytokine production, Th priming and contact hypersensitivity. J Neuroimmunol. 2003;144:91–9. doi: 10.1016/j.jneuroim.2003.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Manni M, Maestroni GJ. Sympathetic nervous modulation of the skin innate and adaptive immune response to peptidoglycan but not lipopolysaccharide: involvement of beta-adrenoceptors and relevance in inflammatory diseases. Brain Behav Immun. 2008;22:80–8. doi: 10.1016/j.bbi.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–62. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dziarski R, Gupta D. Peptidoglycan recognition in innate immunity. J Endotoxin Res. 2005;11:304–10. doi: 10.1179/096805105X67256. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–8. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 11.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–9. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 14.Hasko G, Szabo C, Nemeth ZH, Salzman AL, Vizi ES. Stimulation of beta-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J Neuroimmunol. 1998;88:57–61. doi: 10.1016/s0165-5728(98)00073-3. [DOI] [PubMed] [Google Scholar]
- 15.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Kim BJ, Jones HP. Epinephrine-primed murine bone marrow-derived dendritic cells facilitate production of IL-17A and IL-4 but not IFN-gamma by CD4(+) T cells. Brain Behav Immun. 2010;24:1126–36. doi: 10.1016/j.bbi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–9. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 20.Makhlouf K, Weiner HL, Khoury SJ. Potential of beta2-adrenoceptor agonists as add-on therapy for multiple sclerosis: focus on salbutamol (albuterol) CNS Drugs. 2002;16:1–8. doi: 10.2165/00023210-200216010-00001. [DOI] [PubMed] [Google Scholar]
- 21.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Yang XQ, Cheng J, Hui RS, Gao TW. Increased Th17 cells are accompanied by FoxP3(+) Treg cell accumulation and correlated with psoriasis disease severity. Clin Immunol. 2010;135:108–17. doi: 10.1016/j.clim.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365–70. doi: 10.1007/s11926-009-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS One. 2010;5:e14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamradt T, Chang HD. Diversity and flexibility of Th17 effector functions. Arthritis Res Ther. 2011;13:106. doi: 10.1186/ar3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, Moreno JC, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol. 2009;86:435–43. doi: 10.1189/JLB.0109046. [DOI] [PubMed] [Google Scholar]