Abstract
Cytokine-like factor 1 (CLF1) is a secreted receptor belonging to the interleukin-6 family of cytokines. CLF1 and its physiologic partner, cardiotrophin-like cytokine (CLC) are secreted as a heterodimer and engage the tripartite signaling complex of ciliary neurotrophic factor receptor (CNTFR), leukemia inhibitory factor (LIFR) and gp130. Ligation of this receptor complex leads to activation of the STAT3 and MAPK pathways and mediates survival pathways in neurons. Mutations in CLF1, CLC, or CNTFR in mice lead to the birth of mice that die on post-natal day 1 because of an inability to nurse. These animals exhibit significant decreases in the number of motor neurons in the facial nucleus and the spinal cord. CLF1 or CLC deficiency is associated with the development of the human cold-induced sweating syndromes. A growing body of research suggests that CLF1 expression may be associated with several post-natal disease processes. In this review, we summarize the current understanding of CLF1 expression and suggest future studies to understand the potentially important role of CLF1 in postnatal life and disease.
Keywords: Cytokine-like factor 1, Cardiotrophin-like cytokine, Ciliary neurotrophic factor receptor, Interleukin-27, Crisponi syndrome
INTRODUCTION
Cytokine-like factor 1 (CLF1 or cytokine receptor-like factor 1, CRLF1) is a secreted receptor exhibiting significant homology to the interleukin (IL)-6 family of receptors (1). CLF1 was identified as the physiologic partner for the IL-6 family member, cardiotrophin-like cytokine, CLC (2). To complicate matters further, the CLF1/CLC complex must bind another `c' molecule, ciliary neurotrophic factor receptor (CNTFR) in order to transduce downstream signals. Mutations in the gene encoding CLF1 in both humans and mice have identified a significant role for this protein in neural development. Between 1998 and 2003, several important studies were published that established the structure and most critical functions of CLF1. First, the CLF1 gene in mice and humans was cloned in 1998 by Elson and colleagues (1). Second, Alexander et al. published the CLF1 knockout mouse (NR6−/−) and showed that these mice were born alive, but die on the first day of life due to an inability to suckle (3). And in 2003, mapping studies identified mutations in the CLF1 gene that accounted for an inherited human disease of cold-induced sweating, dysmorphic facial features, and muscle contractions (4). These critical first studies on CLF1 and the ones that followed have shown the importance of this gene in developmental pathways. Yet relatively little is known about CLF1 outside of development. Are there important functions of CLF1 in postnatal and adult life? Does CLF1 signal outside the nervous system? In this review, we will discuss what is known about the discovery, structure, and function of CLF1. We will then review a small but growing body of literature suggesting that there is role for CLF1 in acquired human diseases.
THE IL-6 FAMILY OF CYTOKINES AND CLC: A BRIEF REVIEW
Any discussion of CLF1 (and CLC) requires a brief review of the IL-6 family and its members: IL-6, IL-11, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin (CT-1), and CLC (reviewed in (5)). The IL-6 family members all share significant homology (reviewed in (6)). All members of the family share the common 130 kDa signal transducer, gp130 (reviewed in (7)). In the case of IL-6, -11, and CNTF, these cytokines must bind their specific a-receptor subunit (7). In the case of CNTF and CLF1/CLC, the a-chain is CNTFR. Ligation of the a-receptor and gp130 bv CNTF, CLF1/CLC, LIF, OSM, or CT-1 leads to recruitment and heterodimerization with LIF receptor-β (LIFRβ) (8). This leads to activation of the Janus family kinases, JAK1, 2, 3, and TYK2 and phosphorylation of tyrosine residues on the cytoplasmic domain of gp130 (8). This is followed by recruitment and phosphorylation of signal transducer and activators of transcription (STAT), particularly STAT3 but also STAT1 and STAT5 (9–12). Phosphorylation of gp130 also engages the SH2 domain-containing cytoplasmic protein SHP2 leading to activation of the ERK and PI3K/Akt pathways (reviewed in (7)). The IL-6 family members exhibit a wide variety of activities in immune function, hematopoiesis, oncogenesis, and cell survival and proliferation (5, 7, 13).
In 1999, Senaldi et al. identified a gp130-activating cytokine that they named novel neurotrophin-1/B cell-stimulating factor-3 (NNT-1/BSF-3) because of its ability to stimulate B cell proliferation (14). Independently, Shi et al. cloned this same gene and called it cardiotrophin-like cytokine because of its significant homology with CT-1 (15). CLC contains a signal sequence (14) but is retained in the cell unless complexed with CLF1 or soluble CNTF receptor (sCNTFR) (2, 16). The association of CLC and CLF1 is reviewed in greater detail below.
THE STRUCTURE AND FUNCTION OF CLF1: AN HISTORICAL PERSPECTIVE
The CLF1 gene is encoded by 9 exons contained within a 14 kb region of chromosome 19p12 (1). Two independent groups, using different approaches, cloned both the murine and human CLF1 genes (1, 3). 85% of the human and murine cDNAs for CLF1 are identical, and the amino acid sequences are 96% identical (1). The Elson group found that the amino acid sequence of CLF1 showed close homology to the prolactin and IL-6 cytokine family of receptors (1). Characteristic of the type I cytokine receptor family, in the cytokine receptor-like domain, CLF1 exhibits the four conserved cysteine residues and the W-S-x-W-S motif (1, 3). The N-terminal end of CLF1 contains an immunoglobulin-like domain followed by two fibronectin type III modules (1). Although CLF1 contains a putative signal sequence, both groups found that CLF1 lacked transmembrane or cytoplasmic domains (1, 3). There is significant homology between CLF1 and other secreted receptors including IL-12p40, IL-6 receptor a-chain, human Epstein-Barr virus induced protein (EBI3), and human ciliary neurotrophic factor receptor (hCNTFRa) (3). The similarity in structure of CLF1 to IL-12p40 led Elson and colleagues to hypothesize that a second molecule, similar to the IL-12p35 subunit, required binding to CLF1 for efficient secretion (2). Indeed, they found that CLF1 forms a stable complex with the IL-6 family member cardiotrophin-like cytokine (CLC) and that CLC is secreted from cells only when complexed with CLF1 (2). Investigating this same theme in IL-27, a composite cytokine formed by EBI3 and the p28 subunit, Crabé and colleagues found that similar to CLC, the p28 subunit of IL-27 could associate with CLF1 to form a new composite cytokine that can bind both a tripartite receptor of IL-6Ra, WSX-1, and gp130 (17).
How was CNTFR identified as the specific receptor for the CLF1/CLC complex? CNTF was found to signal through a dimeric cytokine receptor consisting of LIFRβ and gp130 (18). Disruption of CLF1 in mice by Alexander and colleagues resulted in mice that were born live but died within 24 hours because of a failure to suckle (3). A similar observation was made by DeChiara et al when they noted that mice lacking CNTFRa, but not ciliary neurotrophic factor (CNTF), died on postnatal day 1 because of an inability to suckle (19). Elson and colleagues reasoned that there must be a second ligand for CNTFR, so-called CNTF II (20), which compensated for deficiency in CNTF signaling (2). The Elson group found that the CLF1/CLC complex exhibited an absolute requirement to bind CNTFR in order to transduce downstream signaling events via gp130/LIFRβ including phosphorylation of STAT3, Akt, and ERK (2, 21). CLC itself does contain a signal sequence but is inefficiently secreted from cells unless complexed with CLF1 or with soluble CNTFR (22). Recombinant CLC produced in bacteria is physiologically active and is capable of inducing phosphorylation of STAT3 in SK-N-MC cells, suggesting that CLF1 binding, while necessary for CLC secretion, is not necessary for binding to CNTFR (23). By mutating different sites in CLC, Perret and colleagues showed that CLC associates with CLF1 at sites distinct from CNTFR (16). These same sites where CLC associates with CLF1 were also found to be critical for CLC binding to LIFRβ (16).
THE ASSOCIATION BETWEEN CLF1 AND THE COLD-INDUCED SWEATING SYNDROME
Linkage analysis identified mutations in the CLF1 gene in patients with a syndrome of cold-induced sweating (4). This syndrome, first described by Sohar in 1978 (24) exists on a continuum with several syndromes all with overlapping symptoms but different degrees of severity and may be best characterized as “CNTF receptor pathway-related disorders (25).” Supporting the critical association between CLF1 and CLC, mutations in CLC can lead to a similar cold-induced sweating syndrome and has been identified in patients, now described as suffering from cold-induced sweating syndrome 2 (26). Patients with mutations in LIFRβ suffer from the Stuve-Wiedemann syndrome (SWS)/Schwartz-Jampel type 2 syndrome (SJS2), a disease that shares many clinical manifestations with the cold-induced sweating syndromes (25). Perhaps the most severe manifestation is the syndrome described by Giangiorgio Crisponi (Crisponi syndrome) (27, 28) and is characterized by dysmorphic facies, muscle contractions, scoliosis, camptodactyly, hyperthermia, cold-induced sweating, and respiratory abnormalities (25). Patients with Crisponi syndrome have tended to follow a more severe clinical and frequently fatal course in infancy (25). Cases have been described in the Mediterranean (including Spain, Portugal, Italy, Libya, and Israel) (24, 29–32), Yemen (33), India (34), Hungary (35), Canada (31), United States (35), Norway (35), and Japan (36). Patients with SWS/SJS2 exhibit many of the same symptoms as Crisponi syndrome but also have other manifestations including short stature and bowed long bones (25).
The mutations in the disease include missense, single-nucleotide insertions, nonsense, and insertion/deletion mutations (25). In order to answer the question of why CISS1 and Crisponi syndrome exhibit varying degrees of severity, Herholz and colleagues undertook sequencing of multiple cases of CISS1 and CS (27). The observed mutations were cloned into expression vectors and introduced by transfection into COS7 cells (27). Immunoblotting was performed to determine if CLF1 was secreted into the medium and if the mutated protein could induce phosphorylation of Stat3 (27). The authors found that the patients with the most severe disease exhibited mutations that led to the least secretion of CLF1 into the media. In contrast, the patients with the most mild manifestations of disease (originally characterized as CISS patients), showed relatively greater secretion of CLF1 (27).
What can we glean about the function of CLF1 expression in humans (37)? Dysautonomic symptoms seem to predominate in the Crisponi syndrome suggesting that CLF1 functions in the development of the central and peripheral autonomic nervous systems (33). The respiratory disease in Crisponi syndrome has not been well-described. Central apneas in a baby with Crisponi syndrome have been reported (31). Thus, the respiratory abnormalities may reflect the neuromuscular manifestations in Crisponi syndrome. No post-mortem analysis of Crisponi syndrome patients has been published. A detailed analysis of the histopathology of patients with Crisponi syndrome may yield significant insights into how CLF1 functions in the development of the autonomic nervous system. Furthermore, because CLF1 may be active in other organs, the early mortality of many patients with Crisponi syndrome may mask other manifestations of CLF1 deficiency outside the nervous system. Further studies in this syndrome will be necessary to answer important questions on the function of CLF1 in human development.
CLF1 EXPRESSION: WHAT TISSUES EXPRESS CLF1 AND WHEN?
In the mouse embryo, in-situ hybridization experiments have shown expression of CLF1 mRNA in different tissues at different stages of development. At 11.5 days post-conception (dpc), CLF1 was detected in the mesonephric duct, limb buds, first branchial arch, nasal processes, and the dermatomyotome (3). At 14.5 dpc, CLF1 was detected in the lung, kidney, genital tubercle, pre-cartilaginous condensations of the digital metacarpals, intervertebral discs, tongue, and facial mesenchyme (3). 18.5 dpc, CLF1 expression was observed in the cortex and hippocampus regions of the brain (3). It has also been shown that CLF1 and CLC are expressed in developing muscle fibers in embryonic mice from E14.5 through E18.5 (38). In the CNTFR null mice, there was no effect of CNTFR deletion on development of sensory neurons (19). In functional support of this observation, administration of CLC in ovo in developing chicks during the cell death period increased the number of motor neurons but not sensory neurons (38). Furthermore the same authors found in the CLF1 null mice that there are critically important spatial effects of CLF1 expression: CLF1 deletion significantly decreased numbers of spinal motoneurons in the lumbar spinal cord and facial motoneurons but not thoracic or hypoglossal motoneurons (38). Also highlighting the importance of timed expression, CLC mRNA has been shown to vary over the day, exhibiting a circadian rhythm and regulating mammalian locomotor activity (39). This novel observation suggests that cvtokines such as CLC may exhibit neuroendocrine functions. The expression of CLF1 in this system, however, is unknown.
In human tissues, northern blot analysis revealed strong staining for CLF1 mRNA in the spleen, thymus, lymph nodes, stomach, placenta, and fetal lung (1). The timing of expression appears to be important. For example, strong expression of CLF1 was noted in fetal lung but not in adult lung, although there is no known functional consequence to expression of CLF1 in the lung. Further studies will be needed to uncover the effects of CLF1 expression in tissues outside the brain.
CELLULAR FUNCTIONS OF CLF1
It was previously reported that only CNTFRa-expressing cells are responsive to CLF1/CLC stimulation (21). CLC, however, is known to stimulate proliferation of B cells despite the lack of expression of CNTFR in B cells (40). This suggests that CLC may bind another as of yet unknown receptor on B cells. Soluble CNTFR can turn cells that express gp130 and LIFRβ only into CNTF-responders (41) but cannot associate with CLC to do the same, that is stimulate downstream signaling in cells expressing only gp130 and LIFRβ (22). In two CNTFRa-expressing neuroblastoma cell lines, SK-N-GP and IMR-32 cells, gp130, Janus kinases (JAK1, JAK2, and TYK2), and their downstream targets STAT1 and STAT3 are phosphorylated in response to CLF1/CLC (21). Stimulation of these cells with CLF1/CLC also induced phosphorylation of AKT and ERK1/2 (21). These cell signaling pathways are typically associated with proliferation and/or cell survival.
As noted above, CLF1/CLC as well as CNTF promotes survival of motor neurons in vitro (2, 14, 38) and in vivo (38). In animals lacking CLF1, CNTFR, and CLC, there were decreased numbers of neurons in the facial nucleus, and the ventral horns of the lumbar spinal cord suggesting that defects in any component of this signaling axis is necessary and sufficient to lead to the observed abnormalities (3, 19, 38, 42). High throughput genomic screening such as gene expression profiling by microarray has increased the number of associations between CLF1 gene expression and developmental and disease-related pathways. Schmidt-Ott et al. identified by gene expression profiling enrichment of CLF1 in the ureteric bud of the developing rat kidney (43). When applied to undifferentiated rat metanephric mesenchyme, CLF1/CLC induced phosphorylation of STAT3 and development of mature nephron structures including glomerular and tubular markers. The same group described a similar role for LIF (44). There is no published association of Crisponi syndrome or SWS/SJS2 with kidney disease suggesting that LIF and CLF1/CLC can compensate for deficiency of the other in the development of the kidney.
Little is known about the factors governing secretion of CLF1. In fibroblast cultures, CLF1 expression was induced by pro-inflammatory cytokines TNF-a, IL-6, and IFN-γ (1). Explants of rat gubernaculum (a ligament involved in descent of mammalian testes) exposed to insulin-like 3 (INSL3) induced several genes involved in neuronal development including a three-fold increase in CLF1 (45). Gene expression for CLF1 was increased in 293 cells transfected with the proto-oncogene PLAG1 (46). CLF1 was also present at increased levels in pleiomorphic adenomas from salivary glands (high PLAG1-expressing tissues) compared to normal salivary glands (46). The availability of web-based tools to predict targets of microRNAs (miRNA, ~22 nucleotide RNAs that pair to mRNAs coding for protein to repress transcription, reviewed in (47)) may lead to targeted testing of miRNAs that may regulate CLF1 expression. Further studies will be necessary to elucidate epigenetic regulators of CLF1 expression during development and adult life.
IS THERE AN ASSOCIATION OF CLF1 AND ACQUIRED DISEASE IN HUMANS?
The defects that have been observed in mice and humans suggest that the critically important functions of CLF1 expression are limited to developmental pathways. But it begs the question if there are pathologic consequences associated with increased or inappropriate expression of CLF1 or CLC. For example, CLC was identified as a potential permeability factor in the plasma of patients with focal seamental alomerulosclerosis (48). With the relative ease of genomic screening techniques, that assay expression of thousands of genes from small quantities of cells and tissues, CLF1 has been identified in a number of pathologic conditions in animal models and in humans suggesting that there is an important role for CLF1 function in postnatal life, outside of developmental pathways. Experiments in cocaine-exposed H9C2 cells, a rat cardiac myoblast line, showed increases in CLF1 gene expression, as well as pro-apoptotic genes FAS and FAS ligand, that were blocked by pre-treatment with the anti-oxidant N-acetylcysteine (NAC), suggesting a role in cardiomyocyte death in cocaine-induced injury (49). CLF1 gene expression was increased nearly threefold in articular cartilage from patients with osteoarthritis (OA) (50). Supporting the potentially pathogenic role of CLF1 in OA, the authors found that treatment of ATCD5 cells, a chondrocytic line, with transforming growth factor-β induced a 4.5-fold increase in CLF1 expression (50). Furthermore, incubation of ATCD5 cells stimulated proliferation suggesting that CLF1 is a pathogenic response. There are a number of publicly available gene expression profiling datasets that show significant increases (or decreases) in CLF1 gene expression. Verification of CLF1 expression from these microarray findings have not been published. These data do suggest that in a number of pathologic processes, CLF1 gene expression may be part of a program of gene expression that is relevant to the development of disease. Further studies will be necessary to elucidate the function of CLF1 in the context of disease-relevant models. A selection of these datasets is shown in Table 1.
TABLE 1.
Selected NCBI Geo Datasets associated with significant changes in CLF1 expression
| GeoDataSet Number | Species | Conditions | Change in CLF1 relative to the comparison group |
|---|---|---|---|
| GDS1857 | Human | Synovial tissues in RA patients compared to normals | Decreased CLF1 in RA patients |
| GDS2931 | Human | Fibroblats from patients with osteoarthritis (OA) and rheumatoid arthritis (RA) | Increased CLF1 in OA |
| GDS2205 | Human | Myocardial biopsy tissue from non-failing hearts vs dilated cardiomyopathy | Increased CLF1 in dilated cardiomyopathy |
| GDS3661 | Rat | Heart from hypertensive animal compared to control | Increased CLF1 in hypertensive heart tissue |
| GDS3688 | Human | Omental adipose tissue in obese children vs controls | Increased CLF1 in obese children |
| GDS3580 | Human | Lung from patients with sarcoidosis and normal controls | Decreased CLF1 in sarcoid patients |
| GDS2491 | Human | Large airway epithelium in smokers vs. non smokers | Increased CLF1 in smokers |
| GDS1252 | Human | Lung tissue from patients with idiopathic pulmonary fibrosis (IPF) and normal controls | Increased CLF1 in lungs from patients with IPF |
| GDS3627 | Human | Non-small cell carcinoma of the lung: sqamous cell v adenocarcinoma | Increased CLF1 in adenocarcinoma |
| GDS1649 | Mouse | Lung adenocarcinoma induced by urethane vs adjacent normal tissue | Increased CLF1 in adenocarcinoma |
| GDS3224 | Rat | Diaphragm vs normal left ventricle | Increased CLF1 in diaphragms |
FUTURE DIRECTIONS
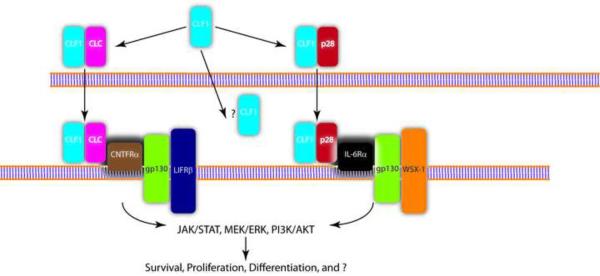
The functions of CLF1 still remain relatively enigmatic. Is CLF1 always complexed with CLC? What determines if CLF1 is secreted with CLC or with the p28 subunit of IL−27? Can CLF1 be secreted on its own and act as a decoy receptor or scavenger? Is there another receptor for CLC? Figure 1 is a schematic representation of CLF1 secretion and signaling and highlights some of the unanswered questions. These challenges will be met with the development of transgenic models. A CLF1 knock-in mouse, as an overexpression model, may help elucidate the role of this protein in conditions such as osteoarthritis. Since the CLF-1 null mouse dies on postnatal day 1, a conditional model using the cre-lox system may be effective in dissecting the organ-specific effects of CLF1 deficiency. A clear understanding is also needed of CLF1 receptor expression, particularly CNTFR, which does exhibit both spatial and temporal changes in expression (51). The recent discovery of the CLF1/p28 complex suggests that CLF1 can stimulate cell populations that may not express CNTFR.
FIGURE 1. Schematic representation of CLF1 secretion and signaling.
Composite cytokines of CLF1 and CLC or CLF1 and the p28 subunit of IL-27 are assembled in the cell and secreted. It is unknown if CLF1 is secreted in the absence of its partners. CLF1/CLC has been found to signal via the tripartite receptor complex of CNTFRa, LIFRβ, and gp130. CLF1/p28 was also found to bind IL-6Ra complexed with gp130 and WSX-1. Engagement of these receptors leads to downstream signaling events via the JAK/STAT pathway (particularly STAT3), MEK/ERK, and PI3K/AKT. The full range of cells that are potentially responsive to CLF1 stimulation is unknown. The effect of CLF1 on these cells also remains unknown.
Signaling through CNTFR has been of interest to drug discovery researchers and has been studied in a therapeutic context in amyotrophic lateral sclerosis/motor neuron disease (reviewed in (52)) and in retinal diseases (53, 54). Results to date in ALS have been disappointing. CLF1/CLC however has not been studied in clinical studies. If CLF1 expression is important in the pathogenesis of heart disease or pulmonary fibrosis or osteoarthritis, common diseases associated with significant degrees of morbidity and mortality, then a more thorough understanding of CLF1 signaling pathways could prove to be critical to the development of novel therapeutic strategies for these diseases.
The author is very appreciative of the assistance of Dr. Rehan Kahloon in the preparation of this manuscript.
CLF1 Review Highlights
CLF1 is a secreted receptor with significant homology to IL-6R.
CLF1 exists complexed with CLC, and this heterodimeric cytokine signals via the tripartite IL-6 family receptor CNTFR, LIFRβ, and gp130.
Mutations in CLF1 are associated with the genetic disease cold induced sweating syndrome/Crisponi syndrome.
CLF1 may be associated with acquired disease in humans.
Acknowledgments
Supported by: HL083085 and the Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WORKS CITED
- 1.Elson GC, Graber P, Losberger C, Herren S, Gretener D, Menoud LN, Wells TN, Kosco-Vilbois MH, Gauchat JF. Cytokine-like factor-1, a novel soluble protein, shares homology with members of the cytokine type I receptor family. J Immunol. 1998;161:1371–1379. [PubMed] [Google Scholar]
- 2.Elson GC, Lelievre E, Guillet C, Chevalier S, Plun-Favreau H, Froger J, Suard I, de Coignac AB, Delneste Y, Bonnefoy JY, et al. CLF associates with CLC to form a functional heteromeric ligand for the CNTF receptor complex. Nat Neurosci. 2000;3:867–872. doi: 10.1038/78765. [DOI] [PubMed] [Google Scholar]
- 3.Alexander WS, Rakar S, Robb L, Farley A, Willson TA, Zhang JG, Hartley L, Kikuchi Y, Kojima T, Nomura H, et al. Suckling defect in mice lacking the soluble haemopoietin receptor NR6. CurrBiol. 1999;9:605–608. doi: 10.1016/s0960-9822(99)80266-8. [DOI] [PubMed] [Google Scholar]
- 4.Knappskog PM, Majewski J, Livneh A, Nilsen PT, Bringsli JS, Ott J, Boman H. Cold-induced sweating syndrome is caused by mutations in the CRLF1 gene. Am J Hum Genet. 2003;72:375–383. doi: 10.1086/346120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 22:347–352. doi: 10.1093/intimm/dxq030. [DOI] [PubMed] [Google Scholar]
- 6.Vlotides G, Zitzmann K, Stalla GK, Auernhammer CJ. Novel neurotrophin-1/B cell-stimulating factor-3 (NNT-1/BSF-3)/cardiotrophin-like cytokine (CLC)-a novel gp130 cytokine with pleiotropic functions. Cytokine Growth Factor Rev. 2004;15:325–336. doi: 10.1016/j.cytogfr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Fischer P, Hilfiker-Kleiner D. Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol. 2008;153(Suppl 1):S414–427. doi: 10.1038/bjp.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT axis. Basic Res Cardiol. 2007;102:393–411. doi: 10.1007/s00395-007-0674-z. [DOI] [PubMed] [Google Scholar]
- 9.Guschin D, Rogers N, Briscoe J, Witthuhn B, Watling D, Horn F, Pellegrini S, Yasukawa K, Heinrich P, Stark GR, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. Embo J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger LC, Hawley TS, Lust JA, Goldman SJ, Hawley RG. Tyrosine phosphorylation of JAK-TYK kinases in malignant plasma cell lines growth-stimulated by interleukins 6 and 11. Biochem Biophys Res Commun. 1994;202:596–605. doi: 10.1006/bbrc.1994.1970. [DOI] [PubMed] [Google Scholar]
- 11.Stahl N, Boulton TG, Farruggella T, Ip NY, Davis S, Witthuhn BA, Quelle FW, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-UF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 12.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–252. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 13.Knight DA, Ernst M, Anderson GP, Moodley YP, Mutsaers SE. The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol Ther. 2003;99:327–338. doi: 10.1016/s0163-7258(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 14.Senaldi G, Varnum BC, Sarmiento U, Starnes C, Lile J, Scully S, Guo J, Elliott G, McNinch J, Shaklee CL, et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc Natl Acad Sci U S A. 1999;96:11458–11463. doi: 10.1073/pnas.96.20.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Wang W, Yourey PA, Gohari S, Zukauskas D, Zhang J, Ruben S, Alderson RF. Computational EST database analysis identifies a novel member of the neuropoietic cytokine family. Biochem Biophys Res Commun. 1999;262:132–138. doi: 10.1006/bbrc.1999.1181. [DOI] [PubMed] [Google Scholar]
- 16.Perret D, Guillet C, Elson G, Froger J, Plun-Favreau H, Rousseau F, Chabbert M, Gauchat JF, Gascon H. Two different contact sites are recruited by cardiotrophin-like cytokine (CLC) to generate the CLC/CLF and CLC/sCNTFRalpha composite cytokines. J Biol Chem. 2004;279:43961–43970. doi: 10.1074/jbc.M407686200. [DOI] [PubMed] [Google Scholar]
- 17.Crabe S, Guay-Giroux A, Tormo AJ, Duluc D, Lissilaa R, Guilhot F, Mavoungou-Bigouagou U, Lefouili F, Cognet I, Ferlin W, et al. The IL-27 p28 subunit binds cytokine-like factor 1 to form a cytokine regulating NK and T cell activities requiring IL-6R for signaling. J Immunol. 2009;183:7692–7702. doi: 10.4049/jimmunol.0901464. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. UFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 19.DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, et al. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83:313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 20.Lesser SS, Lo DC. CNTF II, I presume? Nat Neurosd. 2000;3:851–852. doi: 10.1038/78738. [DOI] [PubMed] [Google Scholar]
- 21.Lelievre E, Plun-Favreau H, Chevalier S, Froger J, Guillet C, Elson GC, Gauchat JF, Gascon H. Signaling pathways recruited by the cardiotrophin-like cytokine/cytokine-like factor-1 composite cytokine: specific requirement of the membrane-bound form of ciliary neurotrophic factor receptor alpha component. J Biol Chem. 2001;276:22476–22484. doi: 10.1074/jbc.M101681200. [DOI] [PubMed] [Google Scholar]
- 22.Plun-Favreau H, Elson G, Chabbert M, Froger J, deLapeyriere O, Lelievre E, Guillet C, Hermann J, Gauchat JF, Gascon H, et al. The ciliary neurotrophic factor receptor alpha component induces the secretion of and is required for functional responses to cardiotrophin-like cytokine. Embo J. 2001;20:1692–1703. doi: 10.1093/emboj/20.7.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elson G. CLF/CLC. Academic Press; 2001. [Google Scholar]
- 24.Sohar E, Shoenfeld Y, Udassin R, Magazanik A, Revach M. Cold-induced profuse sweating on back and chest. A new genetic entity? Lancet. 1978;2:1073–1074. doi: 10.1016/s0140-6736(78)91805-6. [DOI] [PubMed] [Google Scholar]
- 25.Crisponi L, Crisponi G, Meloni A, Toliat MR, Nurnberg G, Usala G, Uda M, Masala M, Hohne W, Becker C, et al. Crisponi syndrome is caused by mutations in the CRLF1 gene and is allelic to cold-induced sweating syndrome type 1. Am J Hum Genet. 2007;80:971–981. doi: 10.1086/516843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rousseau F, Gauchat JF, McLeod JG, Chevalier S, Guillet C, Guilhot F, Cognet I, Froger J, Hahn AF, Knappskog PM, et al. Inactivation of cardiotrophin-like cytokine, a second ligand for ciliary neurotrophic factor receptor, leads to cold-induced sweating syndrome in a patient. Proc Natl Acad Sd U S A. 2006;103:10068–10073. doi: 10.1073/pnas.0509598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herholz J, Meloni A, Marongiu M, Chiappe F, Deiana M, Herrero CR, Zampino G, Hamamy H, Zalloum Y, Waaler PE, et al. Differential secretion of the mutated protein is a major component affecting phenotypic severity in CRLF1-associated disorders. EurJ Hum Genet. doi: 10.1038/ejhg.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crisponi G. Autosomal recessive disorder with muscle contractions resembling neonatal tetanus, characteristic face, camptodactyly, hyperthermia, and sudden death: a new syndrome? Am J Med Genet. 1996;62:365–371. doi: 10.1002/(SICI)1096-8628(19960424)62:4<365::AID-AJMG8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Okur I, Turner L, Crisponi L, Eminoglu FT, Chiappe F, Cinaz P, Yenicesu I, Hasanoglu A. Crisponi syndrome: a new case with additional features and new mutation in CRLF1. Am J Med Genet A. 2008;146A:3237–3239. doi: 10.1002/ajmg.a.32531. [DOI] [PubMed] [Google Scholar]
- 30.Accorsi P, Giordano L, Faravelli F. Crisponi syndrome: report of a further patient. Am J Med Genet A. 2003;123A:183–185. doi: 10.1002/ajmg.a.20292. [DOI] [PubMed] [Google Scholar]
- 31.Delia Marca G., Barone G, Vollono C, Dittoni S, Vasta I, Timpani G, Crisponi G, Zampino G. Central apneas in a case of Crisponi syndrome. Sleep Med. 2008;9:703–704. doi: 10.1016/j.sleep.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Nannenberg EA, Bijlmer R, Van Geel BM, Hennekam RC. Neonatal paroxysmal trismus and camptodactyly: the Crisponi syndrome. Am J Med Genet A. 2005;133A:90–92. doi: 10.1002/ajmg.a.30536. [DOI] [PubMed] [Google Scholar]
- 33.Dagoneau N, Bellais S, Blanchet P, Sarda P, Al-Gazali LI, Di Rocco M, Huber C, Djouadi F, Le Goff C, Munnich A, et al. Mutations in cytokine receptor-like factor 1 (CRLF1) account for both Crisponi and cold-induced sweating syndromes. Am J Hum Genet. 2007;80:966–970. doi: 10.1086/513608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas N, Danda S, Kumar M, Jana AK, Crisponi G, Meloni A, Crisponi L. Crisponi syndrome in an Indian patient: a rare differential diagnosis for neonatal tetanus. Am J Med Genet A. 2008;146A:2831–2834. doi: 10.1002/ajmg.a.32487. [DOI] [PubMed] [Google Scholar]
- 35.Hahn AF, Waaler PE, Kvistad PH, Bamforth JS, Miles JH, McLeod JG, Knappskog PM, Boman H. Cold-induced sweating syndrome: CISS1 and CISS2: manifestations from infancy to adulthood. Four new cases. J Neurol Sd. 293:68–75. doi: 10.1016/j.jns.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki M, Kosho T, Kawachi S, Mikoshiba M, Takahashi J, Sano R, Oka K, Yoshida K, Watanabe T, Kato H, et al. Cold-induced sweating syndrome with neonatal features of Crisponi syndrome: longitudinal observation of a patient homozygous for a CRLF1 mutation. Am J Med Genet A. 152A:764–769. doi: 10.1002/ajmg.a.33315. [DOI] [PubMed] [Google Scholar]
- 37.Hahn AF, Boman H. Cold-Induced Sweating Syndrome including Crisponi Syndrome. [Accessed 23-MAR-2011];GeneReviews at GeneTests: Medical Genetics Information Resource. 2011 (database online). Copyright, University of Washington, Seattle. 1997–2011. Available at A http://www.genetests.org.
- 38.Forger NG, Prevette D, deLapeyriere O, de Bovis B, Wang S, Bartlett P, Oppenheim RW. Cardiotrophin-like cytokine/cytokine-like factor 1 is an essential trophic factor for lumbar and facial motoneurons in vivo. J Neurosd. 2003;23:8854–8858. doi: 10.1523/JNEUROSCI.23-26-08854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraves S, Weitz CJ. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Not Neurosci. 2006;9:212–219. doi: 10.1038/nn1633. [DOI] [PubMed] [Google Scholar]
- 40.Cognet I, Guilhot F, Gabriac M, Chevalier S, Chouikh Y, Herman-Bert A, Guay-Giroux A, Corneau S, Magistrelli G, Elson GC, et al. Cardiotrophin-like cytokine labelling using Bir A biotin ligase: a sensitive tool to study receptor expression by immune and non-immune cells. J Immunol Methods. 2005;301:53–65. doi: 10.1016/j.jim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Davis S, Aldrich TH, Ip NY, Stahl N, Scherer S, Farruggella T, DiStefano PS, Curtis R, Panayotatos N, Gascon H, et al. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993;259:1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- 42.Zou X, Bolon B, Pretorius JK, Kurahara C, McCabe J, Christiansen KA, Sun N, Duryea D, Foreman O, Senaldi G, et al. Neonatal death in mice lacking cardiotrophin-like cytokine is associated with multifocal neuronal hypoplasia. Vet Pothol. 2009;46:514–519. doi: 10.1354/vp.08-VP-0239-B-BC. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Ott KM, Yang J, Chen X, Wang H, Paragas N, Mori K, Li JY, Lu B, Costantini F, Schiffer M, et al. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol. 2005;16:1993–2002. doi: 10.1681/ASN.2004121127. [DOI] [PubMed] [Google Scholar]
- 44.Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999;99:377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KJ, Robbins AK, Wang Y, McCahan SM, Chacko JK, Barthold JS. Insulin-like 3 exposure of the fetal rat gubernaculum modulates expression of genes involved in neural pathways. Biol Reprod. 83:774–782. doi: 10.1095/biolreprod.110.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voz ML, Mathys J, Hensen K, Pendeville H, Van Valckenborgh I, Van Huffel C, Chavez M, Van Damme B, De Moor B, Moreau Y, et al. Microarray screening for target genes of the proto-oncogene PLAG1. Oncogene. 2004;23:179–191. doi: 10.1038/sj.onc.1207013. [DOI] [PubMed] [Google Scholar]
- 47.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 5:2115–2121. doi: 10.2215/CJN.03800609. [DOI] [PubMed] [Google Scholar]
- 49.Lattanzio FA, Jr., Tiangco D, Osgood C, Beebe S, Kerry J, Hargrove BY. Cocaine increases intracellular calcium and reactive oxygen species, depolarizes mitochondria, and activates genes associated with heart failure and remodeling. Cardiovasc Toxicol. 2005;5:377–390. doi: 10.1385/ct:5:4:377. [DOI] [PubMed] [Google Scholar]
- 50.Tsuritani K, Takeda J, Sakagami J, Ishii A, Eriksson T, Hara T, Ishibashi H, Koshihara Y, Yamada K, Yoneda Y. Cytokine receptor-like factor 1 is highly expressed in damaged human knee osteoarthritic cartilage and involved in osteoarthritis downstream of TGF-beta. Caldf Tissue Int. 86:47–57. doi: 10.1007/s00223-009-9311-1. [DOI] [PubMed] [Google Scholar]
- 51.Yang CW, Lim SW, Han KW, Ahn HJ, Park JH, Kim YH, Kirsh M, Cha JH, Kim YS, Kim J, et al. Upregulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha in rat kidney with ischemia-reperfusion injury. J Am Soc Nephrol. 2001;12:749–757. doi: 10.1681/ASN.V124749. [DOI] [PubMed] [Google Scholar]
- 52.Bongioanni P, Reali C, Sogos V. Ciliary neurotrophic factor (CNTF) for amyotrophic lateral sclerosis/motor neuron disease. Cochrone Database Syst Rev. 2004:CD004302. doi: 10.1002/14651858.CD004302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacDonald IM, Sauve Y, Sieving PA. Preventing blindness in retinal disease: ciliary neurotrophic factor intraocular implants. Con J Ophtholmol. 2007;42:399–402. [PubMed] [Google Scholar]
- 54.Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Lujan B, Tao W, Porco TC, Roorda A, Duncan JL. Longitudinal Study of Cone Photoreceptors during Retinal Degeneration and in Response to Ciliary Neurotrophic Factory Treatment. Invest Ophtholmol Vis Sci. doi: 10.1167/iovs.10-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]