Abstract
Background
Gender differences exist in myocardial response to acute ischemia/reperfusion (I/R) injury and estrogen mediates cardioprotection in the female heart following I/R. Accumulating evidence has indicated that stromal cell-derived factor-1 (SDF-1) is increased in the ischemic heart and initiates cardioprotective effects. However, it is unknown whether SDF-1 plays a role in gender-specific response to myocardial I/R and in estrogen-induced acute protection. Therefore, we hypothesize that: 1) increased SDF-1 production will be observed in female hearts compared to males in response to I/R, which is attributable to the effect of estrogen; 2) Estrogen receptor (ER)α, not ERβ mediates estrogen-contributed SDF-1 expression in female hearts following I/R.
Methods
Heart tissue subjected to I/R injury was assessed for myocardial expression of SDF-1(ELISA) and SDF-1 receptor – CXCR4 (Western blot). Groups were as follows: rat hearts from adult male, female, ovariectomized female, and male and ovariectomized female supplemented with chronic 17β-estradiol (E2), and mouse hearts from adult male and female wildtype, ERα knockout (ERαKO) and ERβKO.
Results
I/R significantly increased myocardial SDF-1 expression in both genders. Higher levels of SDF-1 existed in female hearts after I/R compared to males. Depletion of endogenous estrogen by ovariectomy reduced cardiac SDF-1 production in females following I/R. E2 supplementation significantly restored SDF-1 expression in ovariectomized female and males compared to their counterparts. Notably, ablation of ERα, not ERβ, markedly decreased SDF-1 production in females after I/R. Unlike SDF-1, cardiac CXCR4 expression was not affected by gender, sex hormone and ERs in the ischemic heart.
Conclusion
Our study represents the first evidence of that female hearts exhibit higher levels of SDF-1 expression compared to males after acute I/R. This increased myocardial SDF-1 production in females is partly due to effect of estrogen through ERα, not ERβ.
Keywords: CXCR12, sex hormone, myocardial ischemia/reperfusion, estrogen receptor
INTRODUCTION
Gender differences exist in heart diseases in that women have a lower incidence of myocardial infarction (MI) and heart failure, and a higher rate of survival following myocardial ischemia/reperfusion (I/R) injury (1, 2). Although cardiovascular diseases occur uncommonly in premenopausal women, this risk increases in the postmenopausal age group (3, 4), suggesting the beneficial effect of estrogen on the cardiovascular system. Studies from our group and others have demonstrated that estrogen mediates cardioprotection through suppressing cell apoptosis, increasing pro-survival pathway activation, inducing antioxidant activity and decreasing inflammatory cytokine production after myocardial injury (5–11). However, the exact mechanism of estrogen-mediated cardioprotection is still incompletely characterized.
The stromal cell derived factor 1α (SDF-1), an important chemokine, has recently been receiving much interest for its role in the treatment of ischemic diseases. SDF-1 is increased in the heart immediately after MI and has been shown to mediate cardioprotection through mobilization and recruitment of stem cells into the injured heart (12–14). In addition, SDF-1 is able to improve cell survival and promote angiogenesis and tissue regeneration after MI (15–17). However, no information exists regarding whether SDF-1 plays a role in gender-specific response to myocardial I/R. In fact, estrogen has been shown to induce SDF-1 production in a variety of cells (18–21). Increased SDF-1 by estrogen has been reported to promote cancer cell proliferation (18, 21). In addition, evidence has demonstrated that SDF-1 expression pattern is related to estrogen receptor (ER) status in breast cancer (22) and SDF-1 is an estrogen-inducible, ER target gene (20, 21, 23, 24).
Therefore, in the present study, we hypothesized that: 1) gender differences exist in myocardial SDF-1 expression with higher levels of SDF-1 in females compared to male hearts subjected to acute I/R. This upregulated SDF-1 production in females is attributable to effect of estrogen; 2) ERα, not ERβ mediates estrogen-contributed SDF-1 expression in female hearts following I/R.
MATERIALS AND METHODS
All animal studies conformed to the “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 85–23, revised 1996). The protocols were reviewed and approved by the Indiana Animal Care and Use Committee of Indiana University.
Isolated heart perfusion (Langendorff)
Isolated rat or mouse hearts were performed with I/R experiment as previously described (6, 8–10, 25). Briefly, rat or mouse hearts were rapidly excised and the aorta was cannulated. The heart was perfused (70 mm Hg) with oxygenated (95% O2/5% CO2) Krebs-Henseleit solution (37° C) and paced at 350 bpm/min (rat) or 420 bpm/min (mouse) except during ischemia. A three-way stopcock above the aortic root was used to create global ischemia, during which the heart was placed in a 37°C degassed organ bath. Isolated rat hearts were subjected to 15-minute equilibration, 25-minute global ischemia, and 40-minute reperfusion (6, 25), whereas isolated mouse hearts were performed 20-minute global ischemia, followed by 60-minute reperfusion (8–10). Control hearts underwent perfusion only for the same time period as I/R experiment did.
Experimental Groups
A total of 34 Sprague-Dawley rats (9–11 weeks) (Harlan, Indianapolis, IN) were divided into six groups (n=4–8/group): age-matched adult male, female, ovariectomized female (OVX F), OVX F and male treated with 17β-estradiol (E2). Female rats were ovariectomized at 5–6 week-old and were purchased as surgical modified animals from Harlan lab. These animals exhibited significantly lower plasma levels of estradiol compared to age-matched normal female rats (6). Subcutaneous implantation of 21-day release pellets containing 75 mg of E2 was performed in male or OVX F (8 weeks), respectively based on our previous study (6). Those animals were utilized after 21 days.
C57BL/6J mice (16±4 week-old) with and without deficiency of ERα or ERβ (ERαKO, ERβKO and WT) in both genders were purchased from the Jackson Laboratory (Bar Harbor, ME) and were performed with I/R experiment.
Myocardial expression of SDF-1
Heart tissue was homogenized in cold RIPA buffer (Sigma, Saint Louis, MO) and centrifuged at 12000 rpm for 10 minutes. SDF-1 expression in the cardiac tissue was determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available ELISA kit (R&D Systems Inc., Minneapolis, MN). ELISA was performed according to the manufacturer’s instructions. All samples and standards were measured in duplicate.
Western blotting
Heart tissue was homogenized as stated above. The protein extracts (20 μg/lane) were subjected to electrophoresis on a 4–12% Bis-Tris protein gel (Invitrogen, Carlsbad, CA). The membranes were incubated with the primary antibodies: CXCR4 (eBioscience, San Diego, CA) and GAPDH (Biodesign International, Saco, Maine), and then incubated with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG secondary antibody. Detection was performed using supersignal west pico stable peroxide solution (Pierce, Rockford, IL). Films were scanned and band density was analyzed using ImageJ software (NIH).
Presentation of data and statistical analysis
All reported values are mean ± SEM. Data was compared using one-way analysis of variance (ANOVA) with post-hoc Tukey test or Student’s t-test. A probability value of less than 0.05 was considered statistically significant.
RESULTS
Gender-specific difference in myocardial SDF-1 expression after acute I/R
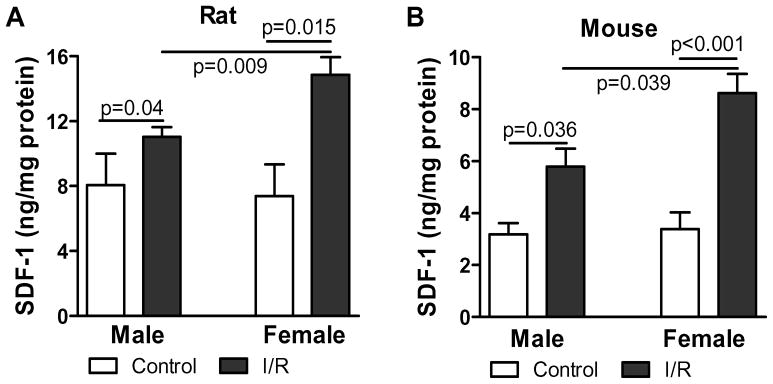
Gender difference has been shown in myocardial function following acute I/R. To determine whether SDF-1 plays a role in this difference, we measured SDF-1 expression between male and female hearts subjected to I/R. Our results indicated that I/R injury significantly increased myocardial levels of SDF-1 in both genders. Interestingly, female hearts exhibited much higher levels of SDF-1 compared to males following I/R in both rat and mouse model, whereas the baseline of SDF-1 expression was similar between male and female hearts (Fig. 1A and B).
Figure 1.
Ischemia/reperfusion (I/R) injury increased cardiac SDF-1 production in both genders. Myocardial SDF-1 expression was assessed in groups of control and I/R by ELISA in both rat and mouse hearts. Results are mean ± SEM, n=4–6/group.
The role of estrogen in mediating myocardial SDF-1 production following I/R
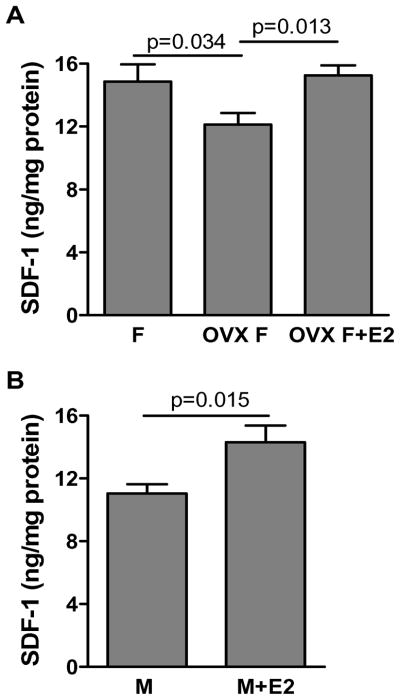
To elucidate whether gender difference in cardiac SDF-1 expression is due to the effect of estrogen, we utilized ovariectomy to deplete endogenous estrogen in female rats. In addition, E2 supplementation was employed in OVX F and male rats. Depletion of endogenous estrogen significantly decreased myocardial SDF-1 expression as shown by lower levels of SDF-1 in OVX F compared to their counterparts in response to acute I/R (Fig. 2A). Additionally, E2 supplementation restored myocardial SDF-1 production in OVX F and increased SDF-1 levels in males following I/R (Fig. 2A and B). These data indicated that estrogen played a role in I/R-induced SDF-1 expression.
Figure 2.
The effect of estrogen on myocardial SDF-1 expression after acute I/R. A. Cardiac SDF-1 levels were analyzed in groups of female, ovariectomized female (OVX F) and 17β-estrodial (E2) supplemented OVX F rat hearts subjected to I/R. B. The effect of E2 supplementation on I/R-increased SDF-1 production was determined in male rat hearts. Results are mean ± SEM, n=5–8/group.
The role of ERα and ERβ on estrogen- induced myocardial SDF-1 expression following I/R
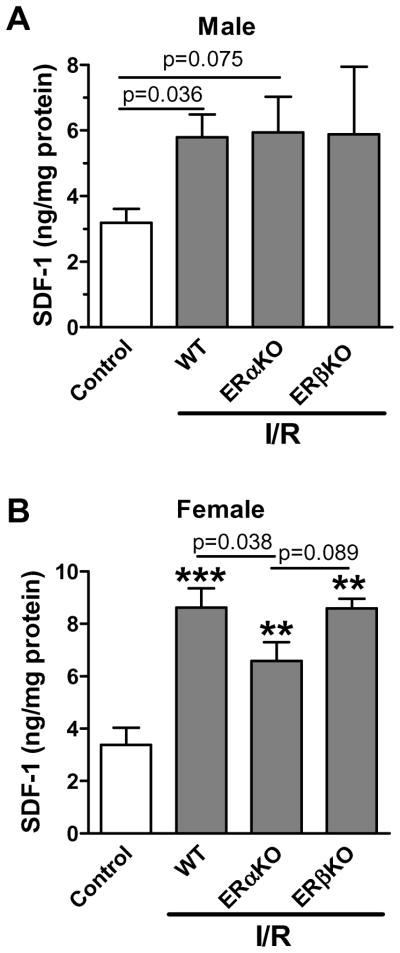
To identify which ER(s) is responsible for estrogen-increased SDF-1 production in the heart subjected to acute I/R, we utilized mice with deficiency of ERα or ERβ since neither highly selective ERβ antagonist is available for being used in rat model, nor the gene knockouts of ERα and ERβ are obtainable for rat species. We observed that ERαKO, not ERβKO, significantly decreased SDF-1 expression in female hearts (Fig. 3B) following I/R. However, ablation of ERα or ERβ did not affect I/R-induced SDF-1 production in male hearts (Fig. 3A).
Figure 3.
The effect of estrogen receptors (ER) on myocardial SDF-1 expression following I/R. A. Cardiac SDF-1 production was analyzed in male mouse hearts with ERαKO or ERβKO. B. Ablation of ERα, not ERβ, decreased SDF-1 levels in female mouse hearts after I/R. Results are mean ± SEM, n=4–6/group, **p<0.01, ***p<0.001 vs. control.
The roles of estrogen/ER(s) in the regulation of cardiac SDF-1 receptor following I/R
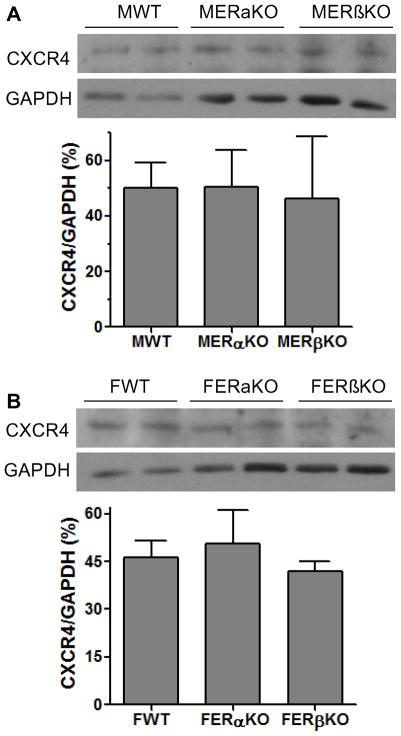
To determine whether estrogen not only mediates SDF-1 production, but regulates expression of the SDF-1 receptor – CXCR4 in the hearts following I/R, we measured myocardial expression of CXCR4 by Western blot assay. Unlike SDF-1, there was no gender difference in cardiac CXCR4 expression after I/R (Fig. 4). Additionally, although depletion of endogenous estrogen by OVX did decrease CXCR4 levels in female hearts (Fig. 5A), E2 supplementation did not restore CXCR4 expression in OVX F and did not upregulate cardiac CXCR4 expression in male hearts, (Fig. 5B, C). Furthermore, ablation of ERα or ERβ did not affect myocardial levels of CXCR4 in either male or female hearts following I/R (Fig. 6).
Figure 4.
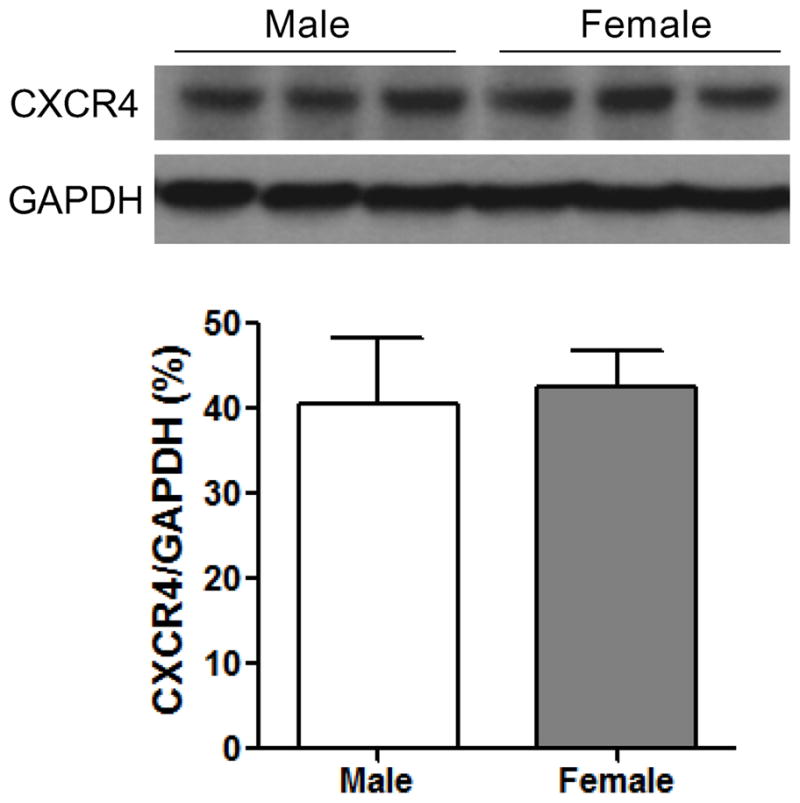
Cardiac CXCR4 (SDF-1 receptor) expression in male and female hearts subjected to I/R. Western blots of CXCR4 and GAPDH between male and female hearts are shown in the top. Bottom is bar graph of densitometry data of CXCR4 represented as % of GAPDH. Results are mean ± SEM, n=4–6/group.
Figure 5.
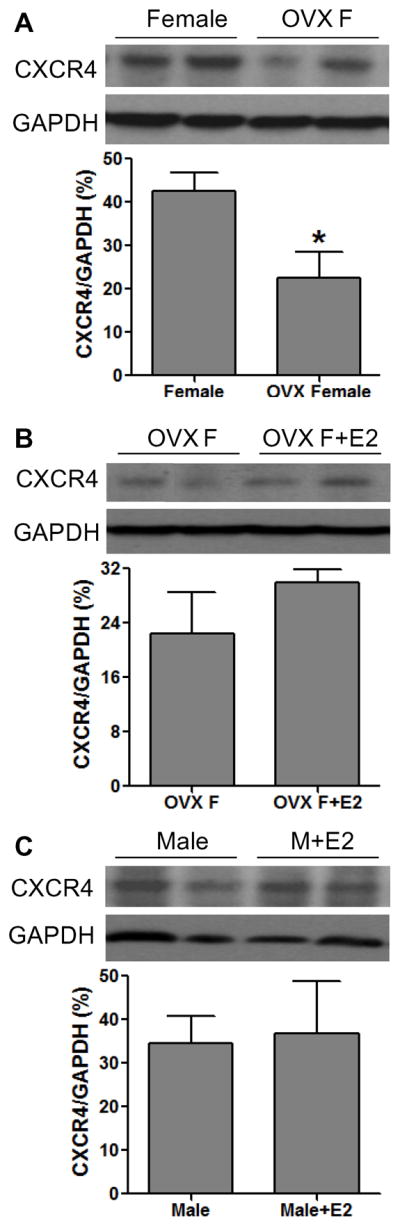
The effect of estrogen on cardiac CXCR4 expression following acute I/R. A. Depletion of endogenous estrogen by OVX decreased CXCR4 protein levels in female hearts. B. E2 supplementation did not restore myocardial CXCR4 expression in OVX F hearts. C. Chronic treatment with E2 did not affect male hearts expressing CXCR4 following I/R. Results are mean ± SEM, n=4–5/group, *p<0.05 vs. Female.
Figure 6.
The effect of ERs on myocardial CXCR4 expression following I/R. A. Cardiac CXCR4 protein levels were analyzed in male WT, ERαKO and ERβKO mouse hearts subjected to I/R. B. Knockouts of ERα or ERβ did not affect myocardial CXCR4 expression in female hearts following I/R. Results are mean ± SEM, n=4/group.
DISCUSSION
This study represents the first evidence of that following acute I/R, there are gender-specific differences in myocardial SDF-1 expression with higher levels of SDF-1 in female hearts compared to males, and this increased myocardial SDF-1 production is likely mediated by estrogen/ERα axis in females, at least in part.
SDF-1, a major chemokine, is required for recruiting of stem/progenitor cells into ischemic tissues (12–14, 26). SDF-1 is upregulated in the heart instantly after MI (12–14). Delivery of SDF-1 protein and gene directly into the heart has been shown to increase stem cells homing to the sites of injury, and thereby, promote angiogenesis, improve cardiac function and advance myocardial structure after MI (12, 14, 27). In addition, SDF-1 is able to suppress cardiomyocyte death through upregulated Akt activation and VEGF production in the infarcted heart (16, 17). Furthermore, SDF-1 is involved in mediating preconditioning-induced cardioprotection (16). Of note, gender differences have been reported in myocardial responses to ischemic injury. Our previous studies have demonstrated that female hearts exhibited improved myocardial function, reduced inflammation and suppressed apoptotic signaling following I/R (6, 8–10, 25, 28). However, it is unknown whether gender-specific differences in the myocardial responses are partly due to different SDF-1 expression between male and female following acute I/R. In this study, we observed that gender-specific difference existed in myocardial SDF-1 expression with higher levels of SDF-1 in female hearts compared to males after acute I/R, indicating that SDF-1 may play a role in protecting the female myocardium during ischemic injury.
It has been well-established that gender differences in myocardial responses to I/R are mainly attributable to estrogen, which exerts cardioprotective effects on females (5–11). Our group has previously indicated that exogenous estrogen supplementation restored cardiac functional recovery in estrogen-deficient animals (males and OVX females) following I/R (6). In fact, estrogen has been shown to exert antioxidant effect, decrease proinflammatory cytokine production and promote cell survival (5–11). In addition, estrogen mediates vasodilation through production of nitric oxide or activation of ion channels (29, 30). Furthermore, estrogen has been reported to inhibit the oxidation of low-density lipoprotein (11). Herein, we demonstrated that depletion of endogenous estrogen by OVX decreased SDF-1 production in female hearts and E2 supplementation restored it in OVX F following I/R, suggesting that estrogen-mediated cardioprotection may be partly contributed through E2-induced SDF-1 expression in the heart subjected to I/R.
Estrogen exerts its biological effects primarily through binding to its receptors ERα and/or ERβ, both of which are expressed in cardiomyocytes (29, 30). Our previous studies have demonstrated that both ERα and ERβ are involved in mediating estrogen-induced cardioprotection following acute I/R by using ERαKO and ERβKO mouse hearts, as well as selective agonists of ERα or ERβ (9, 10, 31, 32). ERα and ERβ are capable of activating PI3K/Akt pathway or MAPK signaling, suppressing apoptosis, reducing inflammation, regulating metabolic gene expression, modulating ion channel expression, and thus, eventually mediating cardioprotection in the ischemic heart (5–11). On the other hand, estrogen has been shown to induce SDF-1 production in a variety of cells through ERα. ERα-upregulated SDF-1 gene expression represents one of estrogenic activities in rat uterine cells (19). In the current study, reduced SDF-1 expression was observed in female ERαKO, but not ERβKO hearts following I/R. In addition, neither ERα nor ERβ affected I/R-induced SDF-1 production in male hearts (presumably with a lower baseline of endogenous estrogen). These results suggested that ERα was likely responsible for estrogen-upregulated SDF-1production in female hearts following acute I/R, and ERα-mediated SDF-1 expression might require a certain level of estrogen in the circulating and/or local tissue/organ. Our findings here further suggested that ERα-induced protection in female hearts might be partly mediated through SDF-1.
SDF-1 functions via binding to its cognate receptor, CXCR4, which has been reported to be expressed and functional in cardiac tissue (16). Similar to SDF-1, myocardial expression of CXCR4 is increased in the ischemic heart (33). However, it remains unclear whether gender/estrogen plays a role in regulating cardiac expression of CXCR4 following I/R as it did in myocardial SDF-1 production. In this study, gender difference was not observed in myocardial expression of CXCR4 after acute I/R. In addition, although depletion of endogenous estrogen decreased CXCR4 protein level in female hearts subjected to I/R, E2 supplementation did not restore it. Furthermore, ablation of ERα or ERβ did not affect CXCR4 expression in either male or female hearts. Taking all together, these results suggested that myocardial expression of CXCR4 was not regulated by estrogen and ERs in both genders following acute I/R.
Given that an in-vitro heart perfusion model was utilized in this study, it is impossible that estrogen-induced SDF-1 mediates protective effect through recruitment of stem cells. In fact, SDF-1 has been shown to promote cardiomyocyte survival via up-regulation of Akt activation following ischemic injury (17). In addition, SDF-1 suppresses cell death through activated ERK pathway in response to hypoxia/reoxygenation injury (16). Notably, our previous studies have indicated that estrogen increased myocardial activation of Akt and ERK, and reduced pro-apoptotic signaling following acute I/R (6, 9). Therefore, taking them together, these elevated activation of Akt and ERK, and decreased apoptotic signaling in female hearts might be partly attributable to the effect of estrogen-increased SDF-1 in response to acute I/R.
In conclusion, the present study provided the initial evidence regarding that gender differences in myocardial responses to ischemic injury may be partly due to estrogen-upregulated SDF-1 expression in female hearts through ERα. Our findings here suggest that gender differences in myocardial response to acute ischemic injury are related to cardiac SDF-1 levels.
Acknowledgments
FUNDING SOURCES
This work was supported by NIH R00 HL0876077 (MW).
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tunstall-Pedoe H, Morrison C, Woodward M, Fitzpatrick B, Watt G. Sex differences in myocardial infarction and coronary deaths in the Scottish MONICA population of Glasgow 1985 to 1991. Presentation, diagnosis, treatment, and 28-day case fatality of 3991 events in men and 1551 events in women. Circulation. 1996;93(11):1981–1992. doi: 10.1161/01.cir.93.11.1981. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308(5728):1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 4.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90(2):786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- 5.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95(7):692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40(2):205–212. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation. 2008;118(14 Suppl):S38–45. doi: 10.1161/CIRCULATIONAHA.107.756890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R972–978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290(6):H2204–2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 11.Sack MN, Rader DJ, Cannon RO., 3rd Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet. 1994;343(8892):269–270. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- 12.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21(12):3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 14.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42(4):792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tillmanns J, Rota M, Hosoda T, Misao Y, Esposito G, Gonzalez A, Vitale S, Parolin C, Yasuzawa-Amano S, Muraski J, De Angelis A, Lecapitaine N, Siggins RW, Loredo M, Bearzi C, Bolli R, Urbanek K, Leri A, Kajstura J, Anversa P. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105(5):1668–1673. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116(6):654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117(17):2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattarozzi A, Gatti M, Barbieri F, Wurth R, Porcile C, Lunardi G, Ratto A, Favoni R, Bajetto A, Ferrari A, Florio T. 17beta-estradiol promotes breast cancer cell proliferation-inducing stromal cell-derived factor-1-mediated epidermal growth factor receptor transactivation: reversal by gefitinib pretreatment. Mol Pharmacol. 2008;73(1):191–202. doi: 10.1124/mol.107.039974. [DOI] [PubMed] [Google Scholar]
- 19.Glace L, Grygielko ET, Boyle R, Wang Q, Laping NJ, Sulpizio AC, Bray JD. Estrogen-induced stromal cell-derived factor-1 (SDF-1/Cxcl12) expression is repressed by progesterone and by Selective Estrogen Receptor Modulators via estrogen receptor alpha in rat uterine cells and tissues. Steroids. 2009;74(13–14):1015–1024. doi: 10.1016/j.steroids.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Coser KR, Chesnes J, Hur J, Ray S, Isselbacher KJ, Shioda T. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc Natl Acad Sci U S A. 2003;100(24):13994–13999. doi: 10.1073/pnas.2235866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17(5):792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Tsuda H, Moriya T, Yamasaki T, Kikuchi R, Ueda S, Omata J, Yamamoto J, Matsubara O. Expression pattern of stromal cell-derived factor-1 chemokine in invasive breast cancer is correlated with estrogen receptor status and patient prognosis. Breast Cancer Res Treat. 2010;123(3):733–745. doi: 10.1007/s10549-009-0672-y. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Ni J, Zhang Y, Muyan M, Yeh S. ERAP75 functions as a coactivator to enhance estrogen receptor alpha transactivation in prostate stromal cells. Prostate. 2008;68(12):1273–1282. doi: 10.1002/pros.20774. [DOI] [PubMed] [Google Scholar]
- 24.Sauve K, Lepage J, Sanchez M, Heveker N, Tremblay A. Positive feedback activation of estrogen receptors by the CXCL12-CXCR4 pathway. Cancer Res. 2009;69(14):5793–5800. doi: 10.1158/0008-5472.CAN-08-4924. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2005;288(2):E321–326. doi: 10.1152/ajpendo.00278.2004. [DOI] [PubMed] [Google Scholar]
- 26.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 27.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109(20):2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Crisostomo PR, Markel T, Wang Y, Lillemoe KD, Meldrum DR. Estrogen receptor beta mediates acute myocardial protection following ischemia. Surgery. 2008;144(2):233–238. doi: 10.1016/j.surg.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 30.Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53(3):709–719. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Wang Y, Abarbanell A, Tan J, Weil B, Herrmann J, Meldrum DR. Both endogenous and exogenous testosterone decrease myocardial STAT3 activation and SOCS3 expression after acute ischemia and reperfusion. Surgery. 2009;146(2):138–144. doi: 10.1016/j.surg.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vornehm ND, Wang M, Abarbanell A, Herrmann J, Weil B, Tan J, Wang Y, Kelly M, Meldrum DR. Acute postischemic treatment with estrogen receptor-alpha agonist or estrogen receptor-beta agonist improves myocardial recovery. Surgery. 2009;146(2):145–154. doi: 10.1016/j.surg.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, Christensen G, Froland SS, Attramadal H, Gullestad L, Aukrust P. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47(4):778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]