Abstract
The medial and lateral perforant path projections to the hippocampal CA3 region display distinct mechanisms of long-term potentiation (LTP) induction, N-methyl-d-aspartate (NMDA) and opioid receptor dependent, respectively. However, medial and lateral perforant path projections to the CA3 region display associative LTP with coactivation, suggesting that while they differ in receptors involved in LTP induction they may share common downstream mechanisms of LTP induction. Here we address this interaction of LTP induction mechanisms by evaluating the contribution of opioid receptors to the induction of associative LTP among the medial and lateral perforant path projections to the CA3 region in vivo. Local application of the opioid receptor antagonists naloxone or Cys2-Tyr3-Orn5-Pen7-amide (CTOP) normally block induction of lateral perforant path-CA3 LTP. However, these opioid receptor antagonists failed to block associative LTP in lateral perforant path-CA3 synapses when it was induced by strong coactivation of the medial perforant pathway which displays NMDAR-dependent LTP. Thus strong activation of non-opioidergic afferents can substitute for the opioid receptor activation required for lateral perforant path LTP induction. Conversely, medial perforant path-CA3 associative LTP was blocked by opioid receptor antagonists when induced by strong coactivation of the opioidergic lateral perforant path. These data indicate endogenous opioid peptides contribute to associative LTP at coactive synapses when induced by strong coactivation of an opioidergic afferent system. These data further suggest that associative LTP induction is regulated by the receptor mechanisms of the strongly stimulated pathway. Thus, while medial and lateral perforant path synapses differ in their mechanisms of LTP induction, associative LTP at these synapses share common downstream mechanisms of induction.
Keywords: long-term potentiation (LTP), CA3, perforant path, NMDA receptor, mu opioid receptor, associative LTP
1.0 Introduction
Long term potentiation (LTP) remains the most likely and intensively studied model for learning and memory (Bliss and Lomo, 1973). An aspect of LTP that makes it attractive as a candidate for memory storage is the property of associativity (McNaughton et al., 1978; Levy and Steward, 1983; Barrionuevo and Brown, 1983). The induction of LTP typically requires synaptic activity and postsynaptic depolarization that are necessary for the activation of N-methyl-d-aspartate (NMDA) receptors. Associative LTP can be explained by properties of the NMDA receptor (NMDAR), such that even weak synaptic activity can induce LTP provided there is strong activation of the postsynaptic neuron by other afferents, resulting in NMDAR activation and LTP of both strong and weakly coactive synapses.
In the CA3 region, medial and lateral perforant path inputs terminate in discrete, thin (~ 100 μm) lamina on the distal apical dendrites of CA3 pyramidal cells (Ishizuka et al., 1995). Anatomic and physiologic studies indicate that the direct perforant path projections to CA3 and CA1 regions of the hippocampus are substantial and thus likely to be of significance in normal hippocampal operation (Amaral and Witter, 1989). Although only a few studies have addressed synaptic plasticity in these cortical-hippocampal projections, these studies indicate that monosynaptic medial perforant path-CA3 and lateral perforant path-CA3 synapses display LTP (Berger and Yeckel, 1991; Breindl et al., 1994; Do et al., 2002; McMahon et al., 2002).
LTP induced at medial and lateral perforant path synapses to both the dentate gyrus and CA3 region display distinct requirements (Bramham et al., 1988; Breindl et al., 1994; Do et al., 2002). Although LTP induction at medial perforant path projections to both the dentate gyrus and CA3 region is typical in that it is blocked by antagonists of the NMDA receptor, the lateral perforant path projections displays mechanisms of LTP induction that are insensitive to NMDAR antagonists in vivo (Bramham et al., 1991; Do et al., 2002; Kosub et al., 2005). However, the lateral perforant path is distinct in that it contains and releases proenkephalin-derived opioid peptides (Drake et al., 2007; Wagner et al., 1990), and lateral perforant path-CA3 LTP induction can be blocked by antagonists selective for mu (μ) opioid receptors in vivo, such as naloxone and CTOP (Do et al., 2002; Breindl et al., 1994). This is similar to LTP in the lateral perforant path projection to the dentate gyrus, which displays opioid receptor-dependent LTP in vivo (Bramham et a., 1991), and is blocked by μ opioid receptor antagonists (Bramham et al., 1988; Bramham and Sarvey, 1996; Xie and Lewis, 1991, 1995).
Previously, we reported associative LTP can be induced among medial and lateral perforant path projections to the CA3 region (Martinez et al., 2002). While the medial and lateral perforant path-CA3 synapses utilize distinct receptor mechanisms for homosynaptic LTP induction, they cooperate in a manner that allows for the mutual induction of associative LTP (McNaughton et al., 1978; Martinez et al., 2002). Because the medial and lateral perforant path-CA3 synapses display distinct mechanisms of LTP induction yet can display associative LTP, we investigated the interaction of these LTP induction mechanisms by evaluating the contribution of opioid receptors to associative LTP induction among medal and lateral perforant path synapses of the hippocampal CA3 region.
2. Materials and Methods
2.1 Experimental Materials
Adult male Sprague-Dawley rats (350-450 g, Harlan laboratories, Raleigh, NC) were anesthetized with pentobarbital sodium (60 mg/kg intraperitoneal) mounted in a stereotaxic frame, and maintained at a 39° C body temperature using a constant temperature heating pad. Anesthesia was maintained throughout the experiments with supplementary doses of pentobarbital (20 mg/kg/hr). All procedures complied with the National Institutes of Health Animal Care and Use Guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at San Antonio.
Electrodes were placed in the selected regions using the stereotaxic coordinates of Paxinos and Watson (1989; Fig. 1). Stimulation (monophasic constant current pulses, 0.2 ms) was delivered via electrodes constructed from twisted Teflon-coated stainless steel wire (0.008 mm diameter, A-M Systems). Field responses were collected using cannulatrode constructed of an Expoxylite coated 30 gauge stainless steel cannula exposed only at the tip (Do et al., 2002). The cannulatrode allows for recording field EPSPs and application of drugs at the same site. Responses evoked by stimulating the medial and lateral perforant path within the angular bundle and recording from the dentate gyrus of the hippocampal formation are well defined (McNaughton and Barnes, 1977; McNaughton, 1980; Breindl et al., 1994; Do et al., 2002). Afferents originating from the medial and lateral entorhinal cortex remain segregated within the angular bundle where they can be stimulated with a high degree of selectively in vivo (McNaughton and Barnes, 1977; McNaughton, 1977; Do et al 2002). By contrast, a similar degree of selectivity is more difficult in vitro due to the proximity of medial and lateral perforant path-CA3 projections in the stratum lacunosum moleculare of area CA3 (Berzhanskaya et al., 1998). Medial perforant path responses were evoked by stimulation of the extreme dorsomedial aspect of the angular bundle (AP −8.0 mm, ML +4.0 mm, DV −2.5 mm; Paxinos and Watson, 1989). Lateral perforant path responses were evoked by stimulation of the extreme ventrolateral aspect of the angular bundle (AP −7.9 mm, ML +4.0 mm, DV −2.5 mm; see Do et al., 2002). In order to isolate medial and lateral perforant path fibers, field responses were first recorded in the dentate gyrus (AP −3.5, ML 2.0, DV 3.3 mm). Due to the lateral perforant path fibers enervating the more distal dendrites, the transition from medial to lateral perforant path fibers can be seen as the stimulating electrode is lowered in the angular bundle, resulting in a decrease in field EPSP slope and peak latency of the lateral perforant path-dentate responses (Bramham et al., 1991). Such a transition is not readily apparent in CA3 responses as both medial and lateral perforant path afferents terminate in close proximity to each other in the distal stratum lacunosum moleculare of area CA3. Once medial and lateral perforant path responses were isolated in the dentate, the recording electrode was then moved to the CA3b region (AP −3.5, ML 3.5, DV 3.4 mm), where CA3 responses were isolated using stereotaxic coordinates, audio localization of the CA1 and CA3 pyramidal layer, and phase reversal of the CA3 response as the electrode entered the pyramidal cell layer of area CA3. Previous studies demonstrated the monosynaptic nature of perforant path responses recorded in the CA3 region (Yeckel and Berger, 1990; Breindl et al., 1994; Do et al., 2002). Perforant path-CA3 responses display characteristic spike and field EPSP peak latencies distinct from the dentate gyrus, displaying field EPSP onset latencies similar to the dentate gyrus, and display phase reversal upon penetration of the CA3 pyramidal cell layer. Additionally, perforant path-CA3 responses follow high frequency stimulation at 50 Hz (Do et al., 2002; Breindl et al., 1994) indicating they are monosynaptically evoked and do not result from either disynaptic activation of the dentate gyrus nor volume conduction from the dentate gyrus (Do et al., 2002). Medial and lateral perforant path responses also are distinguished by a marked facilitation seen with lateral perforant path responses when stimulated at 30-50 msec intervals. Together, these data indicate that perforant path responses observed at CA3 sites are monosynaptic and locally generated (Fig. 1).
Figure 1. Schematic diagram of the in vivo preparation for recording monosynaptic perforant path responses in the dentate gyrus and the CA3 region of the hippocampus.
(a) Field responses were first recorded in the hilar region of the dentate gyrus (DG Record). Following isolation of medial and lateral perforant path-dentate responses, the insulated cannula was placed was placed in the stratum pyramidal of area CA3 (CA3 Record), and perforant path-CA3 responses were evoked by stimulation of the medial (mpp) or the lateral aspect (lpp) of the perforant path within the angular bundle. (b) Medial perforant path responses recorded from both the dentate (dotted line) and the CA3 region (solid line) in the same animal under pentobarbital anesthesia. Note the latency to peak of population spikes in the dentate is approximately 1 ms later than CA3 population spikes. Calibration bars: 0.5 mV, 5 ms.
2.2 Experimental design
Low frequency responses were evoked in each pathway at 0.033 Hz using current intensities (100-600 μA) that elicited responses that were either 25% or 50% of the maximal field EPSP peak amplitude (as determined by estimates of asymptotic field EPSP magnitudes elicited by stimulating at current intensities up to 600 uA). Responses were amplified, filtered at 0.3 Hz-10 KHz, digitized (10 KHz) and stored for off-line analysis (DataWave Technologies, Loveland, CO). Measurement of the magnitude of both medial and lateral perforant path-CA3 responses was confined to the initial slope of field EPSPs measured over a 1 msec period 1 msec following response onset. LTP was induced using a modified theta burst paradigm consisting of five theta burst trains (with each theta burst train comprised of five 50 ms, 400 Hz bursts delivered at 200-ms intervals per 1.05 s train) and 1.05 s interval between trains; Do et al., 2002) delivered to either one pathway or paired in both pathways to induce associative LTP.
The nonselective opioid antagonist naloxone hydrochloride (10 nmole/μl in lactated Ringer’s; RBI Chemicals, St. Louis, MO), the selective μ opioid receptor antagonist Cys2-Tyr3-Orn5-Pen7-amide (CTOP, 3 nmol/μl in lactated Ringer’s, RBI Chemicals), the competitive NMDAR antagonist (+/−)-cyclopiperidine-6-piperiperenzine (CPP, 3 nmol/μl in lactated Ringer’s, Tocris Bioscience), and the lactated Ringer’s vehicle were applied via pressure ejection through the cannulatrode (1 μl total volume delivered at 0.2 μl/min) ten minutes prior to associative LTP induction using paired stimulation of both pathways. Previous studies demonstrate these quantities of naloxone and CTOP are effective in blocking μ opioid receptor agonist effects and the induction of lateral perforant path-CA3 LTP in vivo (Breindl et al., 1994; Do et al., 2002).
For each experiment, baseline responses were evoked at either 25% or 50% intensity for either the medial or lateral perforant path, and were collected for at least 20 minutes, after which the “strong” conditioning pathway evoked by a moderate current intensity (currents that elicit field EPSPs 50% of maximal amplitude) was tetanized first to verify that stimulation was effective in inducing LTP, and that LTP was input specific and confined only to the stimulated pathway. EEG was monitored for at least 1 min following delivery of trains, and no animals displayed afterdischarges following tetanization. Thirty minutes later, the alternate pathway was tetanized at its 25% current intensity to verify that weak stimulation alone was ineffective in inducing LTP. Animals displaying LTP > 20% of baseline 10-15 min following weak trains were eliminated from further study. Fifteen minutes later, drug or lactated Ringer’s vehicle were applied over a 5 minute period. After a 10 min period following cessation of drug delivery, both the medial and lateral perforant path were tetanized simultaneously using the same 25% and 50% current intensities, and responses from both pathways were recorded for an additional 30 minutes.
2.3 Data analysis
Tetanus-induced changes in response amplitudes after stimulation were measured between 26 and 30 minutes after homosynaptic stimulation in the strongly stimulated pathway, 5-10 minutes after delivery of weak stimulation and 26-30 minutes following paired stimulation of both pathways, and are reported as a percent change in response magnitude as compared to baseline response magnitudes measured 5 minutes prior to delivery of high frequency trains. The changes observed following stimulation were evaluated statistically using a repeated measures analysis of variance (ANOVA, Keppel and Zedeck, 1994) and post hoc Newman-Keuls test for pairwise comparisons. Correct electrode placement was verified by stereotaxic coordinates, audio localization of the CA1 and CA3 pyramidal cell layers, and histologic analysis to verify electrode placement in the CA3 region.
3. Results
3.1 Characteristics of monosynaptic medial and lateral perforant path-CA3 responses
Responses evoked by stimulation of the medial or lateral perforant path projections to the CA3 region have been characterized extensively both in vitro and in vivo, and are described in detail elsewhere (Wu and Leung, 1998; Berzhanskaya et al., 1998; Breindl et al., 1994; Do et al, 2002; Martinez et al., 2002). Briefly, previous studies demonstrate that perforant path responses are monosynaptic and locally-generated, as they can be recorded in the CA3 region in vivo without contamination from volume conducted dentate responses nor disynaptically-mediated mossy fiber-CA3 responses (Fig. 1). The slope of perforant path-CA3 responses can be measured without contamination from disynaptically evoked mossy fiber-CA3 responses because (1) the current intensities used to evoke baseline perforant path-CA3 responses did not elicit dentate population spikes, and (2) the onset of perforant path-CA3 and perforant path-dentate field EPSP responses were approximately 2-3 msec post stimulation and were measured 1-2 msec after field EPSP onset, whereas disynaptically evoked perforant path-dentate-CA3 responses are only observed > 7-10 ms later (Yeckel and Berger, 1990).
3.2 Naloxone and CTOP block associative medial perforant path-CA3 LTP induced by strong coactivation of the opioidergic lateral perforant path
We were interested in determining if opioid receptor activation is critical for the induction of associative medial perforant path-CA3 LTP when induced by strong activation of the opioidergic lateral perforant path.
We first verified the ability to induce associative LTP following application of only the lactated Ringer’s vehicle using the paradigm from our previous associativity studies among CA3 afferents (Martinez et al., 2002). Tetanization of the lateral perforant path using theta burst trains delivered at a strong current intensity (50% of maximal) induced an input-specific potentiation of lateral perforant path-CA3 responses (percent change in field EPSP slopes 25-30 minutes after tetanus = 124 ± 3%, (F [1,5] = 39.3, p < 0.01, n = 6; Fig. 2a) and no significant differences in the medial perforant path-CA3 responses (F [3,5] = 6.22, p < 0.01, repeated measures ANOVA; p > 0.05, Newman-Keuls test, n = 6). Tetanization of medial perforant path inputs 30 minutes later using weak current intensities (25% of maximal) did not alter medial perforant path responses significantly from baseline values when measured 10-15 minutes after tetanus (104 ± 9%, p > 0.05, Newman-Keuls test; Fig. 2a). Fifteen minutes following weak medial perforant path stimulation, the lactated Ringer’s vehicle was applied locally within the CA3 region. Ten minutes after the lactated Ringer’s vehicle application was complete, simultaneous stimulation was delivered to both the medial and lateral perforant path using 25% and 50% current intensities, respectively. Pairing weak medial perforant path trains with strong lateral perforant path trains induced associative LTP of the weak medial perforant path responses (139 ± 12%, p < 0.01, Newman-Keuls test; Fig. 2a) in the presence of the lactated Ringer’s vehicle. As demonstrated previously (Martinez et al., 2002), pairing weak medial perforant path trains normally ineffective in inducing LTP together with strong trains delivered to the lateral perforant path induced associative LTP of medial perforant path-CA3 responses.
Figure 2. Opioid receptor antagonists block the associative induction of medial perforant path-CA3 LTP induced by strong coactivation of the lateral perforant path.
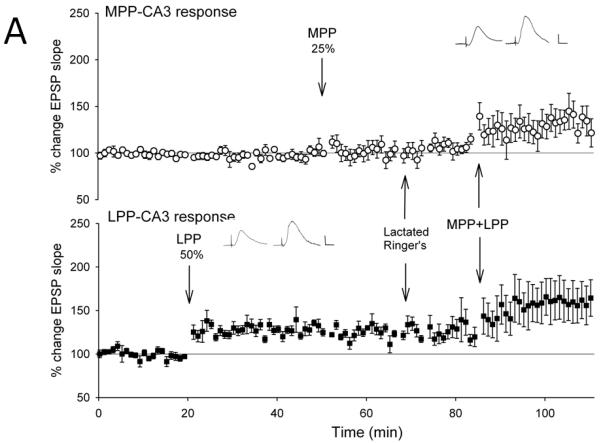


Lateral perforant path-CA3 responses were evoked using current intensities that evoked responses that were 50% of the maximum evoked response (■, n = 5-6), and medial perforant path-CA3 responses were evoked using current intensities that evoked responses that were 25% of the maximum evoked response (○, n= 5-6). Following collection of baseline responses, the lateral perforant path was tetanized at 50% current intensity using theta burst trains, revealing input specific lateral perforant path-CA3 LTP. Thirty minutes later, tetanization of the medial perforant path using theta bursts delivered at 25% current intensity was ineffective in potentiating responses. Fifteen minutes later, the lactated Ringer’s vehicle (A, 1 ul total volume, n = 6), the nonselective opioid antagonist naloxone (B, 10 nmol, n = 5), the selective μ opioid receptor antagonist CTOP (C, 3 nmol, n = 5), or the NMDA receptor antagonist (+/−)-cyclopiperidine-6-piperiperenzine, or CPP (D, 3 nmol, n=4) was applied over a 5 minute period. Ten minutes following cessation of drug delivery, theta burst trains were delivered simultaneously to both medial and lateral perforant path at 25% and 50% current intensities, respectively. Induction of associative LTP of medial perforant path responses was assessed for 25-30 minutes following delivery of the paired trains. Naloxone (p > 0.05) and CTOP (p > 0.05), but not lactated Ringer’s (p < 0.05), blocked associative LTP of medial perforant path-CA3 response following delivery of paired trains. Note that the additional potentiation of lateral perforant path-CA3 responses with paired stimulation (A, p < 0.05) is blocked by both naloxone (B, p > 0.05) and CTOP (C, p > 0.05). Inserts are representative responses. Calibration bars: 0.25 mV, 5 ms.
We then assessed the effects of the nonselective opioid receptor antagonist naloxone using the quantity previously found effective in blocking only lateral perforant path LTP induction (Breindl et al., 1994). Tetanization of the lateral perforant path at strong current intensities (50% of maximal) produced an input-specific potentiation of lateral perforant path responses (146 ± 17%, F [1,4] = 7.97, p < 0.05, n = 5; Fig. 2b) and no significant differences in medial perforant path responses (F [3,4] = 0.125, p > 0.05, n = 5, repeated measures ANOVA; p > 0.05, Newman-Keuls test). Tetanization of medial perforant path inputs using weak current intensities (25% of maximal) did not alter responses significantly from baseline values (102 ± 15%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak medial perforant path stimulation, naloxone was applied locally within the CA3 region. Ten minutes following drug application, associative medial perforant path LTP was induced by simultaneous stimulation of both the medial and lateral perforant path using 25% and 50% current intensities, respectively. Naloxone blocked the induction of associative LTP in medial perforant path-CA3 (101 ± 10%, p > 0.05, Newman-Keuls test; Fig. 2b) when weak medial perforant path trains were paired with strong lateral perforant path trains. Thus, naloxone blocked associative medial perforant path LTP induced by delivery of strong trains to the opioidergic lateral perforant path.
Similar results were observed with the application of the selective μ opioid receptor antagonist CTOP in quantities effective in blocking lateral perforant path-CA3 LTP induction but had no effect on medial perforant path-CA3 LTP induction (Do et al., 2002). Tetanization of strongly stimulated lateral perforant path (50% of maximal) produced an input-specific potentiation of lateral perforant path responses (137 ± 10%, F [1,4] = 12.5, p < 0.05, n = 5; Fig. 2c) but no significant effects on medial perforant path responses (F [3,4] = 0.569, p > 0.05, n = 5, repeated measures ANOVA; p > 0.05, Newman-Keuls test). Subsequent tetanization of the medial perforant path using weak (25% of maximal) current intensities did not alter responses significantly (103 ± 9%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak medial perforant path stimulation, CTOP was applied locally within the CA3 region. Ten minutes after drug application, simultaneous stimulation was delivered to both the medial and lateral perforant path using 25% and 50% current intensities, respectively. Medial perforant path-CA3 associative LTP was blocked (110 ± 16%, p > 0.05, Newman-Keuls test) when weak medial perforant path trains were paired with strong lateral perforant path trains in the presence of CTOP. Together, these data indicate the nonselective opioid receptor antagonist naloxone and the selective μ opioid receptor antagonist CTOP, drugs normally ineffective in blocking medial perforant path-CA3 LTP, block medial perforant path-CA3 LTP when it is induced in an associative manner by strong coactivation of the opioidergic lateral perforant path. Taken together the data suggests that the mechanisms of LTP induction in the strongly stimulated lateral perforant pathway are necessary for inducting associative medial perforant path-CA3 LTP.
3.3 Naloxone and CTOP fail to block associative lateral perforant path-CA3 LTP when induced by coactivation of medial perforant path-CA3 afferents
We determined if opioid receptor antagonists are effective in blocking opioidergic lateral perforant path associative LTP when it is induced by pairing weak lateral perforant path trains with strong trains delivered to the non-opioidergic medial perforant path. We first assessed associative LTP following application of the lactated Ringer’s vehicle. Tetanization of the medial perforant path using theta burst trains delivered at a strong current intensity (50% of maximal) induced an input-specific potentiation of medial perforant path-CA3 responses (138 ± 13%, F [1,4] = 9.28, p < 0.05, n = 5; Fig. 3a) and no significant change in lateral perforant path responses (F [3,4] = 14.30, p < 0.001, n = 5, repeated measures ANOVA, p > 0.05, Newman-Keuls test). Tetanization of lateral perforant path inputs 30 minutes later using weak current intensity (25% of maximal) induced a non-significant depression of lateral perforant path-CA3 responses as measured 10-15 minutes after tetanus (87 ± 9%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak lateral perforant path stimulation, lactated Ringer’s vehicle was applied locally within the CA3 region. Ten minutes after the lactated Ringer’s vehicle application was complete, simultaneous stimulation was delivered to both the medial and lateral perforant path using 50% and 25% current intensities, respectively. As shown in Figure 3a, pairing strong medial perforant path trains with weak lateral perforant path trains induced associative LTP of the weak lateral perforant path responses (127 ± 6%, p < 0.001, Newman-Keuls test) as previously reported (Martinez et al., 2002).
Figure 3. NMDA receptor antagonists, but not opioid receptor antagonists block the induction of lateral perforant path-CA3 LTP induced by strong coactivation of the medial perforant path.

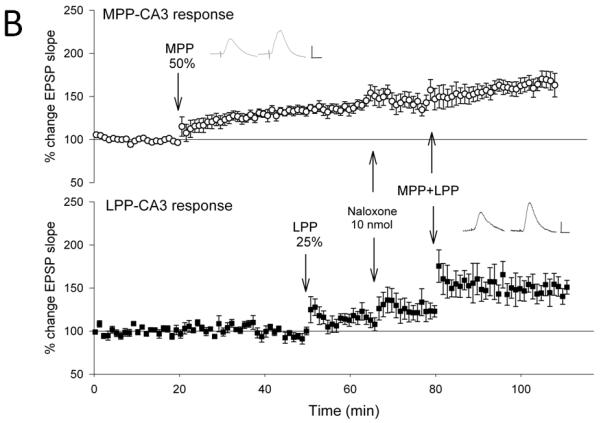
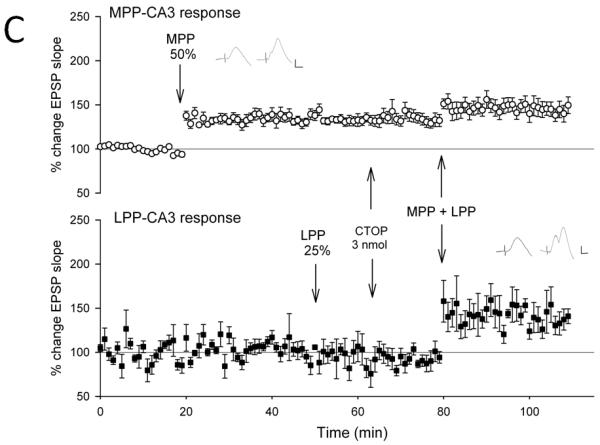
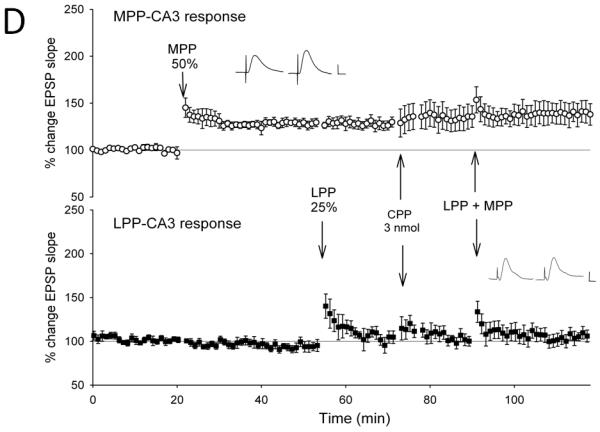
Medial perforant path-CA3 responses were evoked using current intensities that evoked responses that were 50% of the maximum evoked response (○, n= 5-6), and lateral perforant path-CA3 responses were evoked using current intensities that evoked responses that were 25% of the maximum evoked response (■, n = 5-6). Following collection of baseline responses, the medial perforant path was tetanized at 50% current intensity using theta burst trains, revealing input specific medial perforant path-CA3 LTP. Thirty minutes later, tetanization of the lateral perforant path using theta bursts delivered at 25% current intensity was ineffective in potentiating responses. Fifteen minutes later, the lactated Ringer’s vehicle (A, 1 ul total volume, n = 5), the nonselective opioid antagonist naloxone (B, 10 nmol, n = 6), the selective μ opioid receptor antagonist CTOP (C, 3 nmol, n = 6) or the competitive NMDA receptor antagonist CPP (D, 3 nmol, n = 5) was applied over a 5 minute period. Ten minutes following cessation of drug delivery, theta burst trains were delivered simultaneously to both medial and lateral perforant path at 50% and 25% current intensities, respectively. Induction of associative LTP of lateral perforant path responses was observed 25-30 minutes following delivery of the paired trains. In addition, although associative medial perforant path LTP was seen with the Lactated Ringer’s vehicle (A, p < 0.05), both naloxone (B, p < 0.05) and CTOP (C, p < 0.05) failed to block associative LTP of the lateral perforant path-CA3 response following delivery of paired trains. By contrast, blocking NMDA receptors blocked the ability of strong medial perforant path trains to induce associative lateral perforant path-CA3 LTP (D, p > 0.05). Inserts are representative responses. Calibration bars: 0.25 mV, 5 ms.
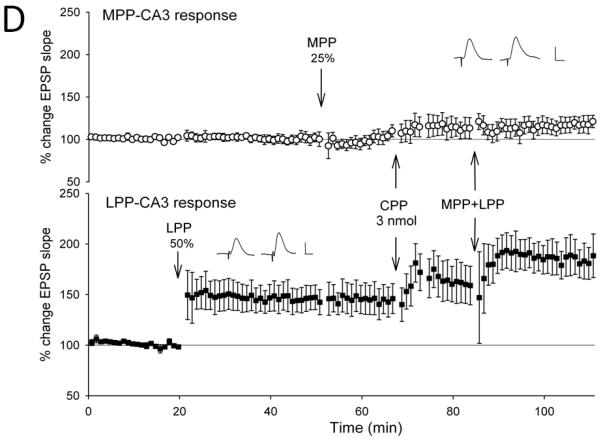
Next, we assessed the effects of the nonselective opioid receptor antagonist naloxone using the quantity previously found effective in blocking only lateral perforant path LTP induction (Breindl et al., 1994). Tetanization of the strongly stimulated medial perforant path responses (50% of maximal) produced an input-specific potentiation of medial perforant path responses (133.8 ± 6%, F [1,5] = 30.1, p < 0.01, n = 6, Fig. 3b) and no significant differences observed in lateral perforant path responses (F [3,5] = 6.71, p < 0.01, repeated measures ANOVA, n = 6; p > 0.05 Newman-Keuls test). Tetanization of lateral perforant path inputs using weak current intensity (25% of maximal) induced did not significantly alter responses compared to baseline values when measured 10-15 minutes after tetanus (114 ± 9%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak lateral perforant path stimulation, naloxone was applied locally within the CA3 region. Ten minutes following drug application, simultaneous stimulation was delivered to both the medial and lateral perforant path using 50% and 25% current intensities, respectively. As shown in Figure 3b, associative LTP of the opioidergic lateral perforant path-CA3 responses (151 ± 14%, p < 0.05, Newman-Keuls test) was induced in the presence of naloxone by pairing weak lateral perforant path trains with strong medial perforant path trains. Thus, the nonselective opioid antagonist naloxone, which normally is effective in blocking induction of homosynaptic lateral perforant path-CA3 LTP, was ineffective in blocking lateral perforant path-CA3 LTP when it was induced in an associative manner by strong coactivation of the medial perforant path.
A similar result was observed with application of the selective μ opioid receptor antagonist CTOP. Tetanization of strongly stimulated medial perforant path responses (50% of maximal) produced an input-specific potentiation of medial perforant path responses (138 ± 5%, F [1,5] = 44.7, p < 0.01, n = 6, Fig. 3c) and no significant differences in lateral perforant path responses (F [3,5] = 4.80, p < 0.05, repeated measures ANOVA, n = 6; p > 0.05, Newman-Keuls test). Subsequent tetanization of lateral perforant path inputs using a weak current intensity (25% of maximal) did not significantly alter responses (107 ± 15%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak lateral perforant path stimulation, CTOP was applied locally to the CA3 region. Ten minutes after drug application, simultaneous stimulation was delivered to both the medial and lateral perforant path using 50% and 25% current intensities, respectively. As shown in Figure 3c, associative LTP of the opioidergic lateral perforant path-CA3 responses was induced in the presence of CTOP by pairing weak lateral perforant path trains with strong medial perforant path trains. (159 ± 27%, p < 0.05, Newman-Keuls test). Thus, the nonselective opioid antagonist naloxone and the selective μ opioid receptor antagonist CTOP, both of which are effective in blocking homosynaptic lateral perforant path-CA3 LTP, were ineffective in blocking associative lateral perforant path LTP induced by the strong coactivation of the convergent medial perforant path. Because strong medial perforant path stimulation induces associative lateral perforant path LTP even when opioid receptors are blocked, this suggests the induction mechanisms of medial perforant path LTP are not only necessary, but are also sufficient, to induce associative lateral perforant path-CA3 LTP.
It is possible that opioid receptor antagonist were ineffective in blocking associative lateral perforant path LTP because of a sustained effect of opioid receptor activation that may have followed the first set of weak lateral perforant path trains. However, the additional potentiation of lateral perforant path responses seen after the second set of strong lateral perforant path trains used to induce associative medial perforant path LTP (154 ± 31%, p < 0.05, Newman-Keuls test, Fig. 2a) was not observed in the presence of either naloxone (p > 0.05, Fig. 2b) or CTOP (p > 0.05, Fig. 2c). Because CTOP and naloxone blocked additional homosynaptic lateral perforant path LTP normally observed following the second paired stimulation of lateral perforant path trains, it is unlikely that opioid receptor antagonist were ineffective in blocking associative lateral perforant path LTP because of a sustained effect of opioid receptor activation that may have occurred with initial LTP induction. If this were the case, then any additional homosynaptic potentiation of lateral perforant-CA3 responses also would have been unaffected by these opioid receptor antagonists.
3.4 The NMDAR antagonist CPP blocks associative lateral perforant path-CA3 LTP when induced by coactivation of medial perforant path-CA3 afferents
Because associative LTP induced by strong lateral perforant path trains is blocked by opioid receptor antagonists, associative LTP among perforant path-CA3 synapses appears to depend upon the induction mechanism of the strongly stimulated pathway. If this is true, then the induction of associative lateral perforant path LTP by strong stimulation of the medial perforant path would be expected to be blocked by NMDAR antagonists.
We tested this hypothesis by using the quantity of CPP (3 nmol) previously identified to be effective in blocking medial perforant path-CA3 LTP in vivo (Do et al., 2002). Tetanization of the strongly stimulated medial perforant path responses (50% of maximal) produced an input-specific potentiation of medial perforant path-CA3 responses (128.6 ± 4%, F [1,4] = 43.77, p < 0.05, n = 5, Fig. 3d), and no significant differences observed in lateral perforant path responses (F [3,4] = 2.17, p > 0.05, n = 5; p > 0.05, Newman-Keuls test). Tetanization of lateral perforant path inputs using weak current intensity (25% of maximal) did not significantly alter responses compared to baseline values when measured 10-15 minutes after tetanus (111.3 ± 5%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak lateral perforant path stimulation, a 3 nmol quantity of CPP was applied locally within the CA3 region. Ten minutes following drug application, simultaneous stimulation was delivered to both the medial and lateral perforant path using 50% and 25% current intensities, respectively. As shown in Figure 3d, associative LTP of the opioidergic lateral perforant path-CA3 responses was blocked by CPP (101.0 ± 3%, n = 5, p > 0.05, Newman-Keuls test). Further potentiation of medial perforant path responses with paired stimulation was not observed with CPP (134.6 ± 1%, p > 0.05 Newman-Keuls test). Thus, just as opioid receptor antagonists blocked associative medial perforant path LTP, the NMDAR antagonist CPP blocked associative lateral perforant path LTP induced by strong coactivation of the medial perforant path. Taken together, this indicates the receptor mechanisms of LTP induction in the strongly stimulated pathway are necessary for inducing associative perforant path LTP at both lateral and medial perforant path synapses.
Although associative lateral perforant path LTP induced by medial perforant path stimulation is blocked by NMDA antagonists, it is also insensitive to μ opioid receptor antagonists, indicating that the receptor mechanisms of LTP induction in the medial perforant pathway are both necessary and sufficient to induce associative lateral perforant path LTP. If the mechanisms of lateral perforant path LTP induction are also sufficient to induce associative medial perforant path LTP, then we would expect that associative medial perforant path LTP induced by strong lateral perforant path stimulation to be unaffected by NMDAR antagonists, just as associative lateral perforant path LTP is unaffected by naloxone when it is induced by strong medial perforant path stimulation. As above, tetanization of the strongly stimulated lateral perforant path responses (50% of maximal) produced an input-specific potentiation of lateral perforant path-CA3 responses (144.9 ± 12%, F [1,3] = 13.38, p < 0.05, n = 4, Fig. 2d), and no significant differences observed in medial perforant path responses (F [3,3] = 2.15, p > 0.05, n = 4; p > 0.05 Newman-Keuls test). Tetanization of medial perforant path inputs using weak current intensity (25% of maximal) did not significantly alter responses compared to baseline values when measured 10-15 minutes after tetanus (100.2 ± 3%, p > 0.05, Newman-Keuls test). Fifteen minutes following weak medial perforant path stimulation, a 3 nmol quantity of CPP was applied locally within the CA3 region. Ten minutes following drug application, simultaneous stimulation was delivered to both the lateral and medial perforant path using 50% and 25% current intensities, respectively. As shown in Figure 2d, associative LTP of the opioidergic lateral perforant path-CA3 responses was blocked by CPP when weak medial perforant path trains were paired with strong lateral perforant path trains (102 ± 3%, n = 4, p > 0.05, Newman-Keuls test). Thus CPP, an NMDA receptor antagonist effective in blocking the induction of homosynaptic medial perforant path-CA3 LTP, was also effective in blocking associative medial perforant path LTP induced by strong coactivation of the lateral perforant path. This is unlike associative lateral perforant path LTP, which is unaffected by opioid receptor antagonists. Thus strong lateral perforant path stimulation and μ opioid receptor activation are necessary, but not sufficient, for inducing associative medial perforant path LTP, as associative medial perforant LTP induction still requires the activation of NMDA receptors.
4. Discussion
Previously we reported that both the medial and lateral perforant path projections to the CA3 region in vivo utilize distinct mechanisms of LTP induction. The medial perforant path-CA3 synapse displays LTP that is blocked by the NMDA receptor antagonist 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid (CPP), but is unaffected by opioid receptor antagonists (Do et al., 2002; Breindl et al., 1994). By contrast, the lateral perforant path-CA3 synapse displays LTP induction that is insensitive to NMDA receptor antagonists (Do et al., 2002; Kosub et al., 2005), but is blocked by the opioid receptor antagonist naloxone and the selective μ opioid receptor antagonist CTOP (Do et al., 2002). In the present studies we first assessed the contribution of opioid receptors to associative LTP among medial and lateral perforant path-CA3 synapses. We found that when medial perforant path-CA3 LTP is induced by the strong coactivation of the lateral perforant path, medial perforant path LTP, which normally is unaffected by opioid receptor antagonists (Do et al., 2002, Kosub et al., 2005) is rendered sensitive to μ opioid receptor antagonists. Thus μ opioid receptor activation is necessary for the induction of associative LTP at synapses normally unaffected by opioid receptor antagonists when it is induced by strong activation of an opioidergic pathway. Conversely, we found that opioid receptor antagonists, in quantities normally effective in blocking homosynaptic lateral perforant path-CA3 LTP, are ineffective in blocking associative lateral perforant path LTP when induced by strong coactivation of the convergent medial perforant path and NMDAR activation. This indicates opioid receptor activation is no longer necessary for the induction of lateral perforant path-CA3 LTP when induced by strong coactivation of the non-opioidergic medial perforant path; rather, strong coactivation of a convergent excitatory path devoid of opioid peptides can substitute for opioid receptor activation normally required for the induction of lateral perforant path-CA3 LTP (Do et al., 2002; Breindl et al., 1994). Thus NMDAR-dependent mechanisms of LTP induction at the medial perforant path-CA3 synapses are both necessary and sufficient for the induction of associative lateral perforant path LTP.
The contribution of NMDA receptors to associative perforant path-CA3 LTP parallels to some degree that observed with opioid receptors. Although the NMDAR antagonist CPP is ineffective in blocking induction of homosynaptic lateral perforant path-CA3 LTP, CPP blocked associative lateral perforant path LTP when it was induced by strong coactivation of the medial perforant pathway. Thus, just as opioid receptor antagonists block associative medial perforant path LTP induced by strong lateral perforant path stimulation, the NMDAR antagonist CPP blocked the induction of associative lateral perforant path LTP induced by strong stimulation of the medial perforant pathway. This indicates that the receptor mechanisms of LTP induction in the strongly stimulated perforant pathway are essential for associative LTP induction among perforant path-CA3 synapses.
However, a different effect was observed with CPP and associative medial perforant path LTP. Although strong activation of the medial perforant pathway can induce associative lateral perforant path LTP, even in the presence of opioid receptor antagonists, associative medial perforant path LTP induced by lateral perforant path stimulation was still blocked by the NMDAR antagonist CPP. Thus, while strong medial perforant path stimulation and NMDAR activation are necessary and sufficient to induce associative lateral perforant path LTP, strong lateral perforant path stimulation and opioid receptor activation are necessary, but not sufficient, for inducing associative medial perforant path LTP, as NMDAR activation is still necessary to induce associative medial perforant path LTP.
The effects of opioid receptor antagonists on LTP induction may be understood from current views of the actions of opioid receptors in the hippocampus. Previous studies indicate LTP induction at lateral perforant path-dentate gyrus synapses is blocked by μ opioid receptor antagonists (Bramham and Sarvey, 1996; Xie and Lewis, 1991, 1995; Bramham et al., 1988). In addition, it is well established that μ opioid receptor agonists can inhibit both GABAergic interneurons (Madison and Nicoll, 1988) and GABA release in hippocampal interneurons (Cohen et al., 1992), resulting in the excitation of hippocampal principal neurons due to a reduction in GABAergic inhibition (Corrigall, 1983). Importantly, a reduction in GABAergic inhibition greatly facilitates postsynaptic depolarization and increases the likelihood of LTP induction (Wigstrom and Gustafsson, 1985). Thus the release of opioid peptides and subsequent μ opioid receptor activation are suggested to contribute to LTP induction by reducing GABAergic inhibition, and facilitating postsynaptic depolarization and subsequent LTP induction (Xie and Lewis, 1995; Bramham and Sarvey, 1996). In support of this view, the induction of lateral perforant path-dentate LTP is blocked by naloxone. However, this effect can be overcome by co-application of a GABAa receptor antagonist (Bramham and Sarvey, 1996; Xie and Lewis, 1995). Thus one mechanism by which opioid peptides are thought to contribute to lateral perforant path-dentate LTP induction is via a reduction in GABAergic inhibition leading to enhanced postsynaptic depolarization necessary for lateral perforant path LTP induction (Bramham and Sarvey, 1996; Xie and Lewis, 1995). Although the induction of lateral perforant path LTP in vivo is insensitive to NMDA receptor antagonists, the mechanisms by which opioid receptor-mediated disinhibition and enhanced postsynaptic depolarization mediate lateral perforant path LTP induction are not known, but may involve postsynaptic calcium influx via voltage-dependent calcium channels (Baratta et al., 2002).
The actions of both opioid and NMDA receptor activation offer a plausible explanation for the present findings. At medial perforant path-CA3 synapses, associative LTP is rendered sensitive to opioid receptor antagonists when it is induced by strong coactivation of the opioidergic lateral perforant path. Thus the requirement for opioid receptor-mediated disinhibition necessary for lateral perforant path LTP induction also is necessary for the induction of associative medial perforant path LTP. Because weakly active medial perforant path synapses allows NMDA receptor activation, but cannot provide the postsynaptic depolarization essential for ion flow through NMDA receptors, the disinhibition and additional postsynaptic depolarization provided by opioid receptor activation may allow for NMDA receptor operation and the associative induction of medial perforant path-CA3 LTP. This view of a cooperative interaction of NMDA and opioid receptors is supported by our data indicating both opioid (Fig. 2b,c) and NMDAR (Fig. 2d) receptor activation are necessary for associative medial perforant path LTP induced by strong lateral perforant path activation. Importantly, this finding supports previously hypothesized roles for opioid peptides in facilitating the induction of LTP at synapses that utilize NMDA receptors for LTP induction (Swearengen and Chavkin, 1987; Milner and Drake, 2001; Segal, 1988). This suggests that opioid receptor activation, and their resultant disinhibitory actions, can modulate LTP induction. This effect may result from an attenuation of GABAergic inhibition and the resultant enhancement of depolarization and dendritic spikes (Kampa et al., 2007) thought crucial for LTP induction at perforant path synapses on apical pyramidal cell dendrites (Golding and Spruston, 2002; Kampa et al., 2007).
We also found that opioid receptor activation is no longer necessary for inducing lateral perforant path LTP when it is induced in an associative manner by the strong coactivation of the non-opioidergic medial perforant path, because the depolarization and NMDA receptor activation provided by medial perforant path stimulation can substitute for μ opioid receptor activation normally required to induce lateral perforant path LTP. Because the principal actions of opioid peptides augment postsynaptic depolarizing via their disinhibitory actions, a likely possibility is postsynaptic depolarization afforded by strong medial perforant path stimulation and NMDAR activation can substitute for the disinhibitory effects of opioid receptor activation required for inducing associative lateral perforant path LTP. Similarly, the disinhibitory actions of GABAa antagonists can substitute for the disinhibitory actions of opioid receptor activation and overcome the naloxone block of lateral perforant path LTP induction (Bramham and Sarvey, 1996; Xie and Lewis, 1995).
Another principal finding derived from these studies is that activation of receptors necessary for LTP induction in the strongly stimulated perforant pathway are also essential for the induction of associative LTP at coactive perforant path synapses. Although lateral perforant path LTP is normally insensitive to NMDAR antagonists, NMDAR antagonist are effective in blocking associative lateral perforant path-CA3 LTP when it is induced by the coactivation of the medial perforant pathway, an afferent system that displays an NMDAR-dependent form of LTP induction. Similarly, opioid receptor antagonists block associative medial perforant path LTP when it is induced by strong coactivation of opioidergic lateral perforant pathway, even though medial perforant path LTP normally is unaffected by opioid receptor antagonists. Taken together, these results indicate that the mechanisms of LTP induction in the strongly stimulated pathway are necessary for the induction of associative LTP at weakly coactive synapses. Thus while medial and lateral perforant path-CA3 synapses display distinct mechanisms of homosynaptic LTP induction, the receptor mechanisms of LTP induction in the strongly stimulated pathway are necessary for associative LTP induction at coactive perforant path synapses. This suggests LTP at these synapses share common downstream mechanisms for associative LTP induction.
One downstream mechanism likely to be shared by these synapses is that activation of the receptors involved in LTP induction in the strong pathway are crucial for achieving levels of postsynaptic depolarization necessary to induce associative LTP at weakly coactive synapses. This may underlie the requirement for opioid receptor activation for inducing associative LTP in medial perforant path synapses when paired with strong lateral perforant path activity. In this case, the enhanced postsynaptic depolarization provided by opioid receptor-mediated disinhibition and necessary for lateral perforant path LTP induction is also necessary for NMDAR activation and associative LTP induction at coactive medial perforant path synapses. Similarly, strong activation of medial perforant path synapses sufficient to activate NMDARs may be necessary to provide levels of postsynaptic depolarization that are normally provided by the disinhibitory effects of opioid peptides and required for lateral perforant path LTP induction (Bramham and Sarvey, 1996; Xie and Lewis, 1995).
The finding that the receptor mechanism of LTP induction at one synapse can induce associative LTP at the other synapse also suggest both medial and lateral perforant path-CA3 synapses not only share common mechanisms of LTP induction, but may also share common mechanisms of LTP maintenance and expression. This would suggest the distinct receptor mechanisms for medial and lateral perforant path LTP induction, rather than reflecting distinct “forms” of LTP, may simply reflect different induction mechanisms underlying a single mechanism of LTP maintenance. This would be of interest as it would indicate LTP at lateral and medial perforant path-CA3 synapses differ only in their mechanisms of initial LTP induction, a finding that would further suggest that the different learning rules reflected in these distinct LTP induction mechanisms are likely to play important roles in regulating associative information storage in the CA3 region. However, while associative medial and lateral perforant path LTP may share common downstream mechanisms for induction, this does not indicate a priori that they also share a common form of LTP maintenance. Further studies are necessary to determine if medial and lateral perforant path-CA3 LTP, and their associative counterparts, display common mechanisms of LTP maintenance. Regardless, the mechanisms underlying the associative induction of medial and lateral perforant path-CA3 LTP appear to share common requirements. Because associative LTP can be induced by strong coactivation of the alternate pathway, it is likely that both synapses share common induction mechanisms for associative LTP, with both receptor mechanisms providing critical levels of postsynaptic depolarization necessary for inducing associative LTP induction at coactive perforant path-CA3 synapses.
The present data suggest an important function of opioid peptides released by the lateral perforant path. It appears that the contribution of opioid peptides to LTP induction is not only confined to afferents that contain and release opioid peptides, as we previously surmised (Derrick et al., 1991). Rather, the release of opioid peptides by opioidergic afferents also appears to play an important role in the induction of associative LTP in other, coactive synaptic populations, including those that normally are insensitive to opioid receptor antagonists. Thus our data suggest that activation of opioidergic afferents and opioid receptors, by virtue of their disinhibitory effects, play a permissive role in the induction of associative LTP in convergent, non-opioidergic CA3 synapses, a role proposed previously for opioid peptides in the hippocampus (Swearengen and Chavkin, 1987; Milner and Drake, 2001; Segal, 1988). In addition, the present data also reveal a hitherto unknown interaction among LTP induction mechanisms in separate afferent systems indicating associative LTP induction among perforant path projections to the CA3 region is regulated by the receptors and induction mechanisms of the strongly stimulated pathway. Because both pathways display LTP that can be induced by the receptor mechanisms of the alternate pathway, this suggests that both medial and lateral perforant path synapses share common downstream mechanisms of associative LTP induction, with postsynaptic depolarization likely being the common factor allowing for associative LTP.
Highlights.
The medial and lateral perforant path projections to the hippocampal CA3 region display distinct mechanisms of long-term potentiation (LTP) induction that require activation of either NMDA receptors or opioid receptors, respectively.
Medial and lateral perforant path projections to the CA3 region display associative LTP with coactivation, suggesting that while they differ in receptors involved in LTP induction they may share common downstream mechanisms of LTP induction.
We address this interaction of LTP induction mechanisms by evaluating the contribution of opioid receptors to the induction of associative LTP among the medial and lateral perforant path projections to the CA3 region in vivo.
Mu opioid receptor antagonists normally block the induction of lateral perforant path-CA3 LTP. However, they fail to block associative LTP in lateral perforant path-CA3 synapses when induced by strong coactivation of the medial perforant path. Thus strong activation of non-opioidergic afferents can substitute for the opioid receptor activation required for lateral perforant path LTP induction.
Mu opioid receptor antagonists blocked the induction of associative medial perforant path-CA3 LTP when induced by strong coactivation of the opioidergic lateral perforant path. This suggests that endogenous opioid peptides contribute to associative LTP at synapses that display NMDAR-dependent LTP when associative LTP is induced by strong activation of an opioidergic afferent system.
These data further suggest that associative perforant path-CA3 LTP induction is regulated by the receptor mechanisms of the strongly stimulated pathway, and that while medial and lateral perforant path synapses differ in mechanisms of LTP induction, associative LTP at these synapses share common downstream mechanisms of induction.
ACKNOWLEDGEMENTS
This research was supported by NIGMS/NINDS (GM082719) to BED.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carlo O. Martinez, The Neuroscience Research Institute The Department of Biology The University of Texas at San Antonio.
Viet H. Do, The Neuroscience Research Institute The Department of Biology The University of Texas at San Antonio.
Brian E. Derrick, The Neuroscience Research Institute The Department of Biology The University of Texas at San Antonio.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–91. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Brown TH. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983;80(23):7347–51. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Lamp T, Tallent MK. Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. J Neurophysiol. 2002;88(6):3078–86. doi: 10.1152/jn.00398.2002. [DOI] [PubMed] [Google Scholar]
- Berzhanskaya J, Urban NN, Barrionuevo G. Electrophysiological and pharmacological characterization of the direct perforant path input to hippocampal area CA3. J Neurophysiol. 1998;79(4):2111–8. doi: 10.1152/jn.1998.79.4.2111. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Errington ML, Bliss TVP. Naloxone blocks the induction of long-term potentiation in the lateral but not in the medial perforant pathway in the anesthetized rat. Brain Res. 1988;449:352–356. doi: 10.1016/0006-8993(88)91052-9. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Milgram NW, Srebro B. Activation of AP5-sensitive NMDA receptors is not required to induce LTP of synaptic transmission in the lateral perforant path. Eur J Neurosci. 1991;3:1300–1308. doi: 10.1111/j.1460-9568.1991.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1opioid receptors is required for long-term potentiation induction in the lateral perforant path: dependence on GABAergic inhibition. J Neurosci. 1996;16:8123–813. doi: 10.1523/JNEUROSCI.16-24-08123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl A, Derrick BE, Rodriguez SB, Martinez JL., Jr. Opioid receptor-dependent long-term potentiation at the lateral perforant path-CA3 synapse in rat hippocampus. Brain Res Bull. 1994;33(1):17–24. doi: 10.1016/0361-9230(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Cohen GA, Doze VA, Madison DV. Opioid inhibition of GABA release from presynaptic terminals of rat hippocampal interneurons. Neuron. 1992;9:325–335. doi: 10.1016/0896-6273(92)90171-9. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Herron CE, Lester RA. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988;399:301–12. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Opiates and the hippocampus: a review of the functional and morphological evidence. Pharmacol Biochem Behav. 1983;18(2):255–62. doi: 10.1016/0091-3057(83)90371-4. [DOI] [PubMed] [Google Scholar]
- Derrick BE, Martinez JL., Jr. Opioid receptors underlie the frequency-dependence of mossy fiber LTP induction. J. Neurosci. 1994;14(7):4359–67. doi: 10.1523/JNEUROSCI.14-07-04359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick BE. Plastic processes in the dentate gyrus: a computational perspective. Prog. Brain Res. 2007;163:417–51. doi: 10.1016/S0079-6123(07)63024-6. [DOI] [PubMed] [Google Scholar]
- Do V, Martinez CO, Martinez JL, Jr., Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J. Neurophysiol. 2002;87:669–674. doi: 10.1152/jn.00938.2000. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–63. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol. 1995;362(1):17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Donnett JG, O’Keefe J. Medial septal control of theta-correlated unit firing in the entorhinal cortex of awake rats. Neuroreport. 1995;6(16):2166–70. doi: 10.1097/00001756-199511000-00017. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30(9):456–63. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Keppel G, Zedeck S. Data Analysis for Research Design. Prentice-Hall; Englewood Cliffs, NJ: 1991. [Google Scholar]
- Kosub KA, Do VH, Derrick BE. NMDA receptor antagonists block heterosynaptic long-term depression (LTD) but not long-term potentiation (LTP) in the CA3 region following lateral perforant path stimulation. Neurosci Lett. 2005;374(1):29–34. doi: 10.1016/j.neulet.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Levy WB, Steward O. Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus. Neuroscience. 1983;8(4):791–797. doi: 10.1016/0306-4522(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Enkephalin hyperpolarizes interneurones in the rat hippocampus. J Physiol. 1988;398:123–30. doi: 10.1113/jphysiol.1988.sp017033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez CO, Do VH, Martinez JL, Jr., Derrick BE. Associative long-term potentiation (LTP) among extrinsic afferents of the hippocampal CA3 region in vivo. Brain Res. 2002;940:86–94. doi: 10.1016/s0006-8993(02)02598-2. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Barrionuevo G. Short- and long-term plasticity of the perforant path synapse in hippocampal area CA3. J Neurophysiol. 2002;88(1):528–33. doi: 10.1152/jn.2002.88.1.528. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard DV. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McNaughton BL. Evidence for two physiologically distinct perforant pathways to the fascia dentata. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- Milner TA, Drake CT. Ultrastructural evidence for presynaptic mu opioid receptor modulation of synaptic plasticity in NMDA-receptor-containing dendrites in the dentate gyrus. Brain Res Bull. 2001;54(2):131–40. doi: 10.1016/s0361-9230(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Atlas of the Rat Brain. Academic Press; New York: 1989. [Google Scholar]
- Segal M. Effects of mu opioid receptor activation in rat hippocampal slice. 1988. (NIDA Research Monograph # 82). [PubMed]
- Swearengen E, Chavkin C. NMDA receptor antagonist D-APV depresses excitatory activity produced by normorphine in rat hippocampal slices. Neurosci. Lett. 1987;78(1):80–4. doi: 10.1016/0304-3940(87)90565-9. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Caudle RM, Neumaier JF, Chavkin C. Stimulation of endogenous opioid release displaces mu receptor binding in rat hippocampus. Neuroscience. 1990;37(1):45–53. doi: 10.1016/0306-4522(90)90190-f. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustaffson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Wu K, Leung S. Monosynaptic activation of CA3 by the medial perforant path. Brain Res. 1999;797(1):35–41. doi: 10.1016/s0006-8993(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Depression of LTP in rat dentate gyrus by naloxone is reversed by GABAa blockade. Brain Res. 1995;688(1-2):56–60. doi: 10.1016/0006-8993(95)00510-w. [DOI] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Opioid-mediated facilitation of long-term potentiation at the lateral perforant path-dentate granule cell synapse. J Pharmacol Exp Ther. 1991;256(1):289–96. [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci USA. 1990;87(15):5832–6. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]



