Abstract
Arachidonic acid (ARA) and docosahexaenoic acid (DHA) are routinely added to infant formula to support growth and development. We evaluated the bioequivalence and safety of three ARA-rich oils for potential use in infant formula using the neonatal pig model. The primary outcome for bioequivalence was brain accretion of ARA and DHA. Days 3 to 22 of age, domestic pigs fed one of three formulas, each containing ARA at ~0.64% and DHA at ~0.34% total fatty acids (FA). Control diet ARA was provided by ARASCO® and all diets had DHA from DHASCO® (Martek Biosciences Corp., Columbia, MD). The experimental diets a1 and a2 provided ARA from Refined Arachidonic acid-rich Oil (RAO; Cargill, Inc., Wuhan, China) and SUNTGA40S (Nissui, Nippon Suisan Kaisha, Ltd., Tokyo, Japan), respectively. Formula intake and growth were similar across all diets, and ARA was bioequivalent across treatments in the brain, retina, heart, liver and day 21 RBC. DHA levels in the brain, retina and heart were unaffected by diet. Liver sections, clinical chemistry, and hematological parameters were normal. We conclude that RAO and SUNTGA40S, when added to formula to supply ~0.64% ARA are safe and nutritionally bioequivalent to ARASCO in domestic piglets.
Keywords: Arachidonic acid, ARASCO, DHASCO, infant nutrition, pig
1. Introduction
Arachidonic acid (ARA) is a long chain polyunsaturated fatty acid (LCPUFA) that is routinely added to infant formula along with docosahexaenoic acid (DHA). Both are natural components of breast milk and are important for growth and development during the perinatal period (Carlson, 2001; Innis, 2005, 2007). In higher vertebrates, ARA and DHA serve as the major LCPUFA in central nervous tissue (Diau et al., 2005; Makrides et al., 1994), and in the heart ARA comprises upwards of 25% total fatty acids (FA) (Tyburczy et al., 2010a). Studies with animals indicate a relative competition between these two LCPUFA for tissue incorporation, especially in the liver (Blank et al., 2002; Hsieh et al., 2007; Tyburczy et al., 2010a) and in some regions of the brain (Hsieh et al., 2007). Thus, a balanced intake of both LCPUFA is recommended to support tissue ARA and DHA accretion, and their associated physiological functions.
Accretion of ARA and DHA during growth occurs via the uptake of preformed dietary LCPUFA or through the endogenous biosynthesis of LCPUFA from the dietary essential FA, linoleic and alpha-linolenic acids. Rates of endogenous biosynthesis appear to be a limiting factor in the accretion of ARA and DHA in tissues (Cunnane et al., 2000; Hsieh et al., 2007; Makrides et al., 1994; Tyburczy et al., 2010a), indicating a requirement for these LCPUFA in the diets of human infants. Recently, dietary ARA was shown to differentially modulate endogenous ARA biosynthesis in a tissue specific manner in piglets (Jacobi et al.). In 1994, the World Health Organization (WHO) and the Food and Agriculture Organization of the United Nations (FAO) set recommendations of 40 mg ARA and 20 mg DHA per kg body weight in formula for term infants, or about 0.66% and 0.33% total FA, respectively (FAO/WHO, 1994). These levels are based largely on mean worldwide breast milk levels (Brenna et al., 2007; Koletzko et al., 1992) and supported by the evidence for benefit in visual and cognitive performance in clinical studies with human infants (Hoffman et al., 2009; Ryan et al., 2010). Re-evaluation of the 1994 FAO/WHO report has produced similar recommendations (FAO/WHO, 2010). Further, for infants 0 – 6 mo of age, the French Food Safety Agency recommended that DHA be included at 0.32% total FA and balanced with 0.5% ARA (AFSSA, 2010).
In the U.S., two ARA sources are considered Generally Recognized as Safe (GRAS) by the U.S. Food and Drug Administration (FDA) for use in infant formula, ARASCO (ARA single-cell oil; Martek Biosciences, Corp., Columbia, MD) (Rulis, 2001; Rulis and Lewis, 2001) and SUNTGA40S (Nissui, Nippon Suisan Kaisha, Ltd., Tokyo, Japan; previously Suntory, Ltd., Osaka, Japan) (Tarantino, 2006). Both are single-cell triglyceride oils derived from the fungus Mortierella alpina (M. alpina) and contain ARA at approximately 40% total FA. A third oil, “Refined Arachidonic acid-rich Oil” (RAO; Cargill, Inc., Wuhan, China) also derived from M. alpina, has been proposed for use in preterm and term infant formulas (Casterton, 2010). These ARA oils may be derived from a common fungal source but differences in the manufacturing process inevitably lead to subtle variations in the final product. Of particular importance here are the levels of trace chemical and fat soluble components, and potentially bioactive microbiological constituents. While an ARA oil may comprise less than 2% of the total fat in formula, the extended consumption of these oils by the vulnerable, growing infant may exaggerate any toxicological or allergenic effect caused by trace or undetected contaminants (Fritsche, 2003; IOM, 2004). Thus, preclinical feeding studies with higher vertebrate animals at a similar stage of development, such as the neonatal pig, are essential for determining the safety of novel ingredients and formulations for use in the diets of human infants.
The present study sought to evaluate in the neonatal pig model the bioequivalence and safety of the ARA-rich oils RAO and SUNTGA40S, relative to ARASCO, formulated in each case with DHASCO as a source of DHA. We hypothesized that the three ARA-rich oils would be nutritionally bioequivalent and equally safe in rapidly-growing neonatal pigs. Piglets were fed one of three ready-to-use formulas that provided ARA at approximately 0.64% and DHA at 0.34% total FA from day 3 to 22 of life, upon which tissues were harvested and analyzed for ARA and DHA accretion. In addition, livers were examined for histopathological changes and clinical chemistry and hematological parameters were measured to assess safety. Given that the key recognized role of LCPUFA in the infant diet is support of proper brain development, the primary biomarker of bioequivalence (i.e. primary outcome) was defined as cerebral cortex accretion of ARA and DHA. Specifically, bioequivalence of the experimental ARA single-cell oils was determined based on 90% confidence intervals on the ratio of the geometric mean ARA levels of each test group compared with the control group. Confidence intervals falling within the 80–125% limit were determined to be bioequivalent based on FDA guidelines (FDA, 2003). Bioavailability was more broadly assessed through secondary supporting outcomes, including ARA and DHA accretion in retina, heart and liver, as well as circulating levels of ARA and DHA in red blood cells (RBC). Thus, to demonstrate overall nutritional bioequivalence of the ARA sources, we aimed to show that their bioavailabilities were similar to such a degree that their effects with respect to safety and nutritional efficacy would be essentially the same.
2. Materials and methods
2.1 Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Cornell University. Domestic piglets were selected for gender and weight from five sows (Yorkshire × Landrace bred to Hampshire boars) at the Cornell University swine facility. One day before the scheduled, term farrowing date, pregnant sows were injected with 2 cc of Lutalyse® (Pfizer Animal Health, Kalamazoo, MI) to induce farrowing and obtain a block of piglets with the same birth date. Newborn piglets were processed according to standard facility practices (i.e. intramuscular injection of iron dextran (100 mg) and penicillin G (½ cc), tooth clip, tail dock, ear notch) and remained with the sow in farrowing pens until day 3 of age, upon which they were matched for weight and assigned to one of three study formulas (n=8 per diet). Piglets were then transported to the campus large animal research facility where they were housed individually in raised stainless steel cages (floor space: 7.1 ft2) with plastic mesh floors, provided appropriate enrichment (plastic and rubber toys) and maintained on a 16/8 hr light/dark cycle. Each cage was equipped with two bowls, one for continuous access to water and one for formula. Most piglets immediately consume formula from bowls; those that do not are handfed by bottle for a few days until they take to bowls. Formula was offered three times daily (6am, 2pm, 10pm).
2.2 Diets
Study formulas were designed to meet or exceed nutrient requirements for growing pigs 3 – 5 kg in body weight (NRC, 1998), and were prepared as ready-to-use formulas that provided 1.0 kcal/ml. The target nutrient composition of study formulas was as follows: (% wt/wt): protein, 4.9; fat, 5.5; ash, 1.2. A detailed composition and nutrient analysis for the study formulas are presented in Table 1. Target specifications for ARA and DHA were 35.8 and 17.9 mg/100 kcal, or approximately 0.64% and 0.32% total FA, respectively, similar to levels currently used in the commercial human infant formula Enfamil® (Mead Johnson Nutrition, Evansville, IN). LCPUFA in the Control diet were supplied by ARASCO® and DHASCO® (Martek Biosciences, Inc., Columbia, MD), as currently used in Enfamil. ARA in the experimental diets a1 and a2 was provided by Refined Arachidonic acid-rich Oil (RAO; Cargill, Inc., Wuhan, China) and SUNTGA40S (Nissui, Nippon Suisan Kaisha, Ltd., Tokyo, Japan), respectively, and both were formulated with DHASCO as a source of DHA. The FA composition of the study formulas is presented in Table 2. Fresh formula was provided three times per day in stainless steel bowls fixed to the cage doors and water was offered ad libitum. Piglets were fed at 80% ad libitum intake, based on pilot data, to support growth rates of approximately 110% of historical growth levels at the Cornell swine farm (Huang et al., 2002). Formula intakes were recorded daily.
Table 1.
Nutrient composition of study formulas.
| Nutrient (% wt/wt)1 | Control | a1 | a2 |
|---|---|---|---|
| Fat | 5.5 | 5.6 | 5.6 |
| Protein | 4.9 | 4.9 | 4.8 |
| Carbohydrate | 6.4 | 6.1 | 6.2 |
| Ash | 1.2 | 1.2 | 1.2 |
| Total solids | 18.1 | 17.7 | 17.8 |
| Mineral (mg/100 kcal) | |||
| Na | 59.3 | 62.6 | 60.6 |
| Mg | 15.0 | 14.5 | 14.6 |
| P | 146.0 | 149.7 | 146.3 |
| K | 306.3 | 301.9 | 304.4 |
| Ca | 217.5 | 214.8 | 208.8 |
| Vitamin (units/100 kcal) | |||
| Vitamin E (IU) | 1.6 | 1.6 | 1.6 |
| Vitamin K (mcg) | 16.7 | 18.3 | 17.5 |
| Vitamin D (IU) | 13.4 | 13.2 | 13.5 |
| Vitamin C (mg) | 6.3 | 6.7 | 6.3 |
| Vitamin A (IU) | 83.0 | 88.0 | 83.0 |
| Thiamine HCl (mcg) | 82.8 | 67.1 | 66.8 |
| Riboflavin (mcg) | 204.5 | 185.7 | 188.3 |
| Vitamin B6 (mcg) | 65.6 | 60.5 | 69.4 |
| Vitamin B12 (mcg) | 0.5 | 0.4 | 0.5 |
Composition taken as percent of total weight (wt) unless indicated otherwise.
Table 2.
Fatty acid (FA) composition of study formulas1
| Diet | Control | a1 | a2 |
|---|---|---|---|
| FA | (% total FA) | ||
| 10:0 | 0.70 ± 0.02 | 0.74 ± 0.02 | 0.46 ± 0.01 |
| 12:0 | 8.19 ± 0.08 | 8.12 ± 0.09 | 7.37 ± 0.07 |
| 14:0 | 4.21 ± 0.04 | 4.24 ± 0.03 | 4.20 ± 0.03 |
| 16:0 | 21.76 ± 0.05 | 21.77 ± 0.08 | 22.07 ± 0.09 |
| 16:1 | 0.18 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.02 |
| 18:0 | 4.58 ± 0.02 | 4.50 ± 0.02 | 4.53 ± 0.04 |
| 18:1 | 38.14 ± 0.12 | 37.96 ± 0.15 | 38.59 ± 0.15 |
| 18:2n-6 | 17.74 ± 0.03 | 17.86 ± 0.06 | 17.96 ± 0.04 |
| Conjugated 18:2 | 0.29 ± 0.03 | 0.31 ± 0.01 | 0.25 ± 0.03 |
| 18:3n-6 | 0.10 ± 0.01 | 0.11 ± 0.00 | 0.10 ± 0.01 |
| 18:3n-3 | 2.00 ± 0.03 | 2.01 ± 0.04 | 2.01 ± 0.03 |
| 20:0 | 0.35 ± 0.01 | 0.35 ± 0.00 | 0.36 ± 0.00 |
| 20:1 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 |
| 20:2n-6 | 0.03 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.00 |
| 20:3n-6 | 0.07 ± 0.00 | 0.08 ± 0.01 | 0.08 ± 0.00 |
| ARA2 | 0.67 ± 0.01 | 0.62 ± 0.00 | 0.67 ± 0.00 |
| 22:0 | 0.27 ± 0.01 | 0.30 ± 0.01 | 0.32 ± 0.01 |
| DHA | 0.35 ± 0.01 | 0.35 ± 0.01 | 0.34 ± 0.01 |
| 23:0 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.00 |
| 24:0 | 0.12 ± 0.00 | 0.21 ± 0.01 | 0.22 ± 0.01 |
| Σ SFA3 | 40.18 ± 0.15 | 40.22 ± 0.20 | 39.53 ± 0.17 |
| Σ MUFA | 38.53 ± 0.13 | 38.36 ± 0.15 | 38.99 ± 0.14 |
| Σ n-6 | 18.62 ± 0.03 | 18.67 ± 0.06 | 18.83 ± 0.04 |
| Σ n-3 | 2.34 ± 0.04 | 2.36 ± 0.03 | 2.35 ± 0.04 |
| 18:2n-6/18:3n-3 | 8.89 ± 0.15 | 8.91 ± 0.18 | 8.94 ± 0.16 |
Values represent means ± SD, n = 3 extractions per diet.
ARA, arachidonic acid; DHA, docosahexaenoic acid; SFA, saturated FA; MUFA, monounsaturated FA.
ΣSFA =Σ 14:0, 16:0, 18:0, 20:0, 22:0, 23:0, 24:0; Σ MUFA = Σ 16:1, 18:1, 20:1; Σ n-6 =Σ 18:2n-6, 20:2n-6, 20:3n-6, ARA; Σ n-3 = Σ 18:3n-3, DHA.
2.3 Sampling
Body weights measurements were taken every other day during the first week of life and thereafter every third day for the remainder of the study. Non-fasted blood samples were collected from the anterior vena cava into EDTA-containing vacutainer tubes (BD, Franklin Lakes, NJ) on days 3, 7, 14 and 21 of age. Whole blood was separated into plasma, red blood cell (RBC) and white cell fractions using Leucosep tubes (Grenier Bio-One, Monroe, NC) with lymphocyte separation media (Mediatech, Inc., Manassas, VA) according to manufacturer’s directions. Piglets were sacrificed on day 22 of age via an intravenous injection of Fatal-Plus (1 ml/4.54 kg body weight; Vortech Pharmaceuticals, Dearborn, MI, USA) followed by exsanguination. Organs were immediately removed and weighed, and samples flash frozen in liquid nitrogen within 10 minutes of cessation of heart beat.
2.4 Fatty acid analysis
FA composition of the study formulas, tissues and RBC fraction were determined by gas chromatography. FA methyl esters (FAME) were prepared from approximately 50 mg tissue or 50 μl of formula or RBC according to the one-step hydrolysis, extraction and methylation procedure (Garces and Mancha, 1993) with modifications by (Zhou et al., 2008). Retinas, which were suspended in saline at necropsy, were first lyophilized using a Savant SpeedVac Concentrator (Thermo Fisher Scientific, Waltham, MA). FAME were quantified on a 5890 Series II gas chromatograph (Hewlett-Packard, Palo Alto, CA) equipped with a BPX70 fused silica column (25 m × 0.22 mm i.d. × 0.25 μm film; SGE Incorporated, Austin, TX) and integrated using PeakSimple 3.78 software (SRI Instruments, Torrance, CA). An equal weight FAME mixture was used daily to verify response factors. FAME were structurally identified by covalent-adduct chemical ionization mass spectrometry on a Saturn 2000 mass spectrometer (Varian, Inc., Walnut Creek, CA) attached to a Varian Star 3400 gas chromatograph (Brenna and Tyburczy, 2010).
2.5 Liver histopathology
Freshly harvested samples from the right central lobe (~ 1 g) were fixed in 10% neutral buffered formalin and submitted to the Histopathology Laboratory (College of Veterinary Medicine, Cornell University) where they were embedded in wax, sectioned and stained with hematoxylin and eosin. Sections were examined under light microscope by a veterinary pathologist for determination of abnormalities.
2.6 Clinical chemistry and hematology analysis
Plasma and EDTA-blood from piglets on day 21 of age were delivered promptly to the Animal Health Diagnostic Center (College of Veterinary Medicine, Cornell University) for analysis of clinical chemistry and hematological parameters.
2.7 Statistical analysis
The objective was to establish the bioequivalence of ARA from RAO and SUNTGA40S by comparing tissue and RBC ARA levels with the ARASCO-fed Control group. The primary outcome was the bioequivalence of cerebral cortex ARA levels; assessment of ARA levels in the retina, liver, heart and RBC were secondary outcomes. Bioequivalence was assessed by 90% confidence intervals around the ratio of the geometric mean ARA levels of each test group compared with the control group. Confidence intervals were calculated from log-transformed ARA data by exponentiating the mean and upper and lower limits. These procedures conform to FDA guidelines (FDA, 2003) and have been previously used to assess the bioequivalence of various sources of supplemental DHA (Arterburn et al., 2007).
Power analysis was based on cerebral cortex DHA means and SD. DHA concentration is known to be more variable than ARA in neural tissue at a given postnatal age, established in animal studies with piglets (Huang et al., 2007), rats (Su et al., 1996) and non-human primates (Diau et al., 2005), and consistent with human autopsy data (Farquharson et al., 1992). In pigs, cerebral cortex DHA is normally distributed with a standard deviation of 0.8% w/w (Tyburczy et al., 2010a). To detect a difference of 10%, e.g. 10 vs 11% w/w, between experimental and control oils, eight piglets were required per study treatment group for 81% power. The Type I error probability associated with this test of the null hypothesis was 0.05. Calculations were made using nQuery Advisor 3.0.
Group differences were tested using the Fit Model platform of JMP (2008 SAS Institute, 8.0) to fit mixed models. Fixed effects were diet, gender, day 3 of age body weight and the full factorial of interactions. Interaction effects were considered significant at P < 0.10 and fixed effects at P < 0.05. For each parameter analyzed, effects not considered significant were removed in a stepwise manner from the final model. Random effects were litter and animal nested within litter for repeated measures of body weight and RBC FA content. Significance of pairwise comparisons was determined using the Student’s t-test. Values are reported as means ± SD. Linear regression analysis was performed to determine the relationship between RBC LCPUFA content and postnatal age.
3. Results
3.1 Formula composition and intake
Study formulas provided equivalent levels of total fat, protein and ash, averaging 5.6%, 4.9% and 1.2% wt/wt, respectively, with variation between the experimental and Control formulas being less than 2%. Carbohydrate levels in the Control formula were 5% greater than the a1 diet and 3% greater than the a2 diet. Target levels of ARA and DHA were set at 35.8 and 17.9 mg/100 kcal, respectively. Actual values were as follows (ARA, DHA; mg/100 kcal): Control 34.6, 17.3; a1 31.3, 16.3; a2, 34.0, 16.5. The content of ARA in the Control, a1 and a2 formulas was 0.67%, 0.62% and 0.67% total FA, respectively, corresponding to a mean difference of 8% between the Control and a1 formulas. A difference of this magnitude may arise from normal variability across product lots and over time due to overages provided to insure minimal nutrient levels at the end of the product lifetime, accounting for nutrient degradation (Cook, 1989). DHA levels averaged 0.35% total FA and were similar among the three formulas.
Total formula intakes over the full study period averaged 29.6 ± 1.7 L and were similar for all three dietary treatment groups. The study formulas provided 1 kcal/ml, corresponding to a mean total energy intake of 29,600 ± 1,700 kcal per pig and a mean daily intake of 1.5 L or 1,500 kcal. Mean total intake of ARA was 10.60 ± 0.59 g, while the mean total intake of DHA was 5.30 ± 0.30 g.
3.2 Clinical observations and growth
All 24 piglets remained on the study until day 21 of age, and very few health ailments were noted during the course of the study. Loose stools during the first week of life were the greatest concern, although by the second week of life, the occurrence of loose stools had cleared in most piglets. One piglet on the a1 diet was given an intramuscular injection of penicillin G benzathine (75,000 IU) by the attending veterinarian on day 11 of age for an undiagnosed, yet persistent disinterest in consuming formula. That animal grew significantly less than all other pigs starting on day 13 of age, as determined using Grubb’s test for statistical outliers, and was removed from the body weight analysis starting at this time point. Data from this piglet were included in all other outcomes.
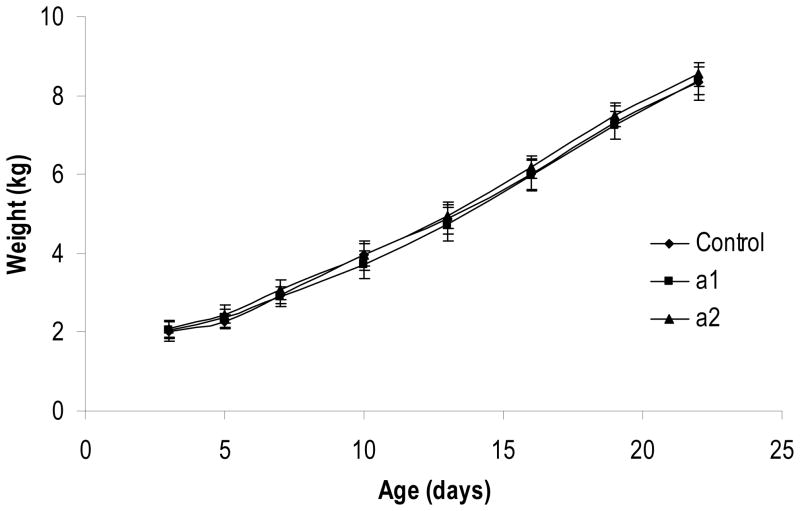
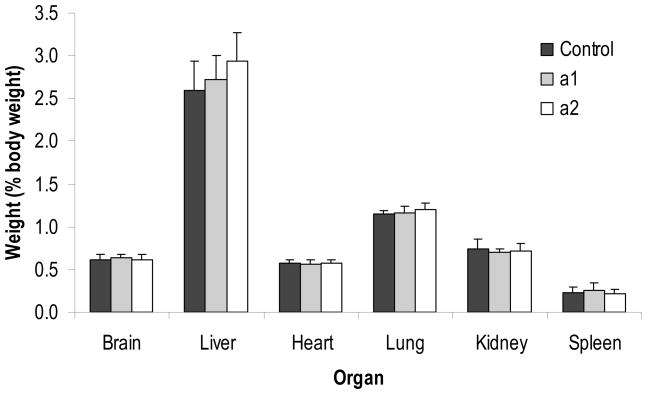
The temporal pattern of body weight gain during the study is presented in Figure 1. Piglets weighed 2.1 ± 0.2 kg at the start of the study (day 3 of age) and rapidly grew to 8.4 ± 0.4 kg by day 22 of age. Mean body weights were similar among the three dietary treatments at every time point measured. Organ weights at necropsy, both relative and absolute, were similar among all dietary treatment groups. Figure 2 presents the summary of relative organ weights (organ weight as a percentage of body weight at necropsy) for piglets sacrificed on day 22 of age.
Figure 1.
Temporal pattern of body weight gain in piglets fed one of three arachidonic acid-rich oils from day 3 to 22 of age. Values represent means ± SD, n = 8/diet. One piglet from Diet a1 was not included in body weight measurements on days 13 – 22 due to a slower rate of growth caused by an undiagnosed, yet persistent disinterest in formula consumption. Body weights were similar among all dietary treatment groups at every time point measured.
Figure 2.
Relative organ weights from piglets on day 22 of age. Values represent means ± SD, n = 8 for Control and Diet a2; n = 7 for Diet a1. Pairwise comparisons determined using Student’s t-test. Organ weights were similar for all dietary treatment groups.
3.3 ARA bioequivalence assessment
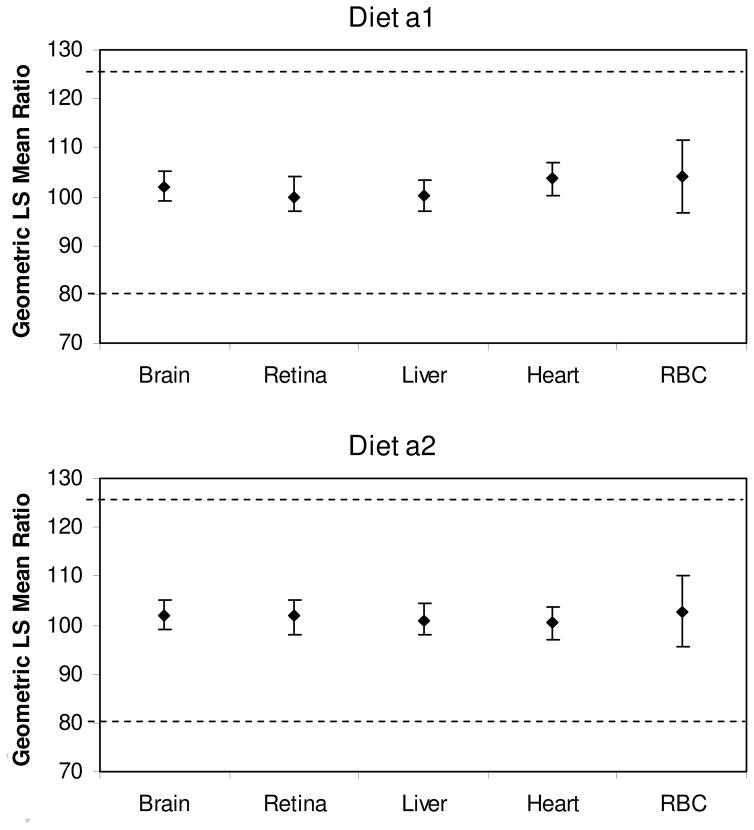
Bioequivalence was assessed by 90% confidence intervals on the least squares geometric mean ratio of tissue ARA from the experimental groups compared with the Control. Bioequivalence is met if the confidence intervals, expressed as percentages with 100% equaling unity (i.e. 1:1 ratio), must fall within the limits of 80 – 125%. Figure 3 presents the 90% confidence intervals for tissue and RBC ARA levels. For both experimental diets, the 90% confidence intervals fall within the 80 – 125% limits for every tissue examined, establishing that RAO and SUNTGA40S are bioequivalent sources of ARA for tissue and RBC ARA accretion compared with ARASCO.
Figure 3.
Arachidonic acid (ARA) bioequivalence assessment. Graphs present geometric least squares (LS) mean ratios of tissue and red blood cell (RBC) ARA from the experimental groups compared with the Control. Values are expressed as percentages with 100% equaling unity (1:1 ratio). Bioequivalence is met if 90% confidence intervals falling within the 80 – 125% limits. RBC ARA levels were determined from blood collected on day 21 of age.
3.4 Tissue LCPUFA accretion
Selected FA of the brain (cerebral cortex), retina, liver and heart harvested from pigs on day 22 of age are presented in Tables 3 and 4. Mean ARA levels in the brain, retina and heart were 10.97 ± 0.36%, 10.50 ± 0.43%, 20.38 ± 0.82% total FA, respectively, and were similar for all three dietary treatment groups. ARA levels in the liver were 2% lower in pigs fed Diet a1 (17.33 ± 0.78% FA) compared with the Control (17.66 ± 0.49% FA), while the a2 pigs showed an intermediary liver ARA content (17.38 ± 0.57% FA; P = 0.009). Study formulas equally supported DHA accretion in the primary target organ of brain, as well as retina and heart, while in the liver, DHA levels were 7% higher in a1 pigs (8.23 ± 0.38) compared with the Control (7.70 ± 0.47% FA) and a2 (7.62 ± 0.62% FA) groups (P = 0.046). No other statistically significant differences in tissue FA accretion were observed among the dietary treatments.
Table 3.
Selected fatty acids (FA) of brain (cerebral cortex) and retina from piglets fed one of three arachidonic acid (ARA)-rich oils 1
| Diet | Control | a1 | a2 | P |
|---|---|---|---|---|
| Cerebral cortex | FA (% total FA) | |||
| Σ SFA2 | 44.49 ± 0.58 | 44.82 ± 0.74 | 44.60 ± 0.42 | NS |
| Σ MUFA | 20.63 ± 0.90 | 20.16 ± 1.06 | 20.28 ± 1.21 | NS |
| 22:3n-9 | 0.31 ± 0.05 | 0.31 ± 0.08 | 0.28 ± 0.04 | NS |
| ARA | 10.83 ± 0.34 | 11.04 ± 0.42 | 11.04 ± 0.33 | NS |
| 22:4n-6 | 3.99 ± 0.34 | 4.12 ± 0.38 | 4.20 ± 0.36 | NS |
| 22:5n-6 | 5.90 ± 0.50 | 5.82 ± 0.70 | 5.58 ± 0.57 | NS |
| Σ n-6 | 23.32 ± 0.73 | 23.62 ± 0.50 | 23.53 ± 0.80 | NS |
| 20:5n-3 | 0.12 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.04 | NS |
| 22:5n-3 | 0.31 ± 0.04 | 0.31 ± 0.05 | 0.31 ± 0.04 | NS |
| DHA | 10.17 ± 0.71 | 9.97 ± 0.79 | 10.24 ± 0.39 | NS |
| Σ n-3 | 10.67 ± 0.70 | 10.48 ± 0.78 | 10.76 ± 0.41 | NS |
| ARA/DHA | 1.07 ± 0.08 | 1.11 ± 0.08 | 1.08 ± 0.04 | NS |
| Σ 22C LCPUFA3 | 20.67 ± 0.52 | 20.54 ± 0.60 | 20.62 ± 0.64 | NS |
| Retina | ||||
| Σ SFA | 41.92 ± 0.92 | 42.65 ± 0.90 | 42.13 ± 1.19 | NS |
| Σ MUFA | 17.64 ± 0.37 | 17.71 ± 0.52 | 17.83 ± 0.60 | NS |
| 22:3n-9 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.08 ± 0.03 | NS |
| ARA | 10.45 ± 0.42 | 10.42 ± 0.43 | 10.63 ± 0.45 | NS |
| 22:4n-6 | 2.51 ± 0.19 | 2.45 ± 0.16 | 2.43 ± 0.11 | NS |
| 22:5n-6 | 2.58 ± 0.41 | 2.41 ± 0.56 | 2.36 ± 0.41 | NS |
| Σ n-6 | 19.06 ± 0.85 | 18.90 ± 0.92 | 18.88 ± 0.68 | NS |
| 20:5n-3 | 0.46 ± 0.07 | 0.48 ± 0.09 | 0.40 ± 0.12 | NS |
| 22:5n-3 | 0.62 ± 0.07 | 0.64 ± 0.07 | 0.62 ± 0.06 | NS |
| DHA | 19.87 ± 0.65 | 19.20 ± 0.83 | 19.75 ± 1.66 | NS |
| Σ n-3 | 21.02 ± 0.69 | 20.38 ± 0.85 | 20.83 ± 1.76 | NS |
| ARA/DHA | 0.53 ± 0.02 | 0.54 ± 0.03 | 0.54 ± 0.05 | NS |
| Σ 22C LCPUFA | 25.58 ± 0.90 | 24.70 ± 1.06 | 25.16 ± 1.58 | NS |
Values represent means ± SD, n = 8 per diet. P < 0.05 indicates that all means are not equal; NS, not significant. Pairwise comparisons determined using Student’s t-test; means not sharing a common superscript are significantly different (P < 0.05).
DHA, docosahexaenoic acid; LCPUFA, long chain polyunsaturated FA; MUFA, monounsaturated FA; SFA, saturated FA.
Σ n-6 =Σ 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, ARA, 22:4n-6, 22:5n-6; Σ n-3 = 18:3n-3, 20:3n-3; 20:5n-3, 22:5n-3, DHA; Σ 22C LCPUFA = Σ 22:3n-9, 22:4n-6, 22:5n-6, 22:5n-3, DHA.
Table 4.
Selected fatty acids (FA) of heart and liver from piglets fed one of three arachidonic acid (ARA)-rich oils1
| Diet | Control | a1 | a2 | P |
|---|---|---|---|---|
| Heart | FA (% total FA) | |||
| Σ SFA2 | 31.92 ± 0.29 | 32.15 ± 0.49 | 32.37 ± 0.39 | NS |
| Σ MUFA | 18.53 ± 0.75 | 18.35 ± 0.77 | 18.09 ± 0.74 | NS |
| ARA | 20.67 ± 0.56 | 19.93 ± 0.79 | 20.57 ± 0.93 | NS |
| 22:4n-6 | 0.92 ± 0.10 | 0.86 ± 0.09 | 0.83 ± 0.13 | NS |
| 22:5n-6 | tr. | tr. | tr. | n/a |
| Σ n-6 | 43.40 ± 0.86 | 43.44 ± 1.12 | 43.59 ± 1.00 | NS |
| 20:5n-3 | 0.42 ± 0.04 | 0.39 ± 0.03 | 0.39 ± 0.04 | NS |
| 22:5n-3 | 0.76 ± 0.08 | 0.71 ± 0.04 | 0.73 ± 0.05 | NS |
| DHA | 4.16 ± 0.39 | 4.04 ± 0.28 | 3.98 ± 0.35 | NS |
| Σ n-3 | 6.05 ± 0.40 | 5.95 ± 0.31 | 5.85 ± 0.40 | NS |
| ARA/DHA | 5.00 ± 0.38 | 4.96 ± 0.40 | 5.22 ± 0.63 | NS |
| Σ 22C LCPUFA3 | 5.84 ± 0.37 | 5.61 ± 0.34 | 5.53 ± 0.37 | NS |
| Liver | ||||
| Σ SFA | 42.15 ± 0.91 | 42.46 ± 0.57 | 41.89 ± 0.74 | NS |
| Σ MUFA | 13.64 ± 1.17 | 13.50 ± 0.71 | 13.89 ± 1.24 | NS |
| ARA | 17.66 ± 0.49a | 17.33 ± 0.78b | 17.38 ± 0.57ab | 0.01 |
| 22:4n-6 | 0.64 ± 0.07 | 0.63 ± 0.10 | 0.65 ± 0.08 | NS |
| 22:5n-6 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.02 | NS |
| Σ n-6 | 34.10 ± 0.69 | 33.49 ± 0.59 | 34.17 ± 0.55 | NS |
| 20:5n-3 | 0.33 ± 0.04 | 0.28 ± 0.08 | 0.31 ± 0.05 | NS |
| 22:5n-3 | 1.03 ± 0.18 | 1.04 ± 0.13 | 1.04 ± 0.06 | NS |
| DHA | 7.70 ± 0.47b | 8.23 ± 0.38a | 7.62 ± 0.62b | 0.046 |
| Σ n-3 | 9.80 ± 0.47 | 10.21 ± 0.43 | 9.70 ± 0.56 | NS |
| ARA/DHA | 2.30 ± 0.12 | 2.11 ± 0.12 | 2.29 ± 0.15 | NS |
| Σ 22C LCPUFA | 9.43 ± 0.52 | 9.96 ± 0.33 | 9.37 ± 0.68 | NS |
Values represent means ± SD, n = 8 per diet. P < 0.05 indicates that all means are not equal; NS, not significant. Pairwise comparisons determined using Student’s t-test; means not sharing a common superscript are significantly different (P < 0.05).
DHA, docosahexaenoic acid; LCPUFA, long chain polyunsaturated FA; MUFA, monounsaturated FA; tr, trace; SFA, saturated FA.
Σ n-6 = Σ 18:2n-6, 18:3n-6, 20:2n-6, 20:3n-6, ARA, 22:4n-6, 22:5n-6; Σ n-3 = 18:3n-3, 20:3n-3; 20:5n-3, 22:5n-3, DHA; Σ 22C LCPUFA = Σ 22:4n-6, 22:5n-6, 22:5n-3, DHA.
Mean ARA levels in the RBC fraction were similar among all dietary treatment groups at every time point examined (days 3, 7, 14 and 21; Table 5). RBC DHA levels were similar among the three dietary treatment groups on days 3 and 21 of age, averaging 1.74 ± 0.32% and 2.57 ± 0.27% total FA, respectively. On day 7 of age, RBC DHA levels were significantly higher in the a1 pigs (1.86 ± 0.19% FA) compared with a2 group (1.69 ± 0.26% FA), while Control pigs had intermediary levels (1.81 ± 0.27% FA; P = 0.03). On day 14 of age, RBC DHA levels were the highest in the a1 pigs (2.85 ± 0.31% FA) and were similar between the Control (2.59 ± 0.37% FA) and a2 (2.49 ± 0.14% FA) groups (P = 0.02). Mean RBC 20:5n-3, 22:5n-3, 22:4n-6 and 22:5n-6 were similar for all dietary treatment groups at every time point examined.
Table 5.
Red blood cell (RBC) long chain polyunsaturated fatty acids (LCPUFA) collected from piglets on days 3, 7, 14 and 21 of life1
| Diet | Control | a1 | a2 | P |
|---|---|---|---|---|
| Day 3 | FA (% total FA) | |||
| ARA2 | 7.06 ± 0.65 | 7.04 ± 0.67 | 6.84 ± 0.52 | NS |
| 22:4n-6 | 0.97 ± 0.17 | 0.99 ± 0.14 | 1.00 ± 0.20 | NS |
| 22:5n-6 | 1.96 ± 0.30 | 1.92 ± 0.12 | 1.84 ± 0.26 | NS |
| 20:5n-3 | 0.16 ± 0.08 | 0.17 ± 0.11 | 0.14 ± 0.03 | NS |
| 22:5n-3 | 0.49 ± 0.08 | 0.53 ± 0.19 | 0.49 ± 0.14 | NS |
| DHA | 1.78 ± 0.37 | 1.70 ± 0.25 | 1.73 ± 0.36 | NS |
| Day 7 | ||||
| ARA | 5.94 ± 0.42 | 6.09 ± 0.47 | 5.76 ± 0.27 | NS |
| 22:4n-6 | 0.72 ± 0.07 | 0.77 ± 0.08 | 0.69 ± 0.08 | NS |
| 22:5n-6 | 1.09 ± 0.25 | 1.23 ± 0.10 | 1.03 ± 0.18 | NS |
| 20:5n-3 | 0.16 ± 0.06 | 0.16 ± 0.06 | 0.14 ± 0.03 | NS |
| 22:5n-3 | 0.50 ± 0.07 | 0.52 ± 0.08 | 0.48 ± 0.07 | NS |
| DHA | 1.81 ± 0.27ab | 1.86 ± 0.19a | 1.69 ± 0.26b | 0.03 |
| Day 14 | ||||
| ARA | 5.04 ± 0.48 | 5.32 ± 0.37 | 4.89 ± 0.22 | NS |
| 22:4n-6 | 0.60 ± 0.08 | 0.61 ± 0.06 | 0.57 ± 0.03 | NS |
| 22:5n-6 | 0.50 ± 0.05 | 0.54 ± 0.12 | 0.46 ± 0.08 | NS |
| 20:5n-3 | 0.22 ± 0.06 | 0.22 ± 0.06 | 0.21 ± 0.03 | NS |
| 22:5n-3 | 0.50 ± 0.06 | 0.55 ± 0.05 | 0.51 ± 0.04 | NS |
| DHA | 2.59 ± 0.37b | 2.85 ± 0.31a | 2.49 ± 0.14b | 0.02 |
| Day 21 | ||||
| ARA | 4.71 ± 0.39 | 4.55 ± 0.49 | 4.59 ± 0.23 | NS |
| 22:4n-6 | 0.48 ± 0.06 | 0.50 ± 0.07 | 0.50 ± 0.07 | NS |
| 22:5n-6 | 0.24 ± 0.05 | 0.25 ± 0.06 | 0.24 ± 0.05 | NS |
| 20:5n-3 | 0.20 ± 0.09 | 0.18 ± 0.08 | 0.15 ± 0.09 | NS |
| 22:5n-3 | 0.48 ± 0.05 | 0.47 ± 0.05 | 0.48 ± 0.04 | NS |
| DHA | 2.61 ± 0.29 | 2.59 ± 0.26 | 2.52 ± 0.27 | NS |
Values represent means ± SD, n = 8 per diet. P < 0.05 indicates that all means are not equal; NS, not significant. Pairwise comparisons determined using Student’s t-test; means not sharing a common superscript are significantly different (P < 0.05).
ARA, arachidonic acid; DHA, docosahexaenoic acid.
Over the full study period, mean RBC ARA levels across the three dietary treatment groups decreased from 6.98 ± 0.60% total FA on day 3 of age to 4.62 ± 0.32% total FA on day 21 of age. One-way analysis of RBC ARA content by day of age confirmed this temporal decline, with each incremental increase in age corresponding to a significant decrease in RBC ARA (P < 0.0001; data not presented). Mean RBC 22:4n-6 (P < 0.0001) and 22:5n-6 (P < 0.0001) also decreased during the study, while 20:5n-3 and 22:5n-3 levels remained constant.
3.5 Liver histopathology
Histological diagnosis
Liver histology was normal for all animals. “Comment: All samples were within normal histopathological limits. The lobular architecture was intact, the portal regions were unremarkable and there was no evidence of cholestasis. Hepatocytes were uniform in size and shape, replete with glycogen and contained no discernable lipid. No mitotic figures were observed and only rare binucleate hepatocytes were noted. Occasional Kupffer cells were present but no hepatic stellate (Ito) cells could be identified. Variable numbers of small foci of extramedullary hematopoietic tissue were randomly dispersed throughout the parenchyma (incidental). No histopathological changes were present in the sections examined. In particular, no changes similar to those described in rodent studies of peroxisome proliferator exposure were detected.”
3.6 Clinical chemistry and hematological analysis
A summary of mean clinical chemistry and hematological parameters is presented in Table 6. Only two parameters, aspartate aminotransferase (AST) and creatine kinase, were determined to be significantly influenced by diet. Control pigs had significantly higher AST (37 ± 13 U/L) than a1 (28 ± 7 U/L) and a2 (25 ± 7 U/L) pigs (P = 0.01), and higher creatine kinase (895 ± 520 U/L) than the a1 (541 ± 227 U/L) and a2 (520 ± 228 U/L) pigs (P = 0.001). AST and creatine kinase levels were similar between the a1 and a2 groups. Additional parameters that were unaffected by diet were mean cell volume, mean cell hemoglobin, mean cell hemoglobin concentration, red cell distribution width, segmented neutrophils, monocytes, eosinophils, basophils, large unstained cells, mean platelet volume, creatinine, phosphate and total bilirubin (data not presented).
Table 6.
Summary of day 21 of age clinical chemistry and hematological parameters from piglets fed one of three arachidonic acid (ARA)-rich oils1–2
| Test | Control | a1 | a2 | P |
|---|---|---|---|---|
| Hematocrit (%) | 43 ± 3 | 46 ± 5 | 42 ± 2 | NS |
| Hemoglobin (g/dL) | 13.2 ± 0.9 | 13.8 ± 1.3 | 12.9 ± 0.5 | NS |
| RBC (mill/μL) | 6.3 ± 0.4 | 6.5 ± 0.5 | 6.3 ± 0.2 | NS |
| WBC (thou/μL) | 10.5 ± 2.2 | 10.9 ± 1.3 | 9.9 ± 2.6 | NS |
| Lymphocytes (thou/μL) | 6.1 ± 1.1 | 6.4 ± 1.1 | 5.7 ± 1.3 | NS |
| Platelet Count (thou/μL) | 709 ± 76 | 697 ± 169 | 667 ± 136 | NS |
| Urea (mg/dL) | 8 ± 2 | 8 ± 1 | 7 ± 2 | NS |
| Creatinine (mg/dL) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.1 | NS |
| Total Protein (g/dL) | 4.6 ± 0.3 | 4.7 ± 0.2 | 4.5 ± 0.2 | NS |
| Albumin (g/dL) | 3.6 ± 0.3 | 3.5 ± 0.2 | 3.5 ± 0.1 | NS |
| Globulin (g/dL) | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.1 | NS |
| Glucose (mg/dL) | 178 ± 37 | 165 ± 31 | 166 ± 33 | NS |
| AST (U/L) | 37 ± 13a | 28 ± 7b | 25 ± 7b | 0.01 |
| GGT (U/L) | 23 ± 11 | 24 ± 14 | 24 ± 16 | NS |
| Creatine Kinase (U/L) | 895 ± 520a | 541 ± 227b | 520 ± 228b | 0.001 |
Values represent means ± SD, n = 8 per diet. P < 0.05 indicates that all means are not equal; NS, not significant. Pairwise comparisons determined using Student’s t-test; means not sharing a common superscript are significantly different (P < 0.05).
Abbreviations: AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; RBC, red blood cell count; WBC, white blood cell count.
4. Discussion
Preclinical studies with animals provide an invaluable tool for evaluating the safety and suitability of food ingredients for use in the diets of humans. In the present study, we evaluated the bioequivalence and safety of two ARA-rich oils, RAO and SUNTGA40S, in formulations providing ARA at about 0.64% total FA plus DHA from DHASCO, compared with the conventional ARASCO and DHASCO blend that is currently being used worldwide in the Enfamil line of infant formulas. Domestic piglets were used because of their rapid rates of growth and similarities in anatomy, physiology and metabolism to human infants, particularly in terms of brain development and metabolism of lipids and LCPUFA (Dobbing and Sands, 1979; Innis, 1993; Moughan and Rowan, 1989).
Growth performance is a well-accepted measure of health and vitality in developing infants. In the present study, the two experimental ARA formulas were readily consumed and equally supported growth in the neonatal pigs compared with the Control formula. Body weights were similar among the three dietary treatment groups at every time point and no differences were observed for organ weights upon sacrifice. Human infants generally double their birth weight by four months of life and grow to four times their birth weight by 24 – 28 months (WHO, 2006). The test piglets grew to four times their initial body weight by day 22 of age, achieving target growth rates that were 110% of values previously observed with formula-reared piglets from this herd (Huang et al., 2002). This rapid rate of growth and concomitant high demand for formula suggest that any trace toxicological constituent of the formulas could have manifested as poorer growth or suboptimal clinical conditions during this short study duration. Rather, pigs in this study appeared healthy and presented with few health ailments. Loose stools in a number of piglets across all three dietary treatment groups were the greatest concern during the first week of the study. Post-weaning diarrhea syndrome is frequently observed in a subset of piglets. Because there was no obvious difference in diarrhea incidence or severity between experimental and control groups, we speculate that the likely cause was stress from the transition period and adaptation to a new diet and environment (Spencer and Howell, 1989) rather than the specific ARA sources.
The three ARA-rich oils equally supported ARA accretion in the brain, retina and heart, consistent with a high degree of similarity in the bioavailabilities of ARA from the experimental sources (RAO and SUNTGA40S) and the Control source (ARASCO). In particular, the similarity in cerebral cortex ARA as well as DHA content at study termination demonstrates the bioequivalence of the three ARA-rich oils in supporting this key measure of relevance to outcomes in human infants. Liver ARA levels, a secondary outcome for the study, showed a remarkable sensitivity to the dietary ARA content, with the Control pigs having significantly higher ARA levels than the a1 group. This difference in liver ARA levels is consistent with the a1 diet providing 8% less ARA (0.62% vs. 0.67% total FA) than the Control and a2 diets, a difference that may arise from normal variability across product lots and over time during the manufacturing process (Cook, 1989). However, liver DHA levels also appeared responsive to the difference in dietary ARA level, with the a1 pigs having the highest DHA content. Numerous studies have demonstrated a sensitivity of liver LCPUFA to dietary FA intake (Blank et al., 2002; de la Presa-Owens et al., 1998; Tyburczy et al., 2010a) that occurs primarily as a result of dietary FA composition but also may be related to the regulation of LCPUFA biosynthesis by dietary LCPUFA (Jump et al., 2005; Nakamura and Nara, 2004). Overall, these results demonstrate that both RAO and SUNTGA40S are nutritionally bioequivalent sources of ARA compared with ARASCO and that subtle variations in the formula content of ARA may influence liver ARA and DHA levels.
Studies with human infants are limited by the information they can provide, especially with regard to the ethical limitations and potential risks associated with frequent blood sampling as well as the general unavailability of tissue from healthy individuals (Howie, 2010). This study provided a unique opportunity to examine acute changes in RBC LCPUFA levels that may otherwise be overlooked in clinical studies with infants. Here, we observed decreases in RBC ARA, 22:4n-6 and 22:5n-6 and an overall increase in RBC DHA compared with baseline values. This decline in RBC ARA has been reported in studies with human infants consuming preformed ARA from breast milk or formula (Carlson, 1996) and may be associated with the accretion of ARA by growing neonatal tissues. In support of this, the infant brain rapidly accumulates ARA over the first few two years of life (Makrides et al., 1994), and in (Tyburczy et al., 2010a) we demonstrated that the growing heart concentrates ARA at rates that appear limited by dietary ARA supply. Concerning DHA, we observed a overall increase in RBC DHA that appeared to reach plateau by day 14 of age. The endogenously low levels of RBC DHA at baseline may be explained in part by the negligible levels of DHA in the sow milk (0.01 ± 0.01% total FA), as were previously determined for this sow herd (Tyburczy et al., 2010a; Tyburczy et al., 2010b). The content of RBC DHA on days 14 and 21 is consistent with most (Amusquivar et al., 2010; Blanaru et al., 2004; Mathews et al., 2002; Mollard et al., 2005), but not all (Huang et al., 2007) studies of rapidly-growing, neonatal pigs with comparable DHA intakes, but is lower than the RBC DHA levels of human infants (Hoffman et al., 2008; Hoffman et al., 2006; Miller et al., 2010; Minns et al., 2010).
Mean values for serum clinical chemistry and hematological parameters were consistent with previous studies in neonatal pigs (Danicke and Doll, 2010; Herfel et al., 2009; Huang et al., 2002; Tyburczy et al., 2010b), and very few differences were observed among the three dietary treatment groups. Control pigs showed elevated plasma AST and creatine kinase compared with pigs fed either of the experimental formulas. For AST, a biomarker of tissue inflammation, Control values were within limits previously observed in nursery pigs (Herfel et al., 2009) and weaned growing pigs (Dubreuil and Lapierre, 1997), suggesting that the numerical difference among diets is biologically insignificant. Creatine kinase levels were highest in the Control pigs compared with the two experimental ARA groups, although all treatment means were at the low end of the normal range for commercial pigs (Carr and Wilbers, 2008) and were comparable to values previously observed in a similar neonatal pig study (Tyburczy et al., 2010b). In all pigs, liver sections were determined to be normal following histopathological examination and showed no indication of gross abnormalities. These results are consistent with other studies in pigs showing that the addition of ARA-rich oil to formula providing ARA up to 5x the level recommended by (FAO/WHO, 1994) is safe and produces no adverse effect on liver histology or serum clinical indicators (Huang et al., 2002; Merritt et al., 2003). Thus, results from the present study indicated that the two experimental ARA oils produced no pathohistological abnormalities in the liver nor had any adverse effects on the serum clinical chemistry or hematological parameters that were measured.
In 2001, ARASCO was determined to be GRAS for use in term infant formula when added with DHASCO and at levels up to 1.88% total fat, providing ARA at 0.75% total fat (Rulis, 2001). More recently, SUNTGA40S was determined to be GRAS for preterm and term infants at levels providing ARA up to 0.40% total FA and in combination with DHA-rich tuna oil (Tarantino, 2006). This study demonstrates that SUNTGA40S is safe and nutritionally bioequivalent to ARASCO when providing ARA at 0.64% total FA and in combination with DHASCO. The third oil, RAO, has been proposed for use in infant formula (Casterton, 2010) although preclinical studies with higher vertebrate neonates have not been previously reported. In rats however, diets supplemented with RAO up to 5% wt/wt were shown to have no adverse effect on reproductive outcomes in the parent generation or effects on clinical chemistry, organ weights or histology in the offspring using 90-day feeding trials (Casterton et al., 2009). Here, we report that formula with RAO supplying 0.64% ARA produced no adverse effect on liver histology or on any of the serum clinical chemistry or hematological outcomes measured in these neonatal piglets.
Overall, this study evaluated the bioequivalence and safety of two ARA sources for potential use in commercial infant formula compared with ARASCO, a source currently used in infant formulas worldwide. Oils provided ARA at approximately 0.64% total FA, were added with DHASCO to supply 0.34% DHA and resulted in no other notable alterations in the formula FA composition. Results demonstrated that the three ARA-rich oils equally supported tissue and RBC ARA accretion, establishing bioequivalence according to FDA guidelines (FDA, 2003), and produced no adverse effect on liver histology or serum clinical indicators in the neonatal pigs. We conclude that ARA supplied by the single-cell oils RAO and SUNTGA40S, when added to formula to provide ~0.64% ARA and in combination with ~0.34% DHA from DHASCO, are nutritionally bioequivalent, equally supporting ARA, as well as DHA accretion in the neonatal pig brain, retina and heart, and are as safe as the current ARASCO source for use as ingredients in infant formula.
Highlights.
Dietary arachidonic acid (ARA) is a component of infant formula.
We examine two commercial food sources of ARA for safety and efficacy in piglets.
Both test ARA sources supported tissue ARA levels as well as a commercial ARA control.
Both test ARA sources were safe.
Acknowledgments
The authors acknowledge the technical assistance of Jessica Kummer and Karl Roneker for assistance with animals, and Françoise Vermeylen of the Cornell Statistical Consulting Unit for assistance with bioequivalence calculations. This project was supported in part by Award T32DK007158 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ARA
arachidonic acid
- ARASCO
ARA single-cell oil
- AST
aspartate aminotransferase
- DHA
docosahexaenoic acid
- DHASCO
DHA single-cell oil
- FA
fatty acid
- FAME
FA methyl ester
- FAO
Food and Agriculture Organization
- FDA
U. S. Food and Drug Administration
- GRAS
Generally Recognized as Safe
- LCPUFA
long chain polyunsaturated FA
- RAO
refined arachidonic acid-rich oil
- RBC
red blood cell
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AFSSA. Opinion of the French Food Safety Agency on the update of French population reference intakes (ANCs) for fatty acids. 2010. Request no. 2006-SA-0359. [Google Scholar]
- Amusquivar E, Laws J, Clarke L, Herrera E. Fatty acid composition of the maternal diet during the first or the second half of gestation influences the fatty acid composition of sows’ milk and plasma, and plasma of their piglets. Lipids. 2010;45:409–418. doi: 10.1007/s11745-010-3415-2. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Oken HA, Hoffman JP, Bailey-Hall E, Chung G, Rom D, Hamersley J, McCarthy D. Bioequivalence of Docosahexaenoic acid from different algal oils in capsules and in a DHA-fortified food. Lipids. 2007;42:1011–1024. doi: 10.1007/s11745-007-3098-5. [DOI] [PubMed] [Google Scholar]
- Blanaru JL, Kohut JR, Fitzpatrick-Wong SC, Weiler HA. Dose response of bone mass to dietary arachidonic acid in piglets fed cow milk-based formula. Am J Clin Nutr. 2004;79:139–147. doi: 10.1093/ajcn/79.1.139. [DOI] [PubMed] [Google Scholar]
- Blank C, Neumann MA, Makrides M, Gibson RA. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res. 2002;43:1537–1543. doi: 10.1194/jlr.m200152-jlr200. [DOI] [PubMed] [Google Scholar]
- Brenna JT, Tyburczy C. Identification of FAME double bond location by covalent adduct chemical ionization (CACI) tandem mass spectrometry. AOCS Lipid Library. 2010 http://lipidlibrary.aocs.org/topics/caci_ms/index.htm.
- Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Arachidonic acid status of human infants: influence of gestational age at birth and diets with very long chain n-3 and n-6 fatty acids. J Nutr. 1996;126:1092S–1098S. doi: 10.1093/jn/126.suppl_4.1092S. [DOI] [PubMed] [Google Scholar]
- Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–449. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- Carr J, Wilbers A. Pet pig medicine: 1. The normal pig. In Practice. 2008;30:160–166. [Google Scholar]
- Casterton PL. Generally regarded as safe notification for the use of refined arachidonic acid rich oil (RAO) as an ingredient in infant formula. 2010. [Google Scholar]
- Casterton PL, Curry LL, Lina BA, Wolterbeek AP, Kruger CL. 90-Day feeding and genotoxicity studies on a refined arachidonic acid-rich oil. Food Chem Toxicol. 2009;47:2407–2418. doi: 10.1016/j.fct.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Cook DA. Nutrient levels in infant formulas: technical considerations. J Nutr. 1989;119:1773–1777. doi: 10.1093/jn/119.suppl_12.1773. discussion 1777–1778. [DOI] [PubMed] [Google Scholar]
- Cunnane SC, Francescutti V, Brenna JT, Crawford MA. Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids. 2000;35:105–111. doi: 10.1007/s11745-000-0501-6. [DOI] [PubMed] [Google Scholar]
- Danicke S, Doll S. A probiotic feed additive containing spores of Bacillus subtilis and B. licheniformis does not prevent absorption and toxic effects of the Fusarium toxin deoxynivalenol in piglets. Food Chem Toxicol. 2010;48:152–158. doi: 10.1016/j.fct.2009.09.032. [DOI] [PubMed] [Google Scholar]
- de la Presa-Owens S, Innis SM, Rioux FM. Addition of triglycerides with arachidonic acid or docosahexaenoic acid to infant formula has tissue- and lipid class-specific effects on fatty acids and hepatic desaturase activities in formula-fed piglets. J Nutr. 1998;128:1376–1384. doi: 10.1093/jn/128.8.1376. [DOI] [PubMed] [Google Scholar]
- Diau GY, Hsieh AT, Sarkadi-Nagy EA, Wijendran V, Nathanielsz PW, Brenna JT. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dubreuil P, Lapierre H. Biochemistry reference values for Quebec lactating dairy cows, nursing sows, growing pigs and calves. Can J Vet Res. 1997;61:235–239. [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO. FAO Food and Nutrition Paper No. 57. Rome: 1994. Fats and oils in human nutrition. Report of a joint expert consultation; pp. 49–55. [PubMed] [Google Scholar]
- FAO/WHO. From the joint FAO/WHO expert consultation on fats and fatty acids in human nutrition. WHO Headquarters; Geneva: 2010. Nov 10–14, Interim summary of conclusions and dietary recommendations on total fat & fatty acids. 2008. [Google Scholar]
- Farquharson J, Cockburn F, Patrick WA, Jamieson EC, Logan RW. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- FDA; U.S. Department of Health and Human Services. Bioavailability and bioequivalence studies for orally administered drug products - general considerations. C.f.D.E.a.R; 2003. Guidance for Industry. [Google Scholar]
- Fritsche R. Animal models in food allergy: assessment of allergenicity and preventive activity of infant formulas. Toxicol Lett. 2003;140–141:303–309. doi: 10.1016/s0378-4274(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Garces R, Mancha M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal Biochem. 1993;211:139–143. doi: 10.1006/abio.1993.1244. [DOI] [PubMed] [Google Scholar]
- Herfel TM, Jacobi SK, Lin X, Walker DC, Jouni ZE, Odle J. Safety evaluation of polydextrose in infant formula using a suckling piglet model. Food Chem Toxicol. 2009;47:1530–1537. doi: 10.1016/j.fct.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Hoffman D, Ziegler E, Mitmesser SH, Harris CL, Diersen-Schade DA. Soy-based infant formula supplemented with DHA and ARA supports growth and increases circulating levels of these fatty acids in infants. Lipids. 2008;43:29–35. doi: 10.1007/s11745-007-3116-7. [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81:151–158. doi: 10.1016/j.plefa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Hoffman DR, Wheaton DK, James KJ, Tuazon M, Diersen-Schade DA, Harris CL, Stolz S, Berseth CL. Docosahexaenoic acid in red blood cells of term infants receiving two levels of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr. 2006;42:287–292. doi: 10.1097/01.mpg.0000189366.91792.64. [DOI] [PubMed] [Google Scholar]
- Howie SRC. Bulletin of the World Health Organization. 2010. Blood sample volumes in child health research: Review of safe limits. BLT.10.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh AT, Anthony JC, Diersen-Schade DA, Rumsey SC, Lawrence P, Li C, Nathanielsz PW, Brenna JT. The influence of moderate and high dietary long chain polyunsaturated fatty acids (LCPUFA) on baboon neonate tissue fatty acids. Pediatr Res. 2007;61:537–545. doi: 10.1203/pdr.0b013e318045bec9. [DOI] [PubMed] [Google Scholar]
- Huang MC, Brenna JT, Chao AC, Tschanz C, Diersen-Schade DA, Hung HC. Differential tissue dose responses of (n-3) and (n-6) PUFA in neonatal piglets fed docosahexaenoate and arachidonoate. J Nutr. 2007;137:2049–2055. doi: 10.1093/jn/137.9.2049. [DOI] [PubMed] [Google Scholar]
- Huang MC, Chao A, Kirwan R, Tschanz C, Peralta JM, Diersen-Schade DA, Cha S, Brenna JT. Negligible changes in piglet serum clinical indicators or organ weights due to dietary single-cell long-chain polyunsaturated oils. Food Chem Toxicol. 2002;40:453–460. doi: 10.1016/s0278-6915(01)00105-3. [DOI] [PubMed] [Google Scholar]
- Innis SM. The colostrum-deprived piglet as a model for study of infant lipid nutrition. J Nutr. 1993;123:386–390. doi: 10.1093/jn/123.suppl_2.386. [DOI] [PubMed] [Google Scholar]
- Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Innis SM. Human milk: maternal dietary lipids and infant development. Proc Nutr Soc. 2007;66:397–404. doi: 10.1017/S0029665107005666. [DOI] [PubMed] [Google Scholar]
- IOM. Institute of Medicine. Infant formula: Evaluating the safety of new ingredients. National Academies Press; Washington D. C.: 2004. [PubMed] [Google Scholar]
- Jacobi SK, Lin X, Corl BA, Hess HA, Harrell RJ, Odle J. Dietary arachidonate differentially alters desaturase-elongase pathway flux and gene expression in liver and intestine of suckling pigs. J Nutr. 141:548–553. doi: 10.3945/jn.110.127118. [DOI] [PubMed] [Google Scholar]
- Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Thiel I, Abiodun PO. The fatty acid composition of human milk in Europe and Africa. J Pediatr. 1992;120:S62–70. doi: 10.1016/s0022-3476(05)81238-7. [DOI] [PubMed] [Google Scholar]
- Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–194. doi: 10.1093/ajcn/60.2.189. [DOI] [PubMed] [Google Scholar]
- Mathews SA, Oliver WT, Phillips OT, Odle J, Diersen-Schade DA, Harrell RJ. Comparison of triglycerides and phospholipids as supplemental sources of dietary long-chain polyunsaturated fatty acids in piglets. J Nutr. 2002;132:3081–3089. doi: 10.1093/jn/131.10.3081. [DOI] [PubMed] [Google Scholar]
- Merritt RJ, Auestad N, Kruger C, Buchanan S. Safety evaluation of sources of docosahexaenoic acid and arachidonic acid for use in infant formulas in newborn piglets. Food Chem Toxicol. 2003;41:897–904. doi: 10.1016/s0278-6915(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Miller MR, Seifert J, Szabo NJ, Clare-Salzler M, Rewers M, Norris JM. Erythrocyte membrane fatty acid content in infants consuming formulas supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA): an observational study. Matern Child Nutr. 2010;6:338–346. doi: 10.1111/j.1740-8709.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns LM, Kerling EH, Neely MR, Sullivan DK, Wampler JL, Harris CL, Berseth CL, Carlson SE. Toddler formula supplemented with docosahexaenoic acid (DHA) improves DHA status and respiratory health in a randomized, double-blind, controlled trial of US children less than 3 years of age. Prostaglandins Leukot Essent Fatty Acids. 2010;82:287–293. doi: 10.1016/j.plefa.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Mollard RC, Kovacs HR, Fitzpatrick-Wong SC, Weiler HA. Low levels of dietary arachidonic and docosahexaenoic acids improve bone mass in neonatal piglets, but higher levels provide no benefit. J Nutr. 2005;135:505–512. doi: 10.1093/jn/135.3.505. [DOI] [PubMed] [Google Scholar]
- Moughan P, Rowan A. The pig as a model for human nutrition research. Proceedings of the Nutrition Society of New Zealand. 1989;14:116–123. [Google Scholar]
- Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- NRC. National Research Council. Nutrient requirements of swine. 10 1998. [Google Scholar]
- Rulis AM. CFSAN/Office of Premarket Approval. 2001. Agency Response Letter GRAS Notice No. GRN 000080. [Google Scholar]
- Rulis AM, Lewis CJ. GRN 000041. CFSAN/Office of Premarket Approval. 2001. Agency Response Letter GRAS Notice No. [Google Scholar]
- Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2010;82:305–314. doi: 10.1016/j.plefa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Spencer BT, Howell PG. Some husbandry factors influencing weaning stresses in piglets. J S Afr Vet Assoc. 1989;60:62–64. [PubMed] [Google Scholar]
- Su HM, Keswick LA, Brenna JT. Increasing dietary linoleic acid in young rats increases and then decreases docosahexaenoic acid in retina but not in brain. Lipids. 1996;31:1289–1298. doi: 10.1007/BF02587915. [DOI] [PubMed] [Google Scholar]
- Tarantino LM. GRN 000094. CFSAN/Office of Premarket Approval. 2006. Agency Response Letter GRAS Notice No. [Google Scholar]
- Tyburczy C, Kothapalli KSD, Park WJ, Blank BS, Bradford K, Zimmer JP, Butt C, Salem JN, Brenna JT. Heart arachidonic acid (ARA) is uniquely sensitive to dietary ARA and docosahexaenoic acid (DHA) content in domestic piglets. J Nutr. 2010a doi: 10.1016/j.plefa.2011.08.005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburczy C, Kothapalli KSD, Park WJ, Blank BS, Liu Y-C, Nauroth JM, Zimmer JP, Salem JN, Brenna JT. Negligible effect of dietary arachidonic acid (ARA) levels on growth, clinical chemistry and immune function in domestic piglets. Brit J Nutr. 2010b doi: 10.1017/S000711451100359X. submitted. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nijland M, Miller M, Ford S, Nathanielsz PW, Brenna JT. The influence of maternal early to mid-gestation nutrient restriction on long chain polyunsaturated fatty acids in fetal sheep. Lipids. 2008;43:525–531. doi: 10.1007/s11745-008-3186-1. [DOI] [PubMed] [Google Scholar]