Abstract
Purpose
The objectives of this study were to examine the differences in health status (HS) of women with breast cancer (BC) at different moments in time, and between women scoring high and not high on trait anxiety, and to identify possible predictors of HS 6 and 12 months after surgery.
Methods
Patients (N = 223) completed a trait anxiety questionnaire before diagnosis. Women who received a diagnosis of BC completed a BC-specific HS questionnaire 1, 3, 6 and 12 months after surgery. ANCOVA for repeated measures and multiple regression analysis were used in the analyses.
Results
Women scoring high on trait anxiety had significant (P < .005) lower Body image, worse Future perspective and Sexual functioning, and more Side-effects than women who did not score high on trait anxiety. At 6 and 12 months after surgery, the same aspects of HS were predicted by higher trait anxiety scores.
Conclusions
Higher scores on trait anxiety resulted in worse scores on four HS domains, indicating that there should be more attention for this group of patients, even before treatment starts.
Keywords: Oncology, Breast cancer, Health status, Trait anxiety, Treatment
Introduction
Breast cancer is the most widespread form of cancer in Europe [1]. Since incidence rates are still increasing but mortality is decreasing [1, 2], more and more women are survivors of breast cancer. For these women, health status (HS), defined as “well-being in terms of physical, mental, and social condition” [3], is an important outcome, next to their medical condition. HS has been examined in different studies with various measures, for instance among long-term survivors (>5 years) of breast cancer [4]. In these women, overall HS was fairly good. The most frequently reported complaints were sexual problems and arm problems [4]. When HS was measured within 2 years after diagnosis, DiSipio et al. [5] found that younger breast cancer survivors (<50 years) 1 year after diagnosis had a HS comparable with the normal population. Women of 50 years and older already had a HS comparable with the normal population 6 months after diagnosis. Inconsistent with these results, other studies noted that 1 year after surgery, cognitive and social functioning in breast cancer survivors were impaired compared with the normal population [6], and the HS of younger women was more impaired than that of older women [7, 8]. There are recent studies that indicate that HS is also affected by socio-economic status and comorbidity [9, 10].
A factor that is associated with the level of HS in breast cancer patients, although not studied frequently, is trait anxiety, i.e. the disposition to experience anxiety in threatening situations. Schreier et al. [11] for example, found that trait anxiety correlates negatively with HS scores in breast cancer patients. Trait anxiety also predicted higher levels of distress in breast cancer patients [12], with higher levels of distress being directly related to a decrease in HS [13]. A recent study in patients with rectal cancer showed that higher trait anxiety levels predicted worse HS [14].
In this longitudinal, prospective study, trait anxiety was measured in patients before they received a diagnosis. Subsequently, HS was measured at different moments in time after diagnosis and treatment. This makes it possible to look at the relation between trait anxiety and the different aspects of HS, as well as looking at the course of HS in time.
The goals of this study were to examine (1) whether women who score high on trait anxiety have a different HS across time compared with women who do not score high on trait anxiety, and (2) which factors predict HS 6 months and 1 year after surgery. The expectation was that women who scored high on trait anxiety had a worse HS than women who did not score high on trait anxiety. Furthermore, trait anxiety was expected to predict HS at 6 and 12 months after surgery.
Methods and patients
Patients
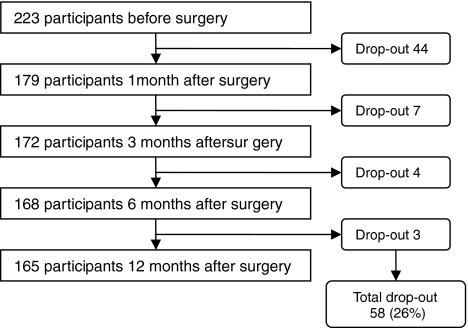
This study was part of a larger study, concerning the relationship between personality, surgical treatment and quality of life in women with early-stage breast cancer. In this larger study, all women visiting the outpatient clinic of the St Elisabeth Hospital in Tilburg (from January 2002 until June 2007), the Maasland Hospital in Sittard (from June 2004 until March 2006), and the Jeroen Bosch Hospital in Den Bosch (from January 2006 until June 2007), The Netherlands, were asked to participate. They were referred by their general practitioner with either a palpable lump in the breast or an abnormality on the screening mammography and a negative medical history for breast disease. At their first appointment with a specialized mamma-care nurse (a nurse who is specialized in the care for breast cancer patients), they were informed about the study and when women agreed to participate, they completed an informed consent and a set of questionnaires (Time 1). This was before they knew the diagnosis of the breast problem they visited the outpatient clinic for. Of the 799 women who visited one of the outpatient clinics with a problem of the breast, 286 (35.8%) were diagnosed with early-stage breast cancer. The other 513 (64.2%) patients appeared to have a benign breast problem. Of the 286 women with breast cancer, 223 (78%) completed the first set of questionnaires, including a questionnaire about trait anxiety. Patients with breast cancer included in this study were in the situation that they had a choice in their surgical treatment: they could choose either for breast conserving therapy (BCT) or for mastectomy (MTC). The breast cancer patients received additional questionnaires at 1 (Time 2), 3 (Time 3), 6 (Time 4) and 12 months (Time 5) after surgical treatment (see Fig. 1). Exclusion criteria for the current study were not being able to speak and/or read Dutch, having cognitive problems, a medical history with breast disease, advanced-stage breast cancer or a benign breast problem. This study was approved by the Medical Ethical Committee (ccMo NL15659.008.06).
Fig. 1.
Flowchart of the study
Questionnaires
HS was measured at Time 2, Time 3, Time 4, and Time 5 using the validated disease-specific European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Breast Cancer module (EORTC QLQ-BR23) [15, 16]. This 23-item questionnaire is developed for breast cancer patients at different stages of their disease and with varying treatment modalities. The EORTC QLQ-BR23 measures the functional scales Body image (4 items), Sexual functioning (2 items), Sexual enjoyment (1 item; only to be completed when patient is sexually active), and Future perspective (1 item; “Were you worried about your health in the future?”) and the disease symptom scales Systemic therapy side-effects (7 items), Breast symptoms (4 items), Arm symptoms (3 items), and Upset by hair loss (1 item; only to be completed if patient had hair loss). Answers vary from 1 (not at all) to 4 (very much). Higher scores on the functional scales represent a higher level of functioning, while higher scores on the symptom scales represent more symptomatology.
Trait anxiety was measured at Time 1 with the trait anxiety subscale of the State-Trait Anxiety Inventory (STAI) at Time 1 [17, 18]. The STAI measures trait anxiety with 20 items. Answers range from 1 (almost never) to 4 (almost always). It is a frequently used questionnaire with good psychometric properties [18]. For some analyses, the scores on trait anxiety were dichotomized in high or not high. A cut-off score of 44 or higher was used to determine high trait anxiety [18]. The definition of this cut-off score was based on normative data for women between 40 and 59 years [19] and was construed using the mean score (34.2) plus 1 SD (9.87).
Demographic variables included in the study were age and marital status. Level of education and work status were assessed to use as an approximation of socio-economic status.
Medical records
The following information was retrieved from patients’ medical files: the disease stage at diagnosis [20], type of operation, and adjuvant treatment (chemotherapy, radiotherapy, hormone treatment).
Statistical procedure
For baseline measures, Chi-square (discrete variables) and independent samples T tests (continuous variables) were used to compare participants and non-participants, and the trait anxiety groups (dichotomized in high or not high) with regard to age, partner, children, education, work, disease stage at diagnosis, level of trait anxiety, surgical treatment, and adjuvant treatment. ANCOVA for repeated measures was used to examine differences in HS scores between measurements in time and between patients with different trait anxiety scores (dichotomized in high or not high). Additionally, univariate regression analysis was used to select factors for the multivariate linear regression analyses (MRA). The dependent variables in the univariate regression were Body image, Sexual functioning, Future perspective, Systemic therapy side-effects, Breast symptoms and Arm symptoms at T4 and T5. Independent variables were age, partner yes/no, children yes/no, education, work yes/no, level of trait anxiety (continuous total score), screening referral yes/no, disease stage at diagnosis, surgical treatment, adjuvant therapy yes/no, chemotherapy yes/no, radiotherapy yes/no, and hormone therapy yes/no. The significant independent variables of the univariate regression analysis were imported in the backward MRA that was performed to identify those factors that best predicted patients’ scores on the EORTC QLQ-BR23 at Time 4 and Time 5. All analyses were performed with the Statistical Package for Social Sciences (SPSS) version 14.0.
Results
Of 286 eligible women with breast cancer, 223 (78%) participated in the study. There were no differences in age, disease stage, type of operation, and receiving adjuvant therapy between participants and non-participants. Demographic and medical information of participants is summarized in Table 1.
Table 1.
Demographic and medical information of the breast cancer patients (N = 223)
| Demographic information | Medical information | ||
|---|---|---|---|
| Age | Screening referral* | ||
| Mean ± SD | 58.7 ± 9.4 | Yes | 151 (67.7%) |
| Partner | No | 72 (32.3%) | |
| Yes | 179 (80.2%) | Disease stage | |
| No | 37 (16.6%) | Stage 0 | 24 (10.8%) |
| Missing | 7 (3.1%) | Stage I | 93 (41.7%) |
| Children | Stage IIa | 70 (31.4%) | |
| Yes | 191 (85.7%) | Stage IIb | 35 (15.7%) |
| No | 29 (13.0%) | Indefinable | 1 (0.4%) |
| Missing | 3 (1.3%) | Type of operation | |
| Educational level | Breast conserving therapy | 107 (48.0%) | |
| Low | 85 (38.1%) | Mastectomy | 114 (51.1%) |
| Middle | 95 (42.6%) | No operation | 2 (0.9%) |
| High | 37 (16.6%) | Adjuvant therapy | |
| Missing | 6 (2.7%) | Received chemotherapy | 60 (26.9%) |
| Work status | Received radiotherapy | 117 (52.2%) | |
| Employed | 85 (38.1%) | Received hormone treatment | 87 (39.0%) |
| Unemployed | 135 (60.5%) | ||
| Missing | 3 (1.3%) | ||
* Screening referral Women referred by the national breast cancer screening program
About 40% was diagnosed with disease stage I. Regarding surgical treatment, 107 women had BCT and 114 women received MTC. Women with BCT and MTC significantly differed on receiving adjuvant therapy and disease stage; i.e. BCT patients more often had adjuvant therapy (P < .001) and were in a lower disease stage at diagnosis (P = .002). Women scoring high on trait anxiety (37%) had a lower education level (P = .005) and were older (P = .041) than women not scoring high on trait anxiety. There were no differences concerning other demographic and medical factors. Disease stage, receiving adjuvant therapy, type of surgery, educational level, and age were included as covariates in the subsequent analyses. During the study, 58 women (26%) dropped out within 12 months after surgery. Reasons for drop-out were ‘not interested’, ‘do not want to be confronted with the past,’ or ‘too much work’ (Fig. 1). Participants and drop-outs only differed on age (P = .008), with drop-outs being older than participants.
In the MRA, the total score on trait anxiety was included following the goal of the study. Age, disease stage, receiving adjuvant therapy, type of surgery, and educational level were included because these factors differed between groups. In addition, significant factors of the univariate analyses were included, namely chemotherapy and screening referral for Body image, marital status for Sexual functioning, chemotherapy and hormone therapy for Systemic therapy side-effects, and children, radiotherapy, and screening referral for Breast symptoms (see Table 2).
Table 2.
Outcomes of the univariate regression analyses of demographic and medical factors on the EORTC QLQ-BR23 domains: P values
| BI T4 | FP T4 | SF T4 | SE T4 | BS T4 | AS T4 | BI T5 | FP T5 | SF T5 | SE T5 | BS T5 | AS T5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | .002 | .025 | .001 | |||||||||
| Screening referral | .014 | .042 | ||||||||||
| STAI total score | .006 | <.001 | <.001 | .004 | .034 | .020 | <.001 | .011 | .008 | .022 | ||
| Adjuvant treatment | .016 | |||||||||||
| Chemotherapy | .001 | <.001 | .038 | |||||||||
| Radiotherapy | .003 | <.001 | ||||||||||
| Hormonal treatment | .029 | |||||||||||
| Type of surgery | .001 | .007 | .005 | <.001 | ||||||||
| Disease stage | .045 | .003 | ||||||||||
| Educational level | .017 | .018 | .012 | |||||||||
| Children | .017 | |||||||||||
| Work | ||||||||||||
| Partner | .007 | <.001 |
BI Body image, FP future perspective, SF sexual functioning, SE systemic therapy side-effects, BS breast symptoms, AS arm symptoms, T4 measurement 6 months after surgical treatment, T5 measurement 12 months after surgical treatment
Note: Only significant results are shown
Functional scales
The high trait anxiety group had a lower Body image than the no-high group (P = .003). The covariate type of surgery was also significant. The BCT group had a higher Body image than the MTC group at all measurements (P = .006). More positive Body image scores at Time 4 and Time 5 was predicted by lower scores on trait anxiety, BCT, and older age. Receiving chemotherapy predicted a more negative Body image, but only at Time 4 (Table 3).
Table 3.
Predictors of the functional scales at 6 and 12 months after surgery
| 6 months after surgery | 12 months after surgery | |||||
|---|---|---|---|---|---|---|
| β | P | Tot. adj. R² | β | P | Tot. adj. R² | |
| Body image | ||||||
| Trait anxiety | −.311 | <.001 | −.257 | .002 | ||
| Type of surgery | −.239 | .002 | −.266 | .001 | ||
| Age | .259 | .001 | .256 | .002 | ||
| Chemotherapy | −.173 | .031 | 23.5% | 15.7% | ||
| Future perspective | ||||||
| Trait anxiety | −.497 | <.001 | −.335 | <.001 | ||
| Age | .166 | .025 | 25.0% | 10.5% | ||
| Sexual functioning | ||||||
| Trait anxiety | −.330 | <.001 | −.232 | .007 | ||
| Partner | .192 | .023 | .277 | .002 | ||
| Age | 13.2% | −.212 | .015 | 17.5% | ||
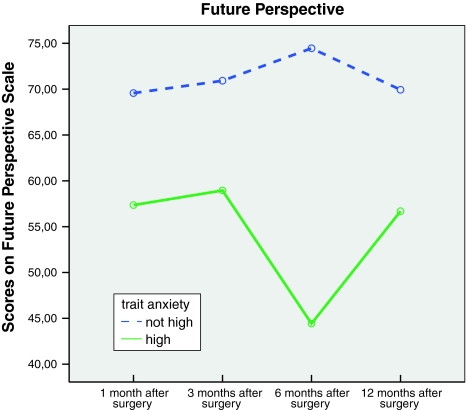
Women who did not score high on trait anxiety had a more positive Future perspective than women who did score high on trait anxiety (P < .001) (see Fig. 2). The latter group had a more unusual course of Future perspective. Between Time 3 and Time 4 their Future perspective dropped seriously (P < .001). At Time 5, their Future perspective had increased to approximately their Time 2 level. This pattern is reflected in the interaction effect of time and trait anxiety (P < .001).
Fig. 2.
Scores on the Future Perspective scale of the EORTC QLQ-BR23 for women scoring high and not high on trait anxiety
More positive Future perspective at Time 4 was predicted by lower scores on trait anxiety and older age. Trait anxiety was the only predictor of more positive Future perspective at Time 5 (Table 3), i.e., lower scores on trait anxiety predicted higher Future perspective.
The women with a high score on trait anxiety reported lower Sexual functioning than the other women (P = .002). There was an interaction effect between time and chemotherapy, showing that the course of Sexual functioning for women with or without chemotherapy differed across time (P = .045). Two different factors predicted better Sexual functioning at Time 4 and Time 5, namely lower scores on trait anxiety and having a partner. Younger age was a predictor of a higher level of Sexual functioning at Time 5 (Table 3).
Symptom scales
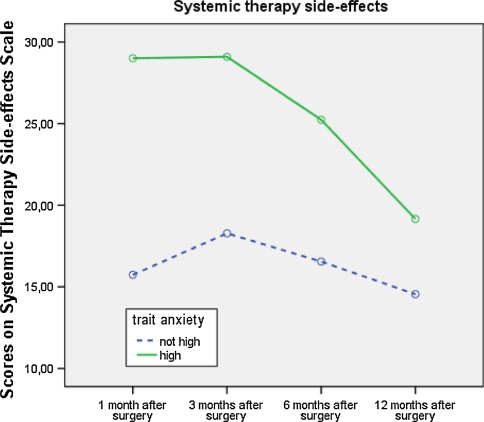
Women high on trait anxiety reported more Side-effects than the other women (P = .001) (Fig. 3). There was an interaction effect between time and chemotherapy (P = .012) reflecting that women without chemotherapy have a constant level of Side-effects across time and women with chemotherapy experienced a major increase in Side-effects 3 months after surgery. Side-effects decreased toward 12 months after surgery, but the level stayed above that of women without chemotherapy. Receiving chemotherapy and having a higher score on trait anxiety were significant predictors of more Side-effects at Time 4. At Time 5, only higher scores on trait anxiety significantly predicted more Side-effects (see Table 4).
Fig. 3.
Scores on the Systemic Therapy Side-effects scale of the EORTC QLQ-BR23 for women scoring high and not high on trait anxiety
Table 4.
Predictors of the symptoms scales at 6 and 12 months after surgery
| 6 months after surgery | 12 months after surgery | |||||
|---|---|---|---|---|---|---|
| β | P | Tot. adj. R² | β | P | Tot. adj. R² | |
| Side-effects | ||||||
| Chemotherapy | .340 | <.001 | ||||
| Trait anxiety | .299 | <.001 | 16.9% | .249 | .005 | 5.3% |
| Breast symptoms | ||||||
| Radiotherapy | .251 | .002 | ||||
| Children | −.233 | .004 | ||||
| Educational level | −.223 | .006 | −.224 | .008 | ||
| Type of surgery | 14.3% | −.378 | <.001 | 17.3% | ||
| Arm symptoms | ||||||
| Educational level | −.204 | .016 | 3.5% | −.177 | .048 | 2.3% |
Trait anxiety did not interfere with Breast symptoms, but type of surgery did. BCT resulted in significantly (P = .007) more Breast symptoms than MTC. More Breast symptoms at Time 4 were predicted by receiving radiotherapy, a lower educational level and not having children. Educational level remained a predictor at Time 5, and receiving BCT predicted more Breast symptoms (Table 4).
Arm symptoms differed between women in different disease stages (P = .015). A higher disease stage resulted in more Arm symptoms. Worse scores on Arm symptoms at Time 4 and Time 5 were predicted by lower educational level (see Table 4).
Discussion
In this study, we examined the relationship between trait anxiety and HS, taking into account multiple demographic, personality, and medical factors. HS contains six domains in this study, and four of those domains, namely Body image, Future perspective, Sexual functioning, and Side-effects were negatively affected by trait anxiety.
Trait anxiety
One of the goals of this study was to examine whether there were differences in HS between women who scored high on trait anxiety and women who did not score high on trait anxiety. Based on earlier studies [11, 12, 21], we expected that women with a high score on trait anxiety would have lower scores on HS. Indeed trait anxiety seemed to play an important role in the HS of women with breast cancer, especially with regard to Body image, Future perspective, Sexual functioning, and Side-effects. On these scales of the EORTC QLQ-BR23, women who scored high on trait anxiety had significant worse outcome than women who did not score high on trait anxiety, even 12 months after surgical treatment. A remarkable result was, for example, the course of Future perspective of women with high scores on trait anxiety (see Fig. 2). There is a significant decrease in their Future perspective 6 months after surgery took place. An explanation could be that when treatment has ended, patients are uncertain about how to pick up their lives.
Furthermore, trait anxiety was the only factor that significantly and negatively predicted Body image, Future perspective and Sexual functioning at 6 and 12 months after surgery, implying that other factors such as disease stage and treatment have less impact on HS than this personality trait. Comparable results were found in three other studies, even though in these studies (HR) QoL [11, 21] and psychological stress [12] were used as outcome measures.
Moreover, higher trait anxiety predicted more Systemic therapy side-effects at 6 and 12 months after surgery (see Fig. 3). This could be due to the fact that patients scoring high on trait anxiety believe that they will experience more side-effects of their systemic therapy, as mentioned in the study of Cameron and colleagues [22]. There was no relation between trait anxiety and Arm and Breast symptoms.
Demographic factors
Another factor besides trait anxiety that predicted more than one domain of HS was age. Older age predicts a better Body image at 6 and 12 months after surgery and a more positive perspective on the future at 6 months after surgery. Multiple explanations could be considered here. One possibility is that younger women more often have an advanced stage of breast cancer, or a more aggressive tumor. In these cases, treatment can be more invasive and mutilating and more often includes chemotherapy, which is also a predictor for worse HS [5, 7]. Another explanation could be that a diagnosis of BC and the subsequent treatment possibly disrupts the course of life more in younger women than in older women [23, 24].
Contrary to the previous result where older age was related to more positive outcomes, better Sexual functioning at 12 months after surgery was predicted by younger age. Presumably, this has a relation with menopause and menopausal symptoms. Most of the older women have past their menopause, but in younger women, only those who had systemic therapy might have menopausal symptoms which can lead to decreased sexual enjoyment [7]. This relation between age and Sexual functioning was also found in other studies that used the EORTC QLQ-BR23 [25, 26].
Other demographic factors with an influence on HS were educational level, having children, and having a partner. A higher educational level predicted less Breast and Arm symptoms at 6 months after surgery, and less Breast symptoms at 12 months after surgery. Earlier research revealed the same relation between education and Breast and Arm symptoms [27]. A possible reason for this could be that higher educated women are more likely to learn about how to take care of their wounds and how to exercise and use their arm. Having children predicted less Breast symptoms at 6 months after surgery, and having a partner predicted better Sexual functioning at 6 and 12 months after surgery.
Disease-specific factors: treatment
Type of surgery had a significant relation with Body image: women that had BCT had a better Body image than women that had MTC, during the 12 months after surgery. This outcome is also supported by multiple studies [28–30]. A more unexpected result was that women with BCT reported much more Breast symptoms than women that had MTC. BCT also significantly predicted more Breast symptoms at Time 5. Since MTC is medically more invasive than BCT, it would be obvious to expect that MTC causes more Breast symptoms. The current outcome could possibly be attributed to treatment features: in BCT radiotherapy is part of regular treatment, while women that have MTC almost never receive radiotherapy. Radiotherapy can therefore not be seen apart from type of surgical treatment. From the regression analysis, we also learned that not receiving radiotherapy was a predictor for less Breast symptoms. These facts together can obviously explain why women with BCT have more Breast symptoms: radiotherapy can cause irritation of the skin and wounds heal slower [31]. Another possible explanation is a linguistic one: in the questionnaire, women are asked about complaints in the area of their affected breast. Perhaps, women that had an MTC reason that they do not have a breast anymore (for MTC removes the breast in total), and they do not report possible complaints as complaints of their breast. In the study of King et al. [27], there was no significant difference reported in Breast symptoms between BCT and MTC, and apart from that study there is no specific literature about this topic, so more research is needed to clarify this result.
Besides type of surgery, there were other treatment factors that predicted HS. Receiving chemotherapy predicted more Side-effects and worse Body image at Time 4. Radiotherapy predicted worse Future perspective and more Breast symptoms at Time 4. These results are in line with a recent study on adjuvant therapy and HR-QoL [32]. In contrast to what Buijs et al. [33] found, there was a relation between hormone therapy and Arm symptoms: receiving hormone therapy predicted less Arm symptoms at Time 5. This could have a relation with other factors, like radiotherapy, but more research needs to be done about this topic.
Limitations of this study
In this study, only women with early-stage breast cancer were included (see Table 1). An advantage is the homogeneity of the data, but the disadvantage is that it is not possible to generalize the conclusions to other patient groups, such as late-stage or recurrent breast cancer patients. Furthermore, there was a selection effect on two items of the EORTC QLQ-BR23: the items about ‘Upset by hair loss’ and ‘Sexual enjoyment’ only had to be answered if women actually did have hair loss or were sexually active, respectively. Due to these criteria, not enough patients answered the questions to evaluate them in this study. To counter this problem and other potential power problems, more women need to be included in future research.
In this study, radiotherapy was introduced as an independent variable. We realize that there is an overlap between radiotherapy and type of surgery, i.e. usually women who receive BCT also get radiotherapy. Likewise, radiotherapy is usually not a part of MTC treatment. However, because in this study there were 10 women (9.3% of BCT) who received BCT but not radiotherapy, and 20 women (17.5% of MTC) who received MTC and radiotherapy, we decided to maintain radiotherapy as an independent variable.
In conclusion, trait anxiety is strongly associated with the HS of breast cancer patients in the first year after surgery. This insight into the personality of patients could be a first indication of better monitoring patients that have a higher risk of having a worse HS after diagnosis and treatment of their disease. Since HS is an important endpoint of treatment in breast cancer patients nowadays, psychosocial support should be deployed to guide these high-risk patients through the process of their disease and possibly thereafter.
Furthermore, it should not be accepted unquestioningly that women who had BCT do better than women with MTC. The results from this study pointed out that even though BCT is, medically spoken, a less invasive treatment than MTC, BCT patients can have serious Breast symptoms. There should therefore be close attention for medical and psychosocial symptoms in women with MTC and BCT.
The results of this study also have clinical implications. Based on the present results, women scoring high on anxiety are prone to experiencing more side-effects. Psychological counseling aimed at these women could probably reduce side-effects. However, in order to provide this treatment, high anxious women should be identified using short, validated easy-to-administer trait anxiety questionnaires, such as the STAI [17, 18].
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of Oncology. 2007;18(3):581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4(4):251–254. doi: 10.1016/S1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 3.An international glossary for general/family practice. WONCA Classification Committee Family Practice. 1995;12(3):341–369. doi: 10.1093/fampra/12.3.341. [DOI] [PubMed] [Google Scholar]
- 4.Mols F, Vingerhoets AJ, Coebergh JW, Van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: a systematic review. European Journal of Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Disipio T, Hayes S, Newman B, Janda M. Health-related quality of life 18 months after breast cancer: comparison with the general population of Queensland, Australia. Supportive Care in Cancer. 2008;16(10):1141–1150. doi: 10.1007/s00520-007-0392-y. [DOI] [PubMed] [Google Scholar]
- 6.Ahn SH, Park BW, Noh DY, Nam SJ, Lee ES, Lee MK, Kim SH, Lee KM, Park SM, Yun YH. Health-related quality of life in disease-free survivors of breast cancer with the general population. Annals of Oncology. 2007;18(1):173–182. doi: 10.1093/annonc/mdl333. [DOI] [PubMed] [Google Scholar]
- 7.Hopwood P, Haviland J, Mills J, Sumo G, Bliss JM. The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2,208 women recruited to the UK START trial (standardisation of breast radiotherapy trial) Breast. 2007;16(3):241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Holzel D. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast Journal. 2004;10(3):223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin HW, Noh DY, Lee ES, Nam SJ, Park BW, Ahn SH, Yun YH. Correlates of existential well-being and their association with health-related quality of life in breast cancer survivors compared with the general population. Breast Cancer Research and Treatment. 2009;118(1):139–150. doi: 10.1007/s10549-009-0326-0. [DOI] [PubMed] [Google Scholar]
- 10.Zebrack BJ, Yi J, Petersen L, Ganz PA. The impact of cancer and quality of life for long-term survivors. Psychooncology. 2008;17(9):891–900. doi: 10.1002/pon.1300. [DOI] [PubMed] [Google Scholar]
- 11.Schreier AM, Williams SA. Anxiety and quality of life of women who receive radiation or chemotherapy for breast cancer. Oncology Nursing Forum. 2004;31(1):127–130. doi: 10.1188/04.ONF.127-130. [DOI] [PubMed] [Google Scholar]
- 12.Bleiker EM, Pouwer F, Van der Ploeg HM, Leer JW, Ader HJ. Psychological distress two years after diagnosis of breast cancer: frequency and prediction. Patient Education and Counseling. 2000;40(3):209–217. doi: 10.1016/S0738-3991(99)00085-3. [DOI] [PubMed] [Google Scholar]
- 13.Hevey D, McGee HM, Horgan J. Relationship of initial level of distress to changes in health-related quality of life during cardiac rehabilitation or usual care. Psychosomatic Medicine. 2007;69(8):793–797. doi: 10.1097/PSY.0b013e318156bcd2. [DOI] [PubMed] [Google Scholar]
- 14.Ristvedt SL, Trinkaus KM. Trait anxiety as an independent predictor of poor health-related quality of life and post-traumatic stress symptoms in rectal cancer. British Journal of Health Psychology. 2009;14(Pt 4):701–715. doi: 10.1348/135910708X400462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprangers MA, Groenvold M, Arraras JI, Franklin J, Te Velde A, Muller M, Franzini L, Williams A, De Haes HC, Hopwood P, Cull A, Aaronson NK. The European organization for research and treatment of cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. Journal of Clinical Oncology. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 16.Montazeri A, Harirchi I, Vahdani M, Khaleghi F, Jarvandi S, Ebrahimi M, Haji-Mahmoodi M. The EORTC breast cancer-specific quality of life questionnaire (EORTC QLQ-BR23): translation and validation study of the Iranian version. Quality of Life Research. 2000;9(2):177–184. doi: 10.1023/A:1008918310251. [DOI] [PubMed] [Google Scholar]
- 17.Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory manual. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 18.Van der Ploeg HM, Defares PB, Spielberger CD. ZBV. A Dutch-language adaptation of the Spielberger State-Trait Anxiety Inventory. Lisse, The Netherlands: Swets&Zeitlinger; 1980. [Google Scholar]
- 19.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State—Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 20.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. American joint committee on cancer staging manual. 6. New York, NY: Springer; 2002. [Google Scholar]
- 21.Van der Steeg AF, De Vries J, Van der Ent FW, Roukema JA. Personality predicts quality of life six months after the diagnosis and treatment of breast disease. Annals of Surgical Oncology. 2007;14(2):678–685. doi: 10.1245/s10434-006-9175-9. [DOI] [PubMed] [Google Scholar]
- 22.Cameron LD, Leventhal H, Love RR. Trait anxiety, symptom perceptions, and illness-related responses among women with breast cancer in remission during a tamoxifen clinical trial. Health Psychology. 1998;17(5):459–469. doi: 10.1037/0278-6133.17.5.459. [DOI] [PubMed] [Google Scholar]
- 23.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. Journal of Clinical Oncology. 2003;21(22):4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 24.Hartl K, Schennach R, Muller M, Engel J, Reinecker H, Sommer H, Friese K. Quality of life, anxiety, and oncological factors: a follow-up study of breast cancer patients. Psychosomatics. 2010;51(2):112–123. doi: 10.1176/appi.psy.51.2.112. [DOI] [PubMed] [Google Scholar]
- 25.Browall MM, Ahlberg KM, Persson LO, Karlsson PO, Danielson EB. The impact of age on HHealth-Related Quality of Life (HRQoL) and symptoms among postmenopausal women with breast cancer receiving adjuvant chemotherapy. Acta Oncologica. 2008;47(2):207–215. doi: 10.1080/02841860701621258. [DOI] [PubMed] [Google Scholar]
- 26.Fehlauer F, Tribius S, Mehnert A, Rades D. Health-related quality of life in long term breast cancer survivors treated with breast conserving therapy: impact of age at therapy. Breast Cancer Research and Treatment. 2005;92(3):217–222. doi: 10.1007/s10549-005-2420-2. [DOI] [PubMed] [Google Scholar]
- 27.King MT, Kenny P, Shiell A, Hall J, Boyages J. Quality of life three months and 1 year after first treatment for early stage breast cancer: influence of treatment and patient characteristics. Quality of Life Research. 2000;9(7):789–800. doi: 10.1023/A:1008936830764. [DOI] [PubMed] [Google Scholar]
- 28.De Haes JC, van Oostrom MA, Welvaart K. The effect of radical and conserving surgery on the quality of life of early breast cancer patients. European Journal of Surgical Oncology. 1986;12(4):337–342. [PubMed] [Google Scholar]
- 29.Curran D, Van Dongen JP, Aaronson NK, Kiebert G, Fentiman IS, Mignolet F, Bartelink H. Quality of life of early-stage breast cancer patients treated with radical mastectomy or breast-conserving procedures: results of EORTC Trial 10801. The European Organization for Research and Treatment of Cancer (EORTC), Breast Cancer Co-operative Group (BCCG) European Journal of Cancer. 1998;34(3):307–314. doi: 10.1016/S0959-8049(97)00312-2. [DOI] [PubMed] [Google Scholar]
- 30.Kenny P, King MT, Shiell A, Seymour J, Hall J, Langlands A, Boyages J. Early stage breast cancer: costs and quality of life 1 year after treatment by mastectomy or conservative surgery and radiation therapy. Breast. 2000;9(1):37–44. doi: 10.1054/brst.1999.0111. [DOI] [PubMed] [Google Scholar]
- 31.Lilla C, Ambrosone CB, Kropp S, Helmbold I, Schmezer P, von Fournier D, Haase W, Sautter-Bihl ML, Wenz F, Chang-Claude J. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Research and Treatment. 2007;106(1):143–150. doi: 10.1007/s10549-006-9480-9. [DOI] [PubMed] [Google Scholar]
- 32.Browall M, Ahlberg K, Karlsson P, Danielson E, Persson LO, Gaston-Johansson F. Health-related quality of life during adjuvant treatment for breast cancer among postmenopausal women. European Journal of Oncology Nursing. 2008;12(3):180–189. doi: 10.1016/j.ejon.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Buijs C, de Vries EG, Mourits MJ, Willemse PH. The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treatment Reviews. 2008;34(7):640–655. doi: 10.1016/j.ctrv.2008.04.001. [DOI] [PubMed] [Google Scholar]