Abstract
The cerebellum’s role in sensory-motor control and adaptation is undisputed. However, a key hypothesis pertaining to the function of cerebellar circuitry lacks experimental support. It is universally assumed that the discharge of mossy fibers accounts for modulation of Purkinje cell “simple spikes” (SSs). This assumption acts as a prism through which all other functions of cerebellar circuitry are viewed. The vestibulo-cerebellum (nodulus and uvula) receives a large, unilateral, vestibular primary afferent mossy fiber projection. We can test its role in modulating Purkinje cell SSs by recording the modulated activity of both mossy fiber terminals and Purkinje cell SSs evoked by identical natural vestibular stimulation. Sinusoidal rotation about the longitudinal axis (roll) modulates the activity of vestibular primary afferent mossy and climbing fibers as well as Purkinje cell SSs and complex spikes (CSs). Remarkably, vestibular primary afferent mossy fibers discharge 180 degrees out of phase with SSs. This indicates that mossy fibers cannot account for SS modulation unless an inhibitory synapse is interposed between mossy fibers or vestibular climbing fibers and Purkinje cells. The authors review several experiments that address the relative contributions of mossy and climbing fiber afferents to the modulation of SSs. They conclude that climbing fibers, not mossy fibers, are primarily responsible for the modulation of SSs as well as CSs and they propose revised functions for these two afferent systems.
Keywords: Golgi cell, granule cell, inferior olive, nodulus, stellate cell, uvula
Cerebellar Charm
The cerebellum charms neurobiologists. A multifoliated structure perched on the brainstem behind the cerebral cortex, the cerebellum has seven neuronal cell types, segregated into three layers. Its afferent and efferent connections are conveyed by three pairs of fiber bundles (peduncles) that fasten the cerebellum to the brainstem and place it at the crossroads of sensory-motor integration. Its crystalline structure attracts cellular physiologists, neuroanatomists, and others interested in structure-function questions. Developmental biologists are absorbed by the migration of cell populations during cerebellar development. Molecular geneticists are attracted by the use of disrupted motor behavior to characterize the phenotypic expression of mutations. Neurologists and neurosurgeons have long been fascinated by the rich variety of motor disorders caused by traumatic cerebellar injury. Systems physiologists and mathematical biologists are enchanted by the cerebellum’s role in sensory-motor integration. Almost all neurobiologists are excited by the use of the cerebellum as a model system for the investigation of neuronal plasticity.
The Purkinje cell is the only output neuron of the cerebellar cortex. The neuronal circuitry in which Purkinje cells are embedded remains remarkably constant in different cerebellar regions. A major factor in how a repeating unit of cerebellar circuitry is deployed depends on its afferent connections. Two distinct afferent pathways convey information to the cerebellar cortex. Mossy fibers originate from multiple brainstem nuclei and terminate on granule cells in a layer just below Purkinje cells in the cerebellar cortex. Granule cell axons ascend through the Purkinje cell layer and into the molecular layer where they bifurcate into parallel fibers that richly innervate Purkinje cell dendrites. Intra-cerebellar branching of mossy fibers makes difficult estimates of the number of extra-cerebellar neurons from which mossy fibers originate. Possibly there are as few as one mossy fiber for every three Purkinje cells or as many as four mossy fibers for each Purkinje cell (Palkovits and others 1972). Climbing fibers originate from only one precerebellar nucleus, the contralateral inferior olive. Each Purkinje cell is directly innervated by a single climbing fiber. One is enough. A climbing fiber makes ~500 synaptic contacts as it entwines the Purkinje cell dendritic tree (Harvey and Napper 1991). Purkinje cells outnumber climbing fibers by a factor of 10 to 15 (Palkovits and others 1972). Here, we will examine how mossy and climbing fibers contribute to the discharge of Purkinje cells under conditions of natural sensory stimulation.
Regulation of Purkinje Cell Complex and Simple Spikes
It is commonly assumed that mossy fibers modulate the relatively high frequency discharge of Purkinje cell simple spikes (SSs) and that this modulation is essential for online correction of movement. Conversely, climbing fibers regulate the relatively low discharge of Purkinje cell complex spikes (CSs) and that this modulation is involved in more abstruse functions. These views are echoed in textbooks (Ghez and Thach 2000), scholarly reviews (Apps and Garwicz 2005; Bloedel and Bracha 2009), and research articles (Armstrong and Edgley 1988; Kano and others 1991b; Lisberger and others 1994; Walter and Khodakhah 2006).
A thorough analysis of how mossy and climbing fibers regulate SSs and CSs requires the use of natural sensory stimulation capable of modulating the discharge of SSs and CSs. We have used natural vestibular stimulation to modulate parametrically the activity in both of these pathways. We have also analyzed activity in one of these pathways after the other has been blocked, providing a unique insight into the operations of cerebellar circuitry. We show that climbing fibers, not mossy fibers, are responsible for the modulation of CSs and SSs.
Mossy Fiber → Granule Cell → Parallel Fiber Circuitry
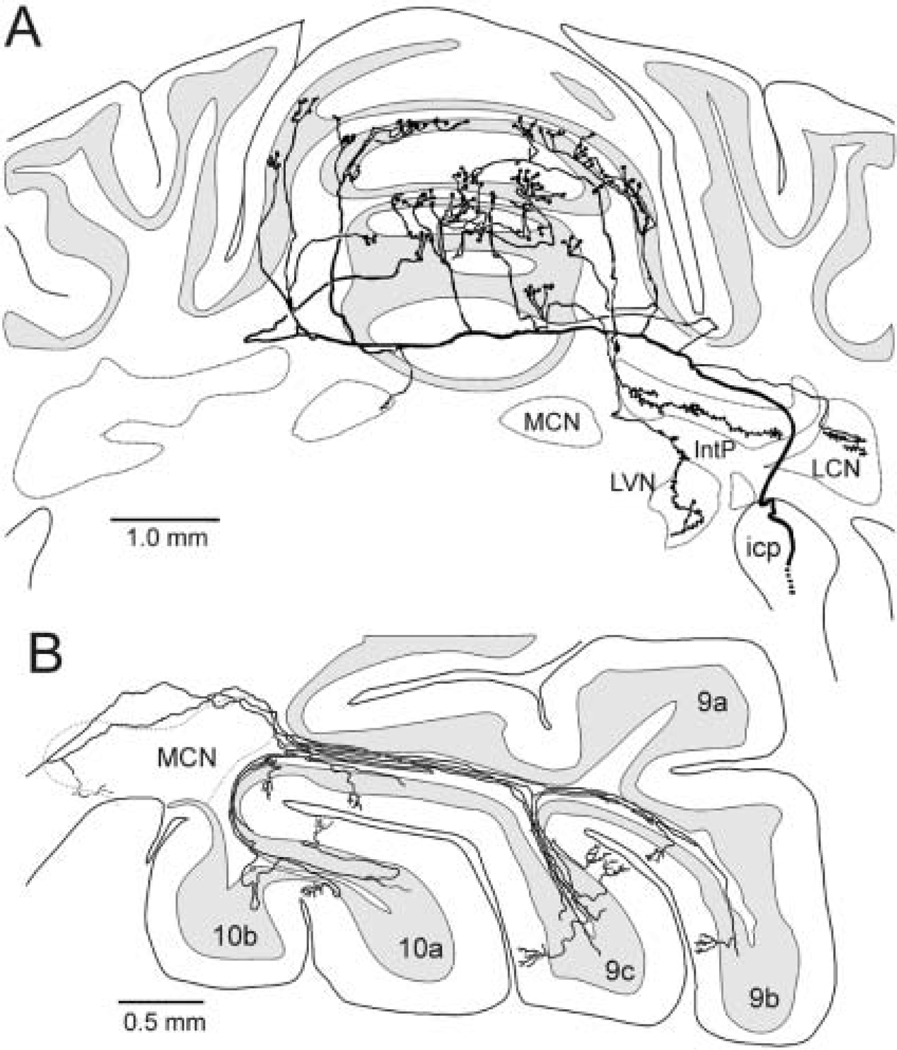
As a mossy fiber exits a cerebellar peduncle, its main stem divides into several branches that penetrate several cerebellar folia, often bilaterally (Fox and others 1967; Wu and others 1999) (Fig. 1A). A mossy fiber branch forms ~40 synaptic rosettes that contact dendrites of ~15 granule cells (Fig. 2A). Each granule cell has three to six dendrites, allowing innervation by more than one mossy fiber (Fox and others 1967). A single mossy fiber projects onto ~600 granule cells (Palkovits and others 1972). This divergence no doubt accounts for the “patchy-mosaic” of granule cell cutaneous receptive fields in which skin surfaces are represented discontinuously in adjacent granule cells (Kassel and others 1984).
Figure 1.
Cerebellar microcircuitry. A, The afferent limbs of cerebellar circuitry consist of an indirect mossy fiber (mf) → granule cell (Gr) → parallel fiber projection to Purkinje cells (Pc) and a direct climbing fiber (cf) projection to the Purkinje cells. Two inhibitory interneurons, basket (Ba) and stellate (Sc) cells, synapse directly on Purkinje cells. Inhibitory Golgi cells (Go) synapse on granule cells. Bergmann (Bg) and NG2+ astrocytes may interact with Purkinje and stellate cells. B, Axial view of a vermal folium illustrates the extensive length of parallel fibers. The two colorized soma of granule cells are located in lateral extremes. Their axons, parallel fibers, could potentially innervate any Purkinje cell within the folium. Modified from Cajal (1911).
Figure 2.
Mossy and climbing fiber projections to the cerebellum and mossy fiber discharge. A, Single cells in the left lateral reticular nucleus were labeled with biotinylated dextran amine. They project bilaterally as mossy fibers to several folia in the anterior vermis. Modified with permission from Wu and others (1999). B, Single cells in the mediocaudal caudal aspect of the medial accessory olive were labeled with biotinylated dextran amine. Several branches of a single climbing fiber project onto a sagittal zone in the contralateral uvula-nodulus. Modified with permission from Sugihara and others (2001). icp = inferior cerebellar peduncle; IntP = nucleus interpositus; LCN = lateral cerebellar nucleus; LVN = lateral vestibular nucleus; MCN = medial cerebellar nucleus.
Granule cell axons ascend to the molecular layer where they bifurcate into parallel fibers that extend through the overlapping dendritic trees of 200 to 1000 Purkinje cells arrayed along a folial axis (Fox and others 1967). In the rat, parallel fibers are 4.7 mm end-to-end (Pichitpornchai and others 1994). In the cat, they are ~6 mm (Brand and others 1976). Each Purkinje cell is contacted by ~150,000 parallel fibers (Harvey and Napper 1991) whose parent granule cell bodies may be several millimeters distant (Fig. 1B).
Based on anatomical considerations alone, the mossy fiber → granule cell → parallel fiber system distributes information to as many Purkinje cells as possible along a folial axis. These anatomical details appear to conflict with electrophysiological studies of cutaneous homunculi mapped onto the cerebellar surface. This apparent conflict is one of scale rather than substance. Homunculi reflect regional patterns of mossy fiber projections. Within these regional patterns a greater functional specificity can be found that may not reflect the topography of the sensory projection.
Some have argued that functional specificity of mossy fiber → granule cell → parallel fiber projections to Purkinje cells is achieved, despite the divergent projections of mossy fiber → granule cell → parallel fibers, by a disproportionate synaptic influence of ascending axons of granule cells on Purkinje cells (Cohen and Yarom 1998; Gundappa-Sulur and others 1999). The premise of this argument is not sustained by experiment. Synaptic currents evoked by ascending axons are roughly comparable to synaptic currents evoked by the parallel fibers (Isope and Barbour 2002; Walter and others 2009). The total number of presynaptic varicosities of parallel fibers in the rat is estimated at 135/mm (Harvey and Napper 1988). The ascending branch of granule cell that is 100 mm long could account for 2% of the total presynaptic granule cell varicosities before it bifurcates into a 5-mm parallel fiber (Walter and others 2009).
The assumption that mossy fiber afferents are responsible for the high discharge frequency (0–80 impulses/s) of Purkinje cell SSs (Granit and Phillips 1956; Eccles and others 1966a) obscures the possible contributions of other factors. Spontaneous SSs occur in isolated Purkinje cells in the absence of any parallel fiber input (Llinás and Sugimori 1980; Raman and Bean 1999). Interneurons in the molecular layer, basket and stellate cells, exert inhibitory control of Purkinje cells (Andersen and others 1964; Midtgaard 1992; Pouzat and Hestrin 1997; Szapiro and Barbour 2007; Fig. 1A). Golgi cells in the granule cell layer indirectly influence SS discharge by inhibiting the discharge of granule cells (Eccles and others 1966b).
Climbing Fiber Circuitry
Unlike the extensive medio-lateral distribution of mossy fiber terminals, climbing fiber terminals are arrayed in narrow sagittal zones (Groenewegen and Voogd 1977; Andersson and Oscarsson 1978; Sugihara and others 1999; Wu and others 1999; Sugihara and others 2001; Fig. 2B). Climbing fibers synaptically evoke the iconic amalgam of sodium and calcium spikes in Purkinje cells that comprise the CS (Llinás and Sugimori 1980; Lasser-Ross and Ross 1992). CSs last 5 to 10 ms and have a relatively low discharge frequency of 0.5 to 8.0 imp/s (Granit and Phillips 1956; Eccles and others 1966a).
CSs have been likened to “phasic motor generators” (Berthoz and Llinás 1974), “threshold devices” (Murphy and others 1973), “event detectors” (Rushmer and others 1976), “error detectors” (Andersson and Armstrong 1987), “frequency synchronizers” (Llinás and Yarom 1986), “gain changers” (Ebner and Bloedel 1981), and “teachers” (Ito and others 1982; Sakurai 1987; Ito and Karachot 1989; Crépel and Jaillard 1991; Narasimhan and Linden 1996). Each of these theoretical descriptions appears guided by an assumption that the discharge frequency of CSs is too low and too variable to encode sensory information parametrically in real time or to account for continuous online correction of movement (Ghez and Thach 2000). Such an assumption should be predicated on knowledge of the range of stimulus frequencies encoded in the modulated discharge of CSs and SSs as well as the range of movement frequencies for which the corrective output of the cerebellum is required.
Climbing fiber discharge frequencies in the flocculus are sufficient to encode optokinetic direction and velocity (Maekawa and Simpson 1973; Leonard and others 1988; Graf and others 1988; Frens and others 2001). Similarly, vestibular end organ specificity and velocity is encoded by climbing fibers in the uvula-nodulus (Barmack and Shojaku 1995; Fushiki and Barmack 1997; Barmack and Yakhnitsa 2003; Yakhnitsa and Barmack 2006). In these systems, climbing fibers encode sensory information over a broad range of stimulus frequencies (0.001–1.000 Hz).
The relatively high discharge frequencies of SSs have often been viewed as evidence that SSs encode higher stimulus frequencies than do CSs. This interpretation would be more persuasive if it were supported by experimental evidence. Higher discharge frequencies of SSs could indicate an ability to transmit higher stimulus frequencies. However, higher discharge frequencies might be unrelated to sensory signaling. Rather they may establish optimal carrier frequencies necessary for effective presynaptic release of transmitter by Purkinje cells onto neurons in the vestibular and cerebellar nuclei.
Anatomy of Uvula-Nodulus
Vestibular Mossy Fiber Pathways to the Uvula-Nodulus
All vestibular primary afferents divide as they approach the brainstem. One branch projects to the vestibular complex. The other branch projects to the ipsilateral uvula-nodulus (Fig. 3). This projection is distributed across multiple folia. Folial branching patterns of mossy fibers have a range of a few millimeters.
Figure 3.
Vestibular primary afferent mossy fiber and climbing fiber projections to uvula-nodulus. Sequences of activation are indicated by solid lines for excitatory pathways and dashed lines for inhibitory pathways, listed numerically. 1, Roll-tilt onto the left side increases primary afferent discharge. 2, Primary afferent mossy fibers (mf) project to ipsilateral parasolitary nucleus (Psol), Y-group (Y), and granule cell layer of nodulus. 3, Psol projects to ipsilateral β-nucleus and dorsomedial cell column (DMCC). 4, Climbing fibers (cf) from the β-nucleus and DMCC project to contralateral nodulus. 5, Vestibular nuclei project bilaterally to the Y-group. 6, The Y-group projects to contralateral dorsal cap (DC), β-nucleus, and DMCC. Fl = flocculus; Gc = granule cell; icp = inferior cerebellar peduncle; IntP = interpositus nucleus; LVN, MVN and SVN = lateral medial and superior vestibular nucleus; LCN and MCN = lateral and medial cerebellar nucleus; LRN = lateral reticular nucleus; Nsol = nucleus solitarius; PFl = paraflocculus; Pc = Purkinje cell; pf = parallel fiber; SpV = spinal trigeminal nucleus; spV = spinal trigeminal tract; VI = abducens nucleus; X = dorsal motor nucleus of the vagus; XII = hypoglossal nucleus; 8n = vestibular nerve.
The maculae of otolith end organs (saccule and utricle) project mainly, but not exclusively to the uvula. Semicircular canal cristae project mainly, but again, not exclusively to the nodulus (Maklad and Fritzsch 2003). The projection of vestibular primary afferent mossy fibers is exclusively ipsilateral. However, the already-dispersed unilateral projections of vestibular primary afferent mossy fibers are further dispersed bilaterally by the extended length of parallel fibers that cross the midline.
Although vestibular primary afferent mossy fibers comprise the largest single mossy fiber projection to the uvula-nodulus, they are not its only projection. Vestibular secondary afferent mossy fibers project bilaterally to the uvula-nodulus from the caudal, descending, medial, and superior vestibular nuclei (Brodal and Torvik 1957; Kotchabhakdi and Walberg 1978; Yamamoto 1979; Brodal and Brodal 1985; Thunnissen and others 1989). The uvula-nodulus also receives a bilateral mossy fiber projection from the nucleus prepositus hypoglossi (Voogd and Barmack 2005). Ponto-cerebellar mossy fibers represent a relatively small projection to the uvula, and an even smaller projection to the nodulus (Serapide and others 2001).
Vestibular Climbing Fiber Projections to the Uvula-Nodulus
The vestibular climbing fiber projection to the uvula nodulus originates from the contralateral β-nucleus and dorsomedial cell column (DMCC) of the inferior olive (Barmack and others 1993; Barmack 1996; Kaufman and others 1996; Fig. 3). The β-nucleus and DMCC receive secondary vestibular projections from the ipsilateral parasolitary nucleus, a small GABAergic nucleus onto which ipsilateral primary vestibular afferents project (Barmack and others 1998; Barmack and Yakhnitsa 2000). The β-nucleus and DMCC also receive excitatory projections from the contralateral Y-group (Voogd and Barmack 2005). Neurons in each Y-group receive ipsilateral vestibular primary afferent projections and bilateral secondary vestibular afferent projections. Consequently each labyrinth projects indirectly to the β-nucleus and DMCC, with the majority of the projection from the ipsilateral parasolitary nucleus. Within the β-nucleus and DMCC, the vestibular projections from posterior and anterior semicircular canals are topographically distinct and include a variable convergence from the utricular otoliths (Barmack and others 1993).
The topographically distinct organization of the inferior olive is preserved in its projection to the uvulanodulus. Climbing fibers from the inferior olive terminate in narrow, functionally distinct, sagittal climbing fiber zones in the contralateral uvula-nodulus (Voogd and others 1996; Sugihara and others 2001). This well-defined climbing fiber termination pattern contrasts with the diffuse pattern of vestibular mossy fiber terminals (Thunnissen and others 1989; Epema and others 1990).
One interesting consequence of the vestibular primary afferent mossy and climbing fiber projections is that a Purkinje cell in the uvula-nodulus likely receives its mossy fiber projections from the ipsilateral labyrinth and its climbing fiber projection from the contralateral labyrinth. This complementary projection pattern can be used to advantage in physiological experiments.
Physiology of Uvula-Nodulus
Vestibularly Modulated Mossy Fiber Terminals and SSs
Vestibular stimulation about an animal’s longitudinal axis effectively modulates primary afferent discharge. When an animal is roll-tilted onto one side, the activity of vestibular afferents that originate from the ipsilateral anterior and posterior semicircular canals as well, as the utricular otolith, increases (Goldberg and Fernandez 1971; Fernandez and Goldberg 1976). In the rabbit and mouse, sinusoidal roll-tilt oscillation evokes modulated activity from single mossy fiber terminals (MFTs; Fig. 4). MFTs characterized by short-duration action potentials (<0.5 ms) with a frequency range of 5 to 30 imp/s have been identified in the granule cell layers of the uvula-nodulus using indium filled glass micropipettes (Barmack and Shojaku 1995). In total, 11 of 12 mossy fibers responded to sinusoidal rotation about a longitudinal axis and 1 of 12 responded to rotation about the vertical axis. Ipsilateral side-down rotation increased MFT discharge (Fig. 4B). Most importantly, MFTs discharged 180 degrees out of phase with SSs, meaning that the discharge of MFTs increased while the discharge of SSs decreased. Under this stimulus condition MFTs could not possibly modulate SSs unless inhibitory synapses were interposed between MFTs and Purkinje cells. We found essentially the same result in the mouse and we were able to identify MFTs unequivocally by juxtacellularly labeling with neurobiotin the mossy fiber rosettes from which we recorded (Fig. 4A; Barmack and Yakhnitsa 2008).
Figure 4.
Vestibular stimulation modulates the discharge of mossy fiber terminals (MFTs). A, Juxtacellularly labeled mouse mossy fiber terminal (MFT) was recorded 700 µm to the left of the midline in folium 9c. The peak MFT discharge led ipsilateral roll-tilt by 45 degrees. The peristimulus histogram (black bars) was fitted with a cosine function (red line). The vertical dashed lines from the stimulus and cosine function indicate how phase was measured. B, Histograms indicate the phases of populations of MFTs, simple spikes (SSs), and complex spikes (CSs) in response to sinusoidal roll-tilt. Note that MFTs and CSs discharge in phase with each other, but out of phase with SSs. Gl = granule cell layer; ml = molecular layer; Pl = Purkinje cell layer.
Sinusoidal vestibular oscillation about the vertical axis (yaw) increases the discharge of horizontal semicircular canal primary afferents during rotation toward the ipsilat-eral labyrinth. Roughly 20% of the MFTs in the uvula-nodulus originate from the ampullae of the horizontal semicircular canals. Despite this substantial population of horizontal semicircular canal afferents, neither Purkinje cell SSs nor CSs are modulated by horizontal angular acceleration (Fushiki and Barmack 1997; Yakhnitsa and Barmack 2006; Yakusheva and others 2007). Horizontal stimulation is also ineffective in modulating the activity of neurons in the known vestibular-related preolivary nuclei (solitary nucleus and Y-group) as well as the vestibular-related olivary nuclei (b-nucleus and DMCC; Barmack and others 1993; Barmack and others 1998; Barmack and Yakhnitsa 2000).
If vestibular primary afferent mossy fibers were responsible for the modulation of SSs, why would horizontally modulated SSs be absent? There are two possibilities: 1) According to an engineering model, mossy fiber activity evoked by horizontal angular acceleration is suppressed by an otolith signal when an animal is maintained in the prone orientation. This suppression is removed only when the animal is placed on its back (Yakusheva and others 2008). This is, of course, an experimental dead end, inasmuch as no one would attempt to investigate the activity of single Purkinje cells in this topsy-turvy orientation—at least not on this planet. 2) Ipsilateral horizontal angular acceleration stimulates horizontal semicircular canal afferents, but these afferents are ineffective in modulating Purkinje cell SSs. This suggests that modulation of SSs depends primarily on climbing fiber signals. This inference is contradicted by reports of weak modulation of CSs in the nodulus during horizontal vestibular stimulation (Precht and others 1976; Robinson and others 1988; Yakusheva and others 2010). However, apparent modulation of CSs evoked by horizontal vestibular stimulation is a likely consequence of an inappropriate alignment of the head of the stimulated animal that results in inadvertent stimulation of the vertical semicircular canals. In such instances, pitching the head forward or backward by only a few degrees will shift the phase of the modulated CSs by 180 degrees if the CSs originate from either of the vertical semicircular canals. With these improbable exceptions noted, modulated climbing fiber activity is essential for SS modulation. Naturally, it follows that the absence of horizontally modulated climbing fiber activity accounts for the lack of horizontally modulated SSs.
There are other reasons to doubt the role of vestibular primary afferent mossy fibers in the modulation of SSs. Sinusoidal roll-tilt modulates the activity of SSs in folia 9a and 9b of the uvula to which vestibular primary and secondary afferents have only sparse projections (Thunnissen and others 1989; Fig. 5C). These folia receive a vestibular climbing fiber projection. Again, if vestibular primary afferent MFTs were essential for modulating the activity of SSs, why would such modulation persist in the absence of significant vestibular primary afferent mossy fiber projection? An alternative explanation is that climbing fibers, acting through inhibitory interneurons, account for the observed antiphasic modulation of SSs.
Figure 5.
Optimal vestibular stimulation of complex spikes (CSs) in uvula-nodulus. Sinusoidal roll-tilt was used to classify optimal response planes of CSs. Figurines illustrate optimal (A) and null (B) CS response planes for a Purkinje cell recorded in the left uvula. Stimulation in the optimal plane (A) evoked increased CSs and decreased simple spikes (SSs) when the rabbit was rotated onto its left side. Stimulation in the null plane (B) failed to modulate CSs or SSs. C, Sagittal view of the rabbit cerebellum. The green shaded area (folia 9d and 10) indicates the region of the uvula-nodulus that receives projections of vestibular primary afferents. D, Folia 9 and 10 are represented in a two-dimensional topographic sheet. Optimal planes for CSs for 205 Purkinje cells are distributed on this surface. Cells with optimal planes corresponding to stimulation in the plane of the ipsilateral posterior semicircular canal (LPC) are illustrated as green. Cells with optimal planes corresponding to stimulation in the plane of the ipsilateral anterior semicircular canal (LAC) are illustrated as red squares. Open symbols indicate cells in which the optimal plane was determined only for sinusoidal stimulation. Filled symbols indicate cells tested for static sensitivity and were positive. Filled diamonds indicate cells that respond to HOKS of the ipsilateral eye in the posterior → anterior direction (LHOK). E, Different postural responses are evoked by vestibular and optokinetic stimulation in different planes. Vestibular stimulation in the plane of LAC evokes a forward and lateral extension of the ipsilateral fore and hind paws. HOKS of the left eye in the posterior → anterior direction evokes a lateral extension of the contralateral paws. Vestibular stimulation in the plane of the ipsilateral posterior semicircular canal evokes a backward extension of the ipsilateral paws. Modified from Barmack and Yakhnitsa (2003).
Climbing Fiber Zones: Vestibularly Modulated CSs and SSs
In contrast to the dispersion of vestibular primary afferent mossy fiber → granule cell → parallel fiber signals, the responses of SSs and CSs are confined to sagittal zones in the uvula-nodulus defined anatomically by climbing fiber projections. These zones can be characterized physiologically using a “null method” to establish a plane of vestibular stimulation least effective in modulating the discharge of CSs. During sinusoidal roll-tilt, the head orientation about the vertical axis is changed systematically in search of a plane of stimulation at which neuronal activity is not modulated. This defines a null plane (Fig. 5A, B). On either side of this null plane, activity is modulated, but phase shifted by 180 degrees. The optimal plane is orthogonal to the null plane. Optimal planes of Purkinje cell CSs can be plotted onto a map of the uvula-nodulus to demonstrate physiological climbing fiber zones in which optimal planes are the defining receptive field characteristic. Interestingly the optimal planes of CSs and SSs are identical, but oppositely polarized (Yakhnitsa and Barmack 2006).
For Purkinje cells that lack static sensitivity, optimal response planes correspond to the anatomical orientation of one of the two pairs of vertical semicircular canals. For Purkinje cells with static sensitivity, optimal planes correspond to a range of polarization vectors reflected in the continuous range of hair cell polarizations in utricular maculae (Barmack and Shojaku 1995; Fushiki and Barmack 1997).
In the rabbit uvula-nodulus, Purkinje cells with similar optimal response planes are clustered into ~800-µm-wide sagittal zones (Fushiki and Barmack 1997; Barmack and Yakhnitsa 2003). In the most medial zone, CSs and SSs have optimal response planes consistent with stimulation of the ipsilateral posterior and contralateral anterior semicircular canals (Fig. 5D). Purkinje cells in this zone are innervated by climbing fibers from the contralateral caudal β-nucleus. In the more lateral zone, CSs and SSs have optimal response planes consistent with stimulation of the ipsilateral anterior and contralateral posterior semicircular canals. Purkinje cells in this zone are innervated by climbing fibers from the contralateral rostral β-nucleus.
A small horizontal optokinetic zone wedged between the two vertical semicircular canal zones in the ventral nodulus of the rabbit. CSs are evoked by posterior —> anterior optokinetic stimulation of the ipsilateral eye. This zone receives its optokinetically modulated climbing fiber input from the contralateral caudal dorsal cap of the inferior olive (Alley and others 1975; Takeda and Maekawa 1989; Barmack and Shojaku 1995).
Different postural responses are evoked by activity evoked in these different zones that project to cerebellar and vestibular nuclei. These projections may be related to appropriate patterns of postural responses necessary to prevent falling in the direction of vestibular stimulation. For example, vestibular stimulation in the plane of the left anterior and right posterior canals evokes a forward and lateral extension of the ipsilateral fore and hind paws (Fig 5E). HOKS of the left eye in the posterior → anterior direction evokes a lateral extension of the contralateral paws. Vestibular stimulation in the plane of the ipsilateral posterior and contralateral anterior semicircular canals evokes a backward extension of the ipsilateral paws. The potentially conflicting postural responses to activity generated in separate climbing fiber zones highlights the importance of maintaining this separation of activity in efferent projections from these zones to subjacent vestibular and cerebellar nuclei (Wylie and others 1994).
Climbing Fibers Induce Antiphasic Responses of SSs
Following the occurrence of a CS there is a decreased probability of SSs. One of the causes of this decreased probability, the “inactivation response,” lasts 5 to 8 ms (Granit and Phillips 1956). However, the SS pause often lasts 15 to 300 ms, an interval much too long to be attributed to an “inactivation response” (Bell and Grimm 1969; Bloedel and Roberts 1971; McDevitt and others 1982; Sato and others 1992).
In fact, it is not necessary for the Purkinje cell to discharge to observe climbing fiber-induced pauses in SSs. Such pauses can be induced by electrical stimulation of the contralateral inferior olive even when the stimulus current is subthreshold for evoking a CS in the recorded Purkinje cell (Bloedel and Roberts 1971). This observation provides additional evidence that SS pauses are not simply a function of a Purkinje cell-specific membrane conductance. SS pauses are generated by inhibitory interneurons or by Purkinje cell axon collaterals or both.
Both decreases and increases in spontaneous SS discharge following a CS have been observed in different regions of the cerebellum (Bell and Grimm 1969; McDevitt and others 1982; Kano and others 1991a; Sato and others 1992). Decreases in SSs occur after electrically evoked CSs (Latham and Paul 1971; Burg and Rubia 1972), under conditions in which CSs are evoked by sensory stimulation and when CSs are evoked during movement (Thach 1970; Rubia and Kolb 1978; Ebner and Bloedel 1981). Variation in the locations of recording electrodes relative to climbing fiber zones can probably account for some of the variability in these data. The depth of modulation of SS discharge in Purkinje cells located at a zonal periphery is less than if the cells were located in a zonal center. In the rabbit uvula-nodulus, the depth of modulation of SSs located at the center of a zone is ~5× greater than at the periphery (Fushiki and Barmack 1997). In some peripherally located Purkinje cells, antiphasic responses of CSs and SSs disappear altogether. SSs in a Purkinje cell located on a zonal periphery are susceptible to interneuronal inhibition evoked by climbing fibers that innervate adjacent zones. Consequently, it is useful to localize Purkinje cells from which recordings are made within a zonal framework. The number of climbing fibers that contribute to the modulation of SSs of a particular Purkinje cell remains unknown, but likely exceeds two.
Improbable Mossy Fiber Zones in the Uvula-Nodulus
In the mouse uvula-nodulus vestibular climbing fiber zones are ~400 mm wide (Yakhnitsa and Barmack 2006). The spatial distribution of granule cells that contribute parallel fibers to these zones is much larger. We have made microinjections of neurobiotin into the dendritic trees of two to four Purkinje cells within a zone with the aim of tracing parallel fiber synapses back to the parent granule cell body. Such microinjections demonstrate a wide distribution of granule cells whose parallel fibers pass through the dendritic trees of the microinjected Purkinje cells. Sixty-two percent of the retrogradely labeled granule cells are located well beyond the bounds of the 400-mm climbing fiber zone. The functional dispersion of signals conveyed by parallel fibers might even be greater. We cannot assume that 38% of the granule cells located within an injected climbing fiber zone have the same optimal response planes as do the climbing fibers that define the zone.
Cutting Vestibular Primary Afferents Does Not Prevent Vestibular Modulation of SSs
However improbable, it remains logically possible that the antiphasic discharge of CSs and SSs is the consequence of two afferent systems with parallel responses modulated out of phase. This idea can be evaluated experimentally. The influence of climbing fiber activity on SSs can be tested independently of vestibular primary afferent mossy fibers by acutely disrupting the vestibular primary afferent pathway. If vestibular primary afferent mossy fibers convey a signal necessary for the modulation of SSs in the ipsilateral uvula-nodulus, then the modulation should be blocked when the ipsilateral labyrinth is destroyed by a unilateral labyrinthectomy (UL). A UL leaves the climbing fiber input to the ipsilateral uvula-nodulus intact because it originates primarily from the contralateral labyrinth (Fig. 3). In rabbits, a UL does not prevent vestibular modulation of SSs in ipsilateral Purkinje cells. In these cells the modulation of SSs remains antiphasic to CSs (Barmack and Yakhnitsa 2003; Fig. 6).
Figure 6.
Complex spikes (CSs) and simple spikes (SSs) are modulated in a Purkinje cell in rabbit uvula after vestibular primary afferent mossy fibers are blocked. A, Purkinje cell was located 1.7 mm lateral to the midline, ipsilateral to the unilateral labyrinthectomy (UL). The optimal plane for CS modulation was orthogonal to the longitudinal axis. The left labyrinth was surgically destroyed. B, CSs and SSs, modulated by sinusoidal roll-tilt at 0.2 Hz, are shown as vertical lines (CSs) and instantaneous frequency (SSs). C, Peristimulus histograms show the persistence of antiphasic modulation of SSs (gray bars) without input from ipsilateral primary vestibular afferent mossy fibers. Modified from Barmack and Yakhnitsa (2003).
Blocking the Vestibular Climbing Fibers Reduces Vestibular Modulation of CSs and SSs
If climbing fibers convey the key signal that modulates CSs directly and SSs indirectly through the action of interneurons, then a unilateral microlesion of the β-nucleus or DMCC should reduce vestibular modulation of both CSs and SSs in the contralateral uvula-nodulus (Fig. 7). In rabbits, we have localized physiologically the β-nucleus of the inferior olive and made electrolytic microlesions that unilaterally destroy most of it (Fig. 7A, B). This microlesion disrupts the climbing fiber projection to contralateral uvula-nodulus while leaving intact the projection from vestibular primary and secondary mossy fiber afferents. Such a microlesion reduces vestibular modulation of both CSs and SSs in the contralateral uvula-nodulus (Barmack and Yakhnitsa 2003; Fig. 7C, D).
Figure 7.
Blocking climbing fiber afferents reduces modulation of complex spikes (CSs) and simple spikes (SSs). Electrolytic microlesions, made in the caudal right β-nucleus reduced modulation of CSs and SSs recorded from Purkinje cells in the contralateral uvula-nodulus. A, Vestibularly modulated responses of a neuron recorded in the right caudal β-nucleus were used to guide the placement of the microlesion. The optimal plane corresponded to the right anterior semicircular canal. B, Microlesion of right β-nucleus is shown. C, Optimal response planes for CSs and depth of modulation for CSs and SSs were measured in Purkinje cells in the uvula-nodulus after microlesions of the right β-nucleus. A key for categorizing responses is located in the upper right corner. Shading in the left half of each symbol characterizes the depth of modulation (Dm) of the CSs. Shading in the right half characterizes the Dm for the SSs. Circles indicate Purkinje cells with optimal responses in the plane of the ipsilateral posterior-contralateral anterior semicircular canals. Squares indicate Purkinje cells with optimal responses in plane of the ipsilateral anterior-contralateral posterior semicircular canals. D, Polar plot compares Dm for CSs and SSs in Purkinje cells recorded in medial zones in the ipsilateral (green) and contralateral (red) uvula-nodulus after microlesions of the right β-nucleus. The vector representing Dm for contralateral SSs was reduced relative to that for ipsilateral SSs. β = β-nucleus; DAO = dorsal accessory olive; dc = dorsal cap of Kooy; MAO = medial accessory olive; Pyr = pyramidal tract; XIIn = hypoglossal nerve. Modified from Barmack and Yakhnitsa (2003).
In the intact system, climbing fiber discharge is associated with a reduction of SSs. If the activity conveyed by vestibular primary afferent mossy fiber → granule cell → parallel fibers had sufficient synaptic strength, it should prevail when the climbing fiber signal is blocked. SSs should then be modulated with a phase shift of 180 degrees. We have looked for such a phase-shifted SS signal following damage to the inferior olive and have found it in only 4 of 30 Purkinje cells (Fig. 7C, blue shaded symbols). This suggests that the vestibular primary afferent mossy fiber → granule cell → parallel fiber signal lacks synaptic efficacy for robust modulation of SSs even in the absence of a “competing” climbing fiber pathway.
Interneuronal Contributions to SS Modulation
How do climbing fibers modulate the discharge of SSs? Return to the observation that electrically evoked olivary activity reduces spontaneous SSs even when the climbing fiber signal is subthreshold for evoking CSs in the recorded Purkinje cell (Bloedel and Roberts 1971). This influence could only be mediated by interneurons or Purkinje cell collaterals. Six cerebellar interneurons could potentially contribute to the modulation of SSs. We have recorded from these interneurons and identified them anatomically by juxtacellular electroporation with neurobiotin (Pinault 1996; Simpson and others 2005; Barmack and Yakhnitsa 2008). One can compare the phase of the vestibularly evoked discharge of separate interneuronal populations and evaluate their phase with respect to that of CSs and SSs (Fig. 8A).
Figure 8.
Response vectors for sinusoidal roll-tilt in Purkinje cells, stellate cells, and Golgi cells. A, Purkinje cell response vectors are plotted separately for complex spikes (CSs; red arrow) and simple spikes (SSs; yellow arrow). The phase of the population vector for CSs leads ipsilateral roll tilt by ~ 45 degrees. The phase of the population vector for SSs leads ipsilateral roll-tilt by ~210 degrees. B, The phase of the population vector for stellate cells leads ipsilateral roll-tilt by ~11 degrees, roughly in phase with CSs. C, The population vector for Golgi cells is 180 degrees out of phase with ipsilateral roll-tilt and is roughly in phase with the population vector for SSs. Modified from Barmack and Yakhnitsa (2008).
Two interneurons, granule cells and unipolar brush cells (UBCs), are excitatory. UBCs are found in abundance in the granule cell layer, particularly in the uvulanodulus (Mugnaini and Floris 1994; Diño and others 2000). They receive vestibular primary afferent projections and amplify vestibular signals through synaptic feed-forward excitation of granule cells. Both of these excitatory interneurons have the wrong phase to account for CS-evoked modulation of SSs.
Four interneurons, stellate cells, Golgi cells, Lugaro cells, and basket cells, are inhibitory. Of these four cell types, stellate cells are the second most numerous cerebellar neurons, exceeded only by granule cells. In the rabbit, stellate cells outnumber Purkinje cells by a factor of 15. Consequently, they are easy to find and identify. Interestingly, the optimal population vector for a sample of 47 stellate cells indicates that their discharge is modulated in phase with CSs and out of phase with SSs. Consequently, as a population, stellate cells have the correct vector to modulate SSs during CS discharge (Fig. 8A, B).
An optimal population vector for a sample of 23 Golgi cells indicates that their discharge was modulated out of phase with CSs and in phase with SSs. Therefore, Golgi cells cannot account for the modulation of SSs by CSs (Fig. 8A, C). Lugaro cells are relatively rare. They are located in the granule cell layer. Basket cells are also encountered relatively infrequently in the lower molecular layer, but are difficult to identify with certainty.
Climbing Fiber-Evoked Modulation of Stellate Cells
One of the problems in establishing a role for climbing fiber-evoked modulation of stellate cells is that ultra-structural studies reveal no conventional synaptic contacts from climbing fibers onto stellate cells (Desclin 1976; Hámori and Szentágothai 1980). Nevertheless, electrical stimulation of the inferior olive excites stellate cells in the contralateral cerebellum. This excitation is 1 to 3 ms delayed from the onset of the climbing fiber field potential and is independent of its amplitude (Jörntell and Ekerot 2003). The discovery that stellate cells are excited by glutamate spillover from the discharge of adjacent climbing fibers provides an obvious solution to this dilemma (Szapiro and Barbour 2007).
The response of stellate cells to glutamate spillover may also depend on Kv4 low voltage-activated A-type potassium channels (Anderson and others 2010). These channels are preferentially distributed on the surface of stellate cell soma and dendrites where climbing fibers make nonsynaptic contact (Kollo and others 2006). These channels are blocked by decreasing external calcium. Hence, the frequency of discharge of stellate cells can be increased by either glutamate spillover from climbing fibers or by decreases in external calcium depleted by Purkinje cell CSs (Anderson and others 2010). These are remarkable observations. They imply that modulation of stellate cells, and consequently the modulation of the cerebellar output signal, depends on a nonclassical synaptic mechanism.
Climbing Fiber Responses in Other Cerebellar Systems
Questions about circuit functionality can be addressed in other cerebellar systems. Cutaneously evoked responses of Purkinje cells in the C3 climbing fiber zone of the cerebellar anterior lobe confirm that granule cells of the anterior lobe receive cutaneously activated mossy fibers from the ipsilateral forelimb and that this projection is somatotopically organized (Garwicz and others 1998). SSs and CSs evoked in Purkinje cells within the C3 climbing fiber zone have remarkably similar receptive fields. This similarity could be attributed to a developmental process during which synapses with like receptive fields are sustained or it could be attributed to the influence of one of the afferent systems preferentially reinforcing inputs of the other afferent system (Jörntell and Ekerot 2002). The only way to choose between these alternatives is to use a time-varying cutaneous stimulus so that it becomes possible to compare the phase of the discharge of cutaneous mossy fiber afferents relative to the phase of the discharge of SSs.
Horizontal optokinetic stimulation is effective in modulating the activity of climbing fibers that project to the flocculus from the contralateral inferior olive (Maekawa and Simpson 1973; Alley and others 1975; Graf and others 1988; Takeda and Maekawa 1989; Barmack and Shojaku 1995). Increased climbing fiber activity is associated with decreased “simple spike” discharge (Barmack and Shojaku 1995; Fushiki and Barmack 1997; Barmack and Yakhnitsa 2003; Yakhnitsa and Barmack 2006). The origins of mossy fiber projections to the flocculus are more varied than in the uvula-nodulus. It may be more difficult to evaluate whether one of several populations of mossy fibers contributes to the modulation of SSs in the flocculus.
One Cerebellum from Two Afferent Pathways
Climbing fiber discharge has two consequences. First, it directly evokes CSs from Purkinje cells arrayed in a sagittal zone. Second, it indirectly reduces SSs in Purkinje cells within this zone through its action on cerebellar inhibitory interneurons, most likely stellate cells. Paradoxically, the influence of inhibitory interneurons appears greater than that conveyed by vestibular primary afferent mossy fiber → granule cell → parallel fiber signals for several reasons: 1) Mossy fiber → granule cell → parallel fiber signals are too diffuse to account for the specificity of SSs; 2) they have the wrong polarity to account for the phase of SSs evoked by vestibular stimulation; and 3) they are too weak to modulate SSs even when climbing fiber signals are blocked by microlesions of the contralateral inferior olive.
Vestibular primary afferent mossy fiber → granule cell → parallel fiber signals optimize a rich diversity of inputs to each climbing fiber zone rather than provide a strict topographic map of the vestibular system that conforms to zonal boundaries. Given the total width of the rabbit uvula-nodulus (~5 mm), the large multifolial and medio-lateral distribution of mossy fiber rosettes, and the length of parallel fibers (~5 mm), it is likely that each uvula-nodular Purkinje cell is contacted by parallel fibers that encode signals from most, if not all, vestibular end organs.
What do mossy fibers do? Possibly they convey a regionally selective level of excitability. The net influence of primary and secondary vestibular mossy fiber afferents could indicate: “Your head is moving.” The details of head movement are encoded in specific climbing fiber vectors: “You are falling forward to the left by 20 degrees.” Modulation of SSs depends not only on the climbing fiber signal, but also on the intensity of activity in the broader population of mossy fiber → granule cell → parallel fiber signals. Increased mossy fiber activity could enhance the spontaneous discharge of SSs. It is likely that a higher spontaneous discharge of SSs would enhance the depth of modulation of SSs by CSs.
A longer term influence of climbing fibers on SS discharge could be achieved through conjunctive inhibition of parallel fiber synapses on Purkinje cell dendrites (Linden and Connor 1993; Ito 2002; Mapelli and D'Angelo 2007; Dean and others 2010). Although mossy fiber → granule cell → parallel fiber signals enhance the level of Purkinje cell excitability, they could also decrease the depth of modulation of SSs if they are persistently out of phase with CSs. This problem may be effectively solved by the conjunctive regulation of the synaptic efficacy of parallel fibers by climbing fiber-induced “long term depression” (LTD; Ito and others 1982; Sakurai 1987; Ito and Karachot 1989; Crépel and Jaillard 1991; Narasimhan and Linden 1996). LTD reduces the synaptic efficacy of parallel fiber signals that occur in phase with CSs while maintaining a level of excitability of Purkinje cells with parallel fiber activity uncorrelated with climbing fibers (Ekerot and Jörntell 2001; Jörntell and Ekerot 2002). LTD enhances the depth of modulation of SSs by minimizing conflicting parallel fiber discharge.
Abnormal Cerebellar Function
Our review presents an alternative framework for understanding the roles of mossy and climbing fibers in cerebellar function. It may shed light on the etiology and treatment of cerebellar disease. Consider the CAG repeat Purkinje cell disease, spinocerebellar ataxia (SCA). SCA is not always restricted to the cerebellum. In SCA-6, degeneration in precerebellar regions occurs (the vestibular complex, motor cortex, and inferior olive; Ishikawa and others 1999). Detailed analysis of cerebellar disease may implicate the inferior olive in other cases, such as in Leigh’s disease (Cavanagh 1994) or in other forms of SCA (Koeppen 2005).
5-HT1A agonists are often administered to provide patient relief from ataxia (Takei and others 2005). It is assumed that the 5-HT1A therapeutic effects are mediated by Purkinje cell 5-HT1A receptors. However, the therapeutic effects could be mediated by 5-HT1A receptors on olivary neurons that receive serotoninergic projections from neurons in the nucleus reticularis paragigantocellularis (Bishop and Ho 1986).
Without knowledge of the particulars of cerebellar disease, it is possible that circumscribed damage to the brainstem (including the inferior olive) could influence the expression of cerebellar symptoms usually attributed to cerebellar cortical pathology. However, alternative possibilities may remain unexplored if we embrace a functional view of cerebellar circuitry that is not supported by experiment.
Acknowledgment
The authors thank Gin McCollum for her comments on an earlier draft of this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This research was supported by grants from the National Institute of Deafness and Communicative Disorders (DC006668) and from the National Eye Institute (EY018561).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- Alley K, Baker R, Simpson JI. Afferents to the vestibulocerebellum and the origin of the visual climbing fibers in the rabbit. Brain Res. 1975;98:582–589. doi: 10.1016/0006-8993(75)90375-3. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Voorhoeve PE. Postsynaptic inhibition of cerebellar Purkinje cells. J Neurophysiol. 1964;27:1138–1153. doi: 10.1152/jn.1964.27.6.1138. [DOI] [PubMed] [Google Scholar]
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. doi: 10.1038/nn.2493. [DOI] [PubMed] [Google Scholar]
- Andersson G, Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis of the cat cerebellum during locomotion. J Physiol (Lond) 1987;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G, Oscarsson O. Projections to lateral vestibular nucleus from cerebellar climbing fiber zones. Exp Brain Res. 1978;32:549–564. doi: 10.1007/BF00239552. [DOI] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Edgley SA. Discharges of interpositus and Purkinje cells of the cat cerebellum during locomotion under different conditions. J Physiol. 1988;400:425–445. doi: 10.1113/jphysiol.1988.sp017130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH. GABAergic pathways convey vestibular information to the beta nucleus and dorsomedial cell column of the inferior olive. In: Highstein SM, Cohen B, Büttner-Ennever J, editors. New directions in vestibular research. New York: New York Academy of Science; 1996. pp. 541–552. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Fagerson M, Fredette BJ, Mugnaini E, Shojaku H. Activity of neurons in the beta nucleus of the inferior olive of the rabbit evoked by natural vestibular stimulation. Exp Brain Res. 1993;94:203–215. doi: 10.1007/BF00230288. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Fredette BJ, Mugnaini E. Parasolitary nucleus: a source of GABAergic vestibular information to the inferior olive of rat and rabbit. J Comp Neurol. 1998;392:352–372. doi: 10.1002/(sici)1096-9861(19980316)392:3<352::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Shojaku H. Vestibular and visual signals evoked in the uvula-nodulus of the rabbit cerebellum by natural stimulation. J Neurophysiol. 1995;74:2573–2589. doi: 10.1152/jn.1995.74.6.2573. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Vestibular signals in the para-solitary nucleus. J Neurophysiol. 2000;83:3559–3569. doi: 10.1152/jn.2000.83.6.3559. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Cerebellar climbing fibers modulate simple spikes in cerebellar Purkinje cells. J Neurosci. 2003;23:7904–7916. doi: 10.1523/JNEUROSCI.23-21-07904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–1152. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Grimm RJ. Discharge properties of Purkinje cells recorded on single and double microelectrodes. J Neurophysiol. 1969;32:1044–1055. doi: 10.1152/jn.1969.32.6.1044. [DOI] [PubMed] [Google Scholar]
- Berthoz A, Llinás R. Afferent neck projection to the cat cerebellar cortex. Exp Brain Res. 1974;20:385–401. doi: 10.1007/BF00237383. [DOI] [PubMed] [Google Scholar]
- Bishop GA, Ho RH. Cell bodies of origin of serotonin-immunoreactive afferents to the inferior olivary complex of the rat. Brain Res. 1986;399:369–373. doi: 10.1016/0006-8993(86)91530-1. [DOI] [PubMed] [Google Scholar]
- Bloedel JR, Bracha V. Cerebellar functions. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedic Reference of Neuroscience. Heidelberg: Springer Verlag; 2009. pp. 667–671. [Google Scholar]
- Bloedel JR, Roberts WJ. Action of climbing fibers in cerebellar cortex of the cat. J Neurophysiol. 1971;34:17–31. doi: 10.1152/jn.1971.34.1.17. [DOI] [PubMed] [Google Scholar]
- Brand S, Dahl AL, Mugnaini E. The length of parallel fibers in the cat cerebellar cortex. An experimental light and electron microscopic study. Exp Brain Res. 1976;26:39–58. doi: 10.1007/BF00235248. [DOI] [PubMed] [Google Scholar]
- Brodal A, Brodal P. Observations on the secondary vestibulocerebellar projections in the macaque monkey. Exp Brain Res. 1985;58:62–74. doi: 10.1007/BF00238954. [DOI] [PubMed] [Google Scholar]
- Brodal A, Torvik A. The origin of secondary vestibulocerebellar fibers in cats; an experimental anatomical study. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1957;195:550–567. doi: 10.1007/BF00343130. [DOI] [PubMed] [Google Scholar]
- Burg D, Rubia FJ. Inhibition of cerebellar Purkinje cells by climbing fiber input. Pflugers Arch. 1972;337:367–372. doi: 10.1007/BF00586652. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. Is Purkinje cell loss in Leigh’s disease an excitotoxic event secondary to damage to inferior olivary nuclei? Neuropathol Appl Neurobiol. 1994;20:599–603. doi: 10.1111/j.1365-2990.1994.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Yarom Y. Patches of synchronized activity in the cerebellar cortex evoked by mossy-fiber stimulation: questioning the role of parallel fibers. Proc Natl Acad Sci U S A. 1998;95:15032–15036. doi: 10.1073/pnas.95.25.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crépel F, Jaillard D. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy in vitro. J Physiol (Lond) 1991;432:123–141. doi: 10.1113/jphysiol.1991.sp018380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jorntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- Desclin JC. Early terminal degeneration of cerebellar climbing fibers after destruction of the inferior olive in the rat. Synaptic relationships in the molecular layer. Anat Embryol (Berl) 1976;149:87–112. doi: 10.1007/BF00315087. [DOI] [PubMed] [Google Scholar]
- Diño MR, Schuerger RJ, Liu YB, Slater NT, Mugnaini E. Unipolar brush cell: A potential feedforward excitatory interneuron of the cerebellum. Neuroscience. 2000;98:625–636. doi: 10.1016/s0306-4522(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Ebner TJ, Bloedel JR. Role of climbing fiber afferent input in determining responsiveness of Purkinje cells to mossy fiber inputs. J Neurophysiol. 1981;45:962–971. doi: 10.1152/jn.1981.45.5.962. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibers on the Purkinje cells of the cerebellum. J Physiol (Lond) 1966a;182:268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Llinás R, Sasaki K. The mossy fibre-granule cell relay of the cerebellum and its inhibitory control by Golgi cells. Exp Brain Res. 1966b;1:82–101. doi: 10.1007/BF00235211. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Jörntell H. Parallel fibre receptive fields of Purkinje cells and interneurons are climbing fibre-specific. Eur J Neurosci. 2001;13:1303–1310. doi: 10.1046/j.0953-816x.2001.01499.x. [DOI] [PubMed] [Google Scholar]
- Epema AH, Gerrits NM, Voogd J. Secondary vestibulocerebellar projections to the flocculus and uvulo-nodular lobule of the rabbit: A study using HRP and double fluorescent tracer techniques. Exp Brain Res. 1990;80:72–82. doi: 10.1007/BF00228849. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Fox CA, Hillman DE, Siegesmund KA, Dutta CR. The primate cerebellar cortex: a Golgi and electron microscopic study. In: Fox CA, Snider RS, editors. Progress in brain research. vol. 25. New York: Elsevier; 1967. pp. 174–225. the cerebellum. [DOI] [PubMed] [Google Scholar]
- Frens MA, Mathoera AL, van der SJ. Floccular complex spike response to transparent retinal slip. Neuron. 2001;30:795–801. doi: 10.1016/s0896-6273(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Fushiki H, Barmack NH. Topography and reciprocal activity of cerebellar Purkinje cells in the uvula-nodulus modulated by vestibular stimulation. J Neurophysiol. 1997;78:3083–3094. doi: 10.1152/jn.1997.78.6.3083. [DOI] [PubMed] [Google Scholar]
- Garwicz M, Jorntell H, Ekerot CF. Cutaneous receptive fields and topography of mossy fibres and climbing fibres projecting to cat cerebellar C3 zone. J Physiol. 1998;512:277–293. doi: 10.1111/j.1469-7793.1998.277bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Thach WT. The cerebellum. In: Kandel ER, Schwartz J, Jessel TM, editors. Principles of neuroscience. New York: Elsevier; 2000. pp. 832–852. [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971;34:635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Graf W, Simpson JI, Leonard CS. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. II. Complex and simple spike responses of Purkinje cells. J Neurophysiol. 1988;60:2091–2121. doi: 10.1152/jn.1988.60.6.2091. [DOI] [PubMed] [Google Scholar]
- Granit R, Phillips CG. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol (Lond) 1956;133:520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J. The parasagittal zonation within the olivocerebellar projection I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol. 1977;174:417–488. doi: 10.1002/cne.901740304. [DOI] [PubMed] [Google Scholar]
- Gundappa-Sulur G, De Schutter E, Bower JM. Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol. 1999;408:580–596. doi: 10.1002/(sici)1096-9861(19990614)408:4<580::aid-cne11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Hámori J, Szentágothai J. Lack of evidence of synaptic contacts by climbing fibre collaterals to basket and stellate cells in developing rat cerebellar cortex. Brain Res. 1980;186:454–457. doi: 10.1016/0006-8993(80)90990-7. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Napper RM. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J Comp Neurol. 1988;274:151–157. doi: 10.1002/cne.902740202. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Napper RM. Quantitative studies on the mammalian cerebellum. Prog Neurobiol. 1991;36:437–463. doi: 10.1016/0301-0082(91)90012-p. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Watanabe M, Yoshizawa K, Fujita T, Iwamoto H, Yoshizawa T, Harada K, Nakamagoe K, Komatsuzaki Y, Satoh A, Doi M, Ogata T, Kanazawa I, Shoji S, Mizusawa H. Clinical, neuropathological, and molecular study in two families with spinocerebellar ataxia type 6 (SCA6) J Neurol Neurosurg Psychiatry. 1999;67:86–89. doi: 10.1136/jnnp.67.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell— Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- Ito M, Karachot L. Long-term desensitization of quisqualate-specific glutamate receptors in Purkinje cells investigated with wedge recording from rat cerebellar slices. Neurosci Res. 1989;7:168–171. doi: 10.1016/0168-0102(89)90058-8. [DOI] [PubMed] [Google Scholar]
- Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression on both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol (Lond) 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci. 2003;23:9620–9631. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Kano M-S, Maekawa K. Optokinetic response of simple spikes of Purkinje cells in the cerebellar flocculus and nodulus of the pigmented rabbit. Exp Brain Res. 1991a;87:484–496. doi: 10.1007/BF00227074. [DOI] [PubMed] [Google Scholar]
- Kano M, Kano M-S, Maekawa K. Simple spike modulation of Purkinje cells in the cerebellar nodulus of the pig-mented rabbit to optokinetic stimulation. Neurosci Lett. 1991b;128:101–104. doi: 10.1016/0304-3940(91)90769-p. [DOI] [PubMed] [Google Scholar]
- Kassel J, Shambes GM, Welker W. Fractured cutaneous projections to the granule cell layer of the posterior cerebellar hemisphere of the domestic cat. J Comp Neurol. 1984;225:458–468. doi: 10.1002/cne.902250311. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Mustari MJ, Miselis RR, Perachio AA. Transneuronal pathways to the vestibulocerebellum. J Comp Neurol. 1996;370:501–523. doi: 10.1002/(SICI)1096-9861(19960708)370:4<501::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4:62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- Kollo M, Holderith NB, Nusser Z. Novel subcellular distribution pattern of A-type K+ channels on neuronal surface. J Neurosci. 2006;26:2684–2691. doi: 10.1523/JNEUROSCI.5257-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchabhakdi N, Walberg F. Cerebellar afferent projections from the vestibular nuclei in the cat: An experimental study with the method of retrograde axonal transport of horseradish peroxidase. Exp Brain Res. 1978;31:591–604. doi: 10.1007/BF00239814. [DOI] [PubMed] [Google Scholar]
- Lasser-Ross N, Ross WN. Imaging voltage and synaptically activated sodium transients in cerebellar Purkinje cells. Proc R Soc Lond Biol. 1992;247:35–39. doi: 10.1098/rspb.1992.0006. [DOI] [PubMed] [Google Scholar]
- Latham A, Paul D. Spontaneous activity of cerebellar Purkinje cells and their responses to impulses in climbing fibres. J Physiol. 1971;213:135–156. doi: 10.1113/jphysiol.1971.sp009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CS, Simpson JI, Graf W. Spatial organization of visual messages of the rabbit’s cerebellar flocculus. I. Typology of inferior olive neurons of the dorsal cap of Kooy. J Neurophysiol. 1988;60:2073–2090. doi: 10.1152/jn.1988.60.6.2073. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Cellular mechanisms of long-term depression in the cerebellum. Curr Opin Neurobiol. 1993;3:401–406. doi: 10.1016/0959-4388(93)90133-j. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol (Lond ) 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones in vitro. J Physiol (Lond) 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa K, Simpson JI. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol. 1973;36:649–666. doi: 10.1152/jn.1973.36.4.649. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Dev Brain Res. 2003;140:223–236. doi: 10.1016/s0165-3806(02)00609-0. [DOI] [PubMed] [Google Scholar]
- Mapelli J, D’Angelo E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci. 2007;27:1285–1296. doi: 10.1523/JNEUROSCI.4873-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt CJ, Ebner TJ, Bloedel JR. The changes in Purkinje cell simple spike activity following spontaneous climbing fiber inputs. Brain Res. 1982;237:484–491. doi: 10.1016/0006-8993(82)90460-7. [DOI] [PubMed] [Google Scholar]
- Midtgaard J. Stellate cell inhibition of Purkinje cells in the turtle cerebellum in vitro. J Physiol (Lond) 1992;457:355–367. doi: 10.1113/jphysiol.1992.sp019382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini E, Floris A. The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol. 1994;339:174–180. doi: 10.1002/cne.903390203. [DOI] [PubMed] [Google Scholar]
- Murphy JT, MacKay WA, Johnson F. Differences between cerebellar mossy and climbing fibre responses to natural stimulation of forelimb muscle proprioceptors. Brain Res. 1973;55:263–289. doi: 10.1016/0006-8993(73)90295-3. [DOI] [PubMed] [Google Scholar]
- Narasimhan K, Linden DJ. Defining a minimal computational unit for cerebellar long-term depression. Neuron. 1996;17:333–341. doi: 10.1016/s0896-6273(00)80164-6. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Magyar P, Szentagothai J. Quantitative histological analysis of the cerebellar cortex in the cat. IV. Mossy fiber-Purkinje cell numerical transfer. Brain Res. 1972;45:15–29. doi: 10.1016/0006-8993(72)90213-2. [DOI] [PubMed] [Google Scholar]
- Pichitpornchai C, Rawson JA, Rees S. Morphology of parallel fibers in the cerebellar cortex of the rat—an experimental light and electron-microscopic study with biocytin. J Comp Neurol. 1994;342:206–220. doi: 10.1002/cne.903420205. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morphofunctional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Hestrin S. Developmental regulation of basket/ stellate cell—Purkinje cell synapses in the cerebellum. J Neurosci. 1997;17:9104–9112. doi: 10.1523/JNEUROSCI.17-23-09104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Simpson JI, Llinás R. Responses of Purkinje cells in rabbit nodulus and uvula to natural vestibular and visual stimuli. Pflugers Arch Ges Physiol. 1976;367:1–6. doi: 10.1007/BF00583649. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FR, Fraser MO, Hollerman JR, Tomko DL. Yaw direction neurons in the cat inferior olive. J Neurophysiol. 1988;60:1739–1752. doi: 10.1152/jn.1988.60.5.1739. [DOI] [PubMed] [Google Scholar]
- Rubia FJ, Kolb FP. Responses of cerebellar units to a passive movement in the decerebrate cat. Exp Brain Res. 1978;31:387–401. doi: 10.1007/BF00237297. [DOI] [PubMed] [Google Scholar]
- Rushmer DS, Roberts WJ, Augter GK. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res. 1976;106:1–20. doi: 10.1016/0006-8993(76)90069-x. [DOI] [PubMed] [Google Scholar]
- Sakurai M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol (Lond ) 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miura A, Fushiki H, Kawasaki T. Short-term modulation of cerebellar Purkinje cell activity after spontaneous climbing fiber input. J Neurophysiol. 1992;68:2051–2062. doi: 10.1152/jn.1992.68.6.2051. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Panto MR, Parenti R, Zappala A, Cicirata F. Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J Comp Neurol. 2001;430:471–484. doi: 10.1002/1096-9861(20010219)430:4<471::aid-cne1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Hulscher HC, Sabel-Goedknegt E, Ruigrok TJ. Between in and out: linking morphology and physiology of cerebellar cortical interneurons. Prog Brain Res. 2005;148:329–340. doi: 10.1016/S0079-6123(04)48026-1. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu H, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–148. [PubMed] [Google Scholar]
- Sugihara I, Wu HS, Shinoda Y. The entire trajectories of single olivocerebellar axons in the cerebellar cortex and their contribution to cerebellar compartmentalization. J Neurosci. 2001;21:7715–7723. doi: 10.1523/JNEUROSCI.21-19-07715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- Takeda T, Maekawa K. Olivary branching projections to the flocculus, nodulus and uvula in the rabbit. II. Retrograde double labeling study with fluorescent dyes. Exp Brain Res. 1989;76:323–332. doi: 10.1007/BF00247892. [DOI] [PubMed] [Google Scholar]
- Takei A, Hamada T, Yabe I, Sasaki H. Treatment of cerebellar ataxia with 5-HT1A agonist. Cerebellum. 2005;4:211–215. doi: 10.1080/14734220500222318. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970;33:537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Thunnissen IE, Epema AH, Gerrits NM. Secondary vestibulocerebellar mossy fiber projection to the caudal vermis in the rabbit. J Comp Neurol. 1989;290:262–277. doi: 10.1002/cne.902900207. [DOI] [PubMed] [Google Scholar]
- Voogd J, Gerrits NM, Ruigrok TJ. Organization of the vestibulocerebellum. Ann N Y Acad Sci. 1996;781:553–579. doi: 10.1111/j.1749-6632.1996.tb15728.x. [DOI] [PubMed] [Google Scholar]
- Voogd J, Barmack NH. Oculomotor cerebellum. Prog Brain Res. 2005;151:231–268. doi: 10.1016/S0079-6123(05)51008-2. [DOI] [PubMed] [Google Scholar]
- Walter JT, Khodakhah K. The linear computational algorithm of cerebellar Purkinje cells. J Neurosci. 2006;26:12861–12872. doi: 10.1523/JNEUROSCI.4507-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JT, Dizon MJ, Khodakhah K. The functional equivalence of ascending and parallel fiber inputs in cerebellar computation. J Neurosci. 2009;29:8462–8473. doi: 10.1523/JNEUROSCI.5718-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Wylie DR, DeZeeuw CI, DiGiorgi PL, Simpson JI. Projections of individual Purkinje-cells of identified zones in the ventral nodulus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349:448–463. doi: 10.1002/cne.903490309. [DOI] [PubMed] [Google Scholar]
- Yakhnitsa V, Barmack NH. Antiphasic Purkinje cell responses in mouse uvula-nodulus are sensitive to static roll-tilt and topographically organized. Neuroscience. 2006;143:615–626. doi: 10.1016/j.neuroscience.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yakusheva T, Blazquez PM, Angelaki DE. Frequency-selective coding of translation and tilt in macaque cerebellar nodulus and uvula. J Neurosci. 2008;28:9997–10009. doi: 10.1523/JNEUROSCI.2232-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakusheva T, Blazquez PM, Angelaki DE. Relationship between complex and simple spike activity in macaque caudal vermis during three-dimensional vestibular stimulation. J Neurosci. 2010;30:8111–8126. doi: 10.1523/JNEUROSCI.5779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54:973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto M. Topographical representation in rabbit cerebellar flocculus for various afferent inputs from the brainstem investigated by means of retrograde axonal transport of horseradish peroxidase. Neurosci Lett. 1979;12:29–34. doi: 10.1016/0304-3940(79)91475-7. [DOI] [PubMed] [Google Scholar]