Abstract
BACKGROUND:
Various digestive tract procedures effectively improve metabolic syndrome, especially the control of type 2 diabetes mellitus. Very good metabolic results have been shown with vertical gastrectomy and entero-omentectomy; however, the metabolic effects of an isolated entero-omentectomy have not been previously studied.
METHODS
: Nine patients with type 2 diabetes mellitus and a body mass index ranging from 29 to 34.8 kg/m2 underwent an entero-omentectomy procedure that consisted of an enterectomy of the middle jejunum and exeresis of the major part of the omentum performed through a mini-laparotomy. Glucagon-like peptide-1 and peptide YY were measured preoperatively and three months following the operation. Fasting and postprandial variations in glycemia, insulinemia, triglyceridemia, hemoglobin A1c, and body mass index were determined in the preoperative period and 3, 18 and, 36 months after the operation.
RESULTS
: All patients significantly improved the control of their type 2 diabetes mellitus. Postprandial secretion of peptide YY and Glucagon-like peptide-1 were enhanced, whereas hemoglobin A1c, fasting and postprandial glucose, insulin, and triglyceride levels were significantly reduced. Mean body mass index was reduced from 31.1 to 27.3 kg/m2. No major surgical or nutritional complications occurred.
CONCLUSIONS
: Entero-omentectomy is easy and safe to perform. A simple reduction in jejunal extension and visceral fat causes important improvements in the metabolic profile.
Keywords: Type 2 diabetes mellitus, GLP-1, PYY, Enterectomy, Omentectomy, Metabolic surgery
INTRODUCTION
The combination of a vertical gastrectomy, omentectomy and enterectomy has been proposed as a surgical strategy for obesity and associated diseases.1 The goals of this method are to improve gastrointestinal hormone levels rather than cause mechanical restriction or malabsorption. The results of five years of experience with this procedure have been published and are promising, especially in resolving metabolic syndrome.1 Indeed, this procedure was proposed in 2002 as the first surgical technique designed to treat obesity by exclusively aiming at a metabolic rearrangement and avoiding mechanical restriction (such as prostheses and/or calibrated anastomoses) and malabsorption.
Vertical gastrectomy adjusts the gastric chamber volume without narrowing passages to combat the higher caloric density of a modern diet. Vertical gastrectomy aims to reduce levels of ghrelin, an orexigenic hormone whose secretion is supposedly suppressed after meals but is not properly inhibited in the obese.2,3
Omentectomy leads to purely metabolic actions, and experimental work has continuously shown that it reduces insulin resistance.4,5
Jejunectomy has many effects, but the main one is to diminish nutrient exposure in the proximal gut and move a significant portion of the absorptive work to the distal small bowel (as if an unrefined diet were consumed), thereby enhancing the secretion of Glucagon-like peptide-1 (GLP-1). GLP-1 and peptide YY (PYY)6 are enterohormones that are secreted postprandially, satiating and provoking intense insulinotropic and glucagonostatic actions.7 Both obese and type 2 diabetic (T2DM) individuals have a deficiency in the secretion of GLP-1.8,9
Jejunectomy is possible because there is a very wide variance of the dimensions of the small bowel;10 an isolated partial jejunectomy is very safe (a much more extensive enterectomy is necessary, affecting also the ileum to generate disease.) Jejunectomies, indeed cause an intense rise in the production of GLP-1.11
The sum of these interventions, i.e., vertical gastrectomy, omentectomy and jejunectomy, has beneficial effects on both obesity and metabolic syndromes without significant restriction or malabsorption.1
In this model, unlike the vertical gastrectomy, the enterectomy has received severe criticism. The former was already part of a well-known procedure, the biliopancreatic bypass with a duodenal switch. However, based exclusively on objective data, the opposite result was expected, because reducing the stomach is more dangerous than reducing the jejunum. The jejunum has a wider range of dimensions than the stomach. Vertical gastrectomy alters the organ conformation and changes the His angle, whereas after a jejunectomy, as in the proposed pattern, the structure and dimensions of the small bowel remain within the normal range among surgery-naïve patients; not even a radiologist could identify this pattern of partial jejunectomy. Enteric functions are maintained, and the bowel responds with a strong compensatory growth facilitated by Glucagon-like peptide-2 (GLP-2), another entero-hormone co-secreted with GLP-1.12 None of these benefits apply to a sleeve gastrectomy.
In addition, biological, physiological, anthropological, epidemiological, and clinical evidence indicates that the metabolic damage caused by abundant quantities of refined food is linked to an overstimulation of the proximal gut and an understimulation of the distal small bowel. Specifically, the evolutionary adaptation to a richer diet involves the selection of smaller abdomens and digestive tracts.13
Finally, when vertical gastrectomy and enterectomy were performed together,1 all complications were related to the gastrectomy; none were related to the enterectomy as would be expected. Therefore, although both may produce very interesting metabolic effects, there is little doubt that partial jejunectomies are safer than vertical gastrectomies.
Indeed, vertical gastrectomy is not simple and can cause severe surgical complications such as fistulas. These dangers raise the possibility of using just entero-omentectomy (EO), the simpler and safer part of the aforementioned combined procedures, avoiding the sleeve gastrectomy. The goal would be to improve metabolic parameters, especially in T2DM. Thus, eliminating the vertical gastrectomy would significantly reduce the complexity, costs, risk and weight loss such that merely mildly obese diabetic patients could benefit.
This study shows and discusses the metabolic effects of an isolated EO. It presents the results of a research protocol in mildly obese patients with T2DM who were excluded from traditional bariatric surgery for exhibiting only “overweight” or “Grade I” obesity.
MATERIALS AND METHODS
Patients
The present study includes nine patients with T2DM who underwent surgery between February and March 2006 and were followed until the present date. The patients, diagnosed and treated for T2DM for at least two years, were between 30 and 60 years of age and had a body mass index (BMI) between 29 and 34.8 kg/m2. Six of these patients used insulin; the other three used oral hypoglycemia medication. Moreover, some of the patients used medications to control hypertension.
The inclusion criteria were as follows: insufficient control of T2DM with clinical treatment; ability to understand the procedure and associated risks; having a surgical risk considered acceptable by the medical assistant (ASA I and II); and having signed an informed consent form. All patients were submitted to the same collection methodology and exams in the preoperative period and 3, 18, and 36 months following surgery.
Technique
The surgery was performed through a mini-laparotomy with a median supra-umbilical incision of 8 to 10 cm to release the epiplon from the large gastric curvature while preserving the gastroepiploic artery. The duodenojejunal angle was located, measuring 40 cm from the proximal to the distal end of the jejunum, which remained preserved. Having located the ileocecal valve, 260 cm of the ileum was measured in the cranial direction and was also preserved. The intestine between these two points was measured, and the enterectomy was then performed. An end-to-end jejunoileal anastomosis was then performed on two planes, approximating the mesentery. The small intestine was measured prior to resection from the duodenal-jejunal angle to the ileocecal valve.
Anthropometric parameters
Patient weight values were recorded one day prior to hospital admission and at 3, 18, and 36 months following surgery. BMI was calculated during the first consultation; before the surgery, the patients were classified as overweight or having Grade I obesity.
Hormonal and laboratory studies
Blood samples of all patients were analyzed after two different twelve-hour fasting periods. Venous blood samples were collected after fasting and 30, 60, 90, and 120 minutes after the ingestion of a 300 kcal standard meal (200 mL Nutridrink®, Nutricia, Bornem, Belgium). Plasma samples were used to measure GLP-1 and PYY with a radioimmunoassay at the Hammersmith Hospital in London as previously described.14 On a different occasion, after a twelve-hour fast, all nine patients were submitted to another series of blood sampling: fasting and 1, 2, 3, 4, 5, and 6 hours after the ingestion of a lipid-enriched meal (250 g meal, 65.95 g of fat, 7 g of carbohydrate, 13.15 g of protein and 674 total kcal) to measure triglyceride levels. Both the Nutridrink and the lipid-enriched meal studies were performed preoperatively and five to six months postoperatively. Weight loss, changes in bowel habits, feces for abnormal fat excretion (Sudam III), nutritional parameters (hemoglobin, albumin, iron, B12 vitamin, and folic acid), and patient satisfaction were measured on both occasions. Plasma glucose levels (fasting and 120 min postprandial) were measured using the hexokinase method; fasting insulin was measured using immunofluorimetry.
In the preoperative and postoperative evaluations (at 3, 18, and 36 months), glucose levels were determined by blood enzymatic colorimetric analysis after 8 to 12 hours of fasting and also two hours after eating a standardized lunch containing 414 kcal. Fasting and postprandial insulin concentrations were determined at the same time as the glucose by chemoluminescence. Glycated hemoglobin A1c (HbA1c) levels were determined at the same time as the fasting glucose level analysis with the method certified by the US National Glycohemoglobin Standardization Program using standard reference values (Bio-Rad Variant II HbA1c Program). The homeostatic model assessment - insulin resistance (HOMA-IR) index was indirectly derived from fasting glycemia and insulinemia through the following equation: HOMA-IR = glycemia (mMol) x insulin (uU/mL) ÷ 22.5.15 The reference value used for the diagnosis of insulin resistance by the HOMA–IR index was defined in the BRAMS study (Brazilian Metabolic Syndrome Study) as 2.71.16
Statistical analysis
Analyses of GLP1, PYY, fasting and postprandial glycemia, fasting and postprandial insulin, HbA1c, HOMA-IR and BMI were based on the mean values of the laboratory analyses in the preoperative and postoperative periods. Linear regression analysis with mixed fixed effects and random effects was used. The area under the curve (AUC) was used to assess the peptides GLP1 and PYY. In the present study, the AUC values were used to identify changes in the analyzed parameters from the preoperative period through the three month post-surgical evaluation. The AUC analysis was conducted using SAS 9.1.3 for Windows.
To compare each variable of interest (fasting and postprandial glycemia, fasting and postprandial insulin, HbA1c, BMI, and HOMA-IR index) over time, an analysis of variance (ANOVA) for repeated measures was used. Differences between mean values were compared through an analysis of residuals; when the supposition of normality was not satisfied, a logarithmic transformation was performed on the variable. A paired Student's t-test was used to compare the variables between evaluation periods. The level of significance was set at p<0.05.
Nutritional parameters
In the 18th and 36th months after the EO, the patients' circulating blood was used to determine the following nutritional values: albumin (g/dL), folic acid (ng/mL), vitamin B12 (pg/mL), iron (µg/dL), hemoglobin (g/dL), and globule volume (%). Fecal fat was assessed using a qualitative study (Sudam III).
Ethical considerations
The study received approval from the Ethics Committee of the Universidade Estadual de Ponta Grossa (COEP authorization n°. 51/2005, process n°. 0416905) and from the Hospital Vicentino da Sociedade Beneficente São Camilo, where the surgeries were performed. All patients gave their signed, informed consent.
RESULTS
Surgical results
The total length of the small intestine measured during surgery, the length and weight of the removed intestinal segment with the related mesentery and the weight of the removed epiplon were 7.53±0.83 meters, 4.53±0.83 meters and 892.22±273.53 grams, and 431.11±150.70 grams, respectively (values given as means ± standard deviation). No deaths occurred in the study. Postoperative complications were restricted to one case of a small incisional hernia six months following surgery.
Clinical results
Table 1 displays the BMI comparisons between the preoperative period and the evaluations at 3, 18, and 36 months after surgery. The patients who underwent the EO exhibited a total weight reduction of 8.43±2.88% by the 3rd month, 11.17±6.33% by the 18th month and 12.07±7.75% by the 36th month following the EO.
Table 1.
Comparison of BMI between the preoperative period and at 3, 18 and 36 months after surgery, expressed as means and standard deviations. Based on ANOVA with repeated measures, there is a significant difference between the initial BMI and the 36-month postoperative BMI (p = 0.0039).
| Patient | Initial BMI | BMI after 3 months | BMI after 18 months | BMI after 36 months |
| 1 | 30.3 | 27.89 | 27.02 | 23.15 |
| 2 | 29.5 | 28.20 | 27.27 | 28.60 |
| 3 | 30.5 | 28.09 | 28.30 | 29.02 |
| 4 | 33.8 | 31.41 | 31.56 | 30.09 |
| 5 | 30.3 | 28.08 | 28.59 | 29.40 |
| 6 | 33.2 | 30.55 | 30.93 | 27.73 |
| 7 | 34.8 | 29.53 | 26.28 | 27.05 |
| 8 | 29.1 | 26.29 | 24.94 | 24.96 |
| 9 | 29,0 | 26.56 | 24.03 | 26.01 |
| M (SD) | 31.17 (2.18) | 28.51 (1.70) | 27.66 (2.51) | 27.33 (2.28) |
In the immediate postoperative period, NPH insulin was suspended, and the patients controlled their diabetes with regular insulin as needed. The six patients who regularly used insulin were discharged from the hospital without a prescription for insulin. After 18 months, no patients were taking insulin, but three patients were taking oral hypoglycemia medication. Arterial hypertension improved in all eight previously hypertensive patients; five no longer used medication, and three controlled their hypertension using a lower dose.
Hormonal assay results
Enterohormones
PYY and GLP-1 secretion were enhanced in all nine patients. Concentrations of both PYY and GLP-1 at fasting and at 30, 60, 90, and 120 minutes after a standard meal were higher in the postoperative period than before the operation.
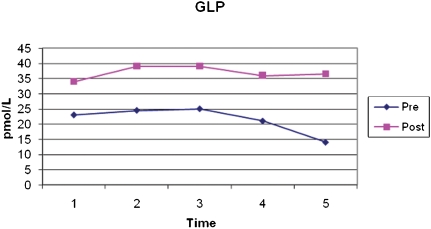
The average fasting preoperative GLP-1 was 23.2±16.3 pmol/L, whereas the fasting postoperative average was 33.9±18.3 pmol/L (p<0.001). Figure 1 shows the postprandial secretion of GLP-1. The AUC was also significantly higher postoperatively (4487.43±2033.8 pmol/L/min) compared to preoperatively (2657.67±1436.5 pmol/L/min) (p = 0.005).
Figure 1.
Mean levels of GLP-1 at fasting and 30, 60, 90, and 120 minutes after a standard meal, pre- and postoperatively. *p<0.005.
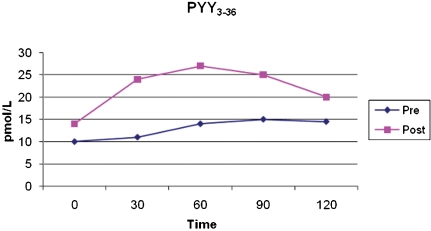
Fasting postoperative PYY (13.6±7.6 pmol/L) was not statistically higher than preoperative PYY (9.9±3.6 pmol/L). However, postprandial secretion of PYY, analyzed by comparing the AUC after the standard meal, was significantly higher (preoperatively 1546.33±672.32 pmol/L/min; postoperative 2793.17±1787.68 pmol/L/min; p<0.005), as shown in Figure 2.
Figure 2.
Mean levels of PYY at fasting and 30, 60, 90, and 120 minutes after a standard meal, pre- and postoperatively. *p<0.005.
Triglycerides
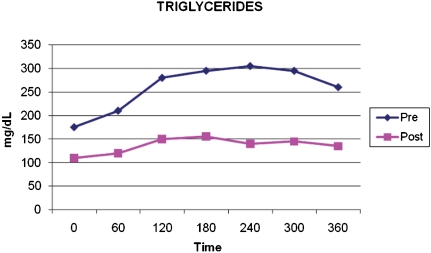
Analysis of plasma triglycerides showed that the procedure significantly reduced fasting and postprandial levels. Figure 3 shows that the AUC was significantly smaller in the postoperative period (mean preoperative 79644.00±27992.67 g/dL/min; mean postoperative 42009.00±16954.33 g/dL/min; p = 0.002).
Figure 3.
Mean levels of triglycerides (mg/dL) at fasting and 60, 120, 180, 240, 300, and 360 minutes after a standard meal, pre- and postoperatively. *p = 0.002.
Other laboratory assay results
Thirty-six months following the EO surgery, there was a significant reduction in fasting and postprandial glycemia and insulinemia, HbA1c, and HOMA-IR compared with the preoperative period (Tables 2356 and 4 respectively).
Table 2.
Fasting glycemia: Comparison between preoperative values and values at 3, 18, and 36 months after surgery. All postoperative values were significantly lower (p<0.05). M = mean; (SD) = standard deviation.
| Patient | Pre-op (mg/dl) | After 3 months(mg/dl) | After 18 months (mg/dL) | After 36 months (mg/dl) |
| 1 | 206 | 98 | 128 | 104 |
| 2 | 114 | 93 | 74 | 86 |
| 3 | 238 | 132 | 119 | 110 |
| 4 | 265 | 135 | 121 | 114 |
| 5 | 98 | 134 | 133 | 138 |
| 6 | 140 | 120 | 106 | 97 |
| 7 | 135 | 132 | 87 | 94 |
| 8 | 211 | 98 | 137 | 123 |
| 9 | 161 | 123 | 126 | 118 |
| M (SD) | 174.2 (58.1) | 118.3 (17.3) | 114.6 (21.5) | 109.3 (16.1) |
Table 3.
Post-prandial glycemia: Comparison between preoperative values and values at 3, 18, and 36 months after surgery. All postoperative values were significantly lower (p<0.05).
| Patient | Pre-op (mg/dl) | After 3 months(mg/dl) | After 18 months (mg/dL) | After 36 months (mg/dl) |
| 1 | 491 | 173 | 184 | 179 |
| 2 | 264 | 188 | 81 | 128 |
| 3 | 338 | 185 | 143 | 154 |
| 4 | 265 | 198 | 108 | 160 |
| 5 | 304 | 117 | 155 | 185 |
| 6 | 225 | 125 | 68 | 151 |
| 7 | 311 | 146 | 129 | 135 |
| 8 | 383 | 160 | 176 | 145 |
| 9 | 241 | 131 | 152 | 166 |
| M (SD) | 313.6 (82.9) | 158.1 (29.8) | 132.9 (40.3) | 155.9 (18.9) |
Table 5.
Fasting insulin levels: Comparison between preoperative values and values at 3, 18, and 36 months after surgery. All postoperative values were significantly lower (p<0.05). M = mean; (SD) = standard deviation.
| Patient | Pre-op (µU/dl) | After 3 months (µU/dl) | After 18 months (µU/dl) | After 36 months (µU/dl) |
| 1 | 32.5 | 16.8 | 8.5 | 8.3 |
| 2 | 29.8 | 5.3 | 18.2 | 10.4 |
| 3 | 10.9 | 17.2 | 9.8 | 8.6 |
| 4 | 17.5 | 13.2 | 10.1 | 8.0 |
| 5 | 16.7 | 6.3 | 5.5 | 4.8 |
| 6 | 19.3 | 25.7 | 7.6 | 8.4 |
| 7 | 56.2 | 9.0 | 3.1 | 3.0 |
| 8 | 12.0 | 3.1 | 5.2 | 3.4 |
| 9 | 15.5 | 3.8 | 4.4 | 5.0 |
| M (SD) | 35.4 (34.6) | 14.3 (9.4) | 8.0 (4.5) | 6.7 (2.6) |
Table 6.
Post-prandial insulin levels: Comparison between preoperative values and values at 3, 18, and 36 months after surgery. All postoperative values were significantly lower (p<0.05). M = mean; (SD) = standard deviation.
| Patient | Pre-op (µU/dl) | After 3 months (µU/dl) | After 18 months (µU/dl) | After 36 months (µU/dl) |
| 1 | 56.0 | 41.1 | 30.0 | 38.0 |
| 2 | 67.0 | 18.4 | 8.3 | 14.1 |
| 3 | 51.6 | 66.2 | 22.6 | 17.7 |
| 4 | 113.0 | 62.4 | 9.3 | 16.8 |
| 5 | 22.6 | 28.7 | 15.8 | 19.3 |
| 6 | 65.5 | 79.6 | 17.9 | 15.2 |
| 7 | 64.9 | 52.0 | 9.9 | 15.9 |
| 8 | 191.0 | 58.8 | 8.9 | 10.0 |
| 9 | 45.1 | 11.9 | 10.9 | 12.2 |
| M (SD) | 75.2 (49.6) | 46.6 (23.1) | 14.8 (7.5) | 17.7 (8.1) |
Table 4.
Glycated hemoglobin (HbA1c%): Comparison between preoperative values and values at 3, 18, and 36 months after surgery. All postoperative values were significantly lower (p<0.05). M = mean; (SD) = standard deviation.
| Patient | Pre-op (mg/dl) | After 3 months(mg/dl) | After 18 months (mg/dL) | After 36 months (mg/dl) |
| 1 | 11.49 | 8.20 | 7.0 | 7.2 |
| 2 | 8.16 | 5.80 | 6.7 | 5.6 |
| 3 | 9.20 | 6.80 | 6.2 | 6.6 |
| 4 | 8.90 | 7.70 | 7.4 | 6.8 |
| 5 | 9.00 | 7.40 | 7.2 | 7.4 |
| 6 | 12.20 | 6.40 | 6.6 | 6.3 |
| 7 | 10.44 | 6.60 | 5.6 | 5.3 |
| 8 | 11.30 | 6.16 | 7.8 | 6.5 |
| 9 | 10.65 | 5.80 | 6.9 | 6.7 |
| M (SD) | 10.15 (1.39) | 6.76 (0.85) | 6.82 (0.65) | 6.49 (0.68) |
Nutritional aspects
Throughout the 36-month follow-up, the patients did not exhibit fecal fat at any evaluation, as assessed through a qualitative investigation (Sudam III). The values of nutritional parameters such as albumin, folic acid and iron were normal in all patients in the three periods of postoperative evaluation.
Two female patients exhibited low levels of hemoglobin and globule volume 18 months after surgery. One reported not eating red meat due to a taste sensation change; the other underwent gynecological treatment for 30 days due to functional metrorrhagia. All patients had serum levels of vitamin B12 within the normal range. However, one patient was excluded from the calculation of the B12 mean and standard deviation because of a chronic intra-muscle B12 replacement as part of a treatment for previous lower limb neuropathy as directed by a neurologist.
DISCUSSION
The modern diet is related to an intense rise in the incidence of obesity and T2DM. Refined and concentrated nutrients comprise much of the modern diet, and refinement is a form of pre-digestion. These resultant high glycemic index nutrients are rapidly absorbed by the proximal small intestine, leaving less absorptive work for the distal gut.
There is much evidence that an overstimulation of the proximal gut and simultaneous understimulation of the distal gut might be related to obesity and T2DM.14 Procedures that exclude parts of the foregut are progressively more potent in resolving T2DM. Gastric banding (which does not exclude the bowel) somewhat improves T2DM but is dependent on weight loss and takes time. Roux-en-Y gastric bypass, which excludes part of the proximal gut, is fast and efficient in resolving T2DM. However, biliopancreatic bypass, which excludes the entire duodenum and jejunum, is the most efficient procedure for resolving obesity, DM2 and dyslipidemia.17
Some researchers who advocate the “foregut hypothesis” attribute this rapid improvement to undiscovered products of the proximal gut that have an anti-incretinic effect.18 Others, advancing the “hindgut hypothesis,” simply attribute this fast improvement to an enhancement of distal bowel hormones like GLP-1.19
The evidence supporting the hindgut hypothesis is very strong. As mentioned, obese and T2DM patients are deficient in GLP-1.8,9 GLP-1, either endogenous or exogenous, leads to important improvements in T2DM; its analogues have become an alternative in the medical treatment of T2DM.
Additionally, the exclusion of the duodenum and proximal jejunum is not required for fast resolution of T2DM if the distal gut is flooded with nutrients. For example, the jejunoileal bypass, an abandoned bariatric procedure, also caused a fast remission of T2DM20 and an elevation in the distal bowel hormones that lasted for many years without a duodenal exclusion.21 However, significant evidence suggests that the proximal gut plays a role in the development of metabolic syndrome; thus, both the foregut and hindgut theories are probably correct.
Glucose-dependent insulinotropic polypeptide (GIP) is hyper-secreted in overeating situations, especially in response to fatty foods. The obese have an increased postprandial secretion of GIP.22 GIP is a strong inducer of fat storage in adipocytes and plays a role in the development of obesity; blocking GIP results in a certain protection against diet-induced obesity.23 GIP is also an incretin, but T2DM patients are resistant to its incretinic effects.24 With this resistance, GIP becomes detrimental because its obesogenic properties remain, but its insulinotropic actions are inactivated (perhaps because of insufficient confirmatory response from GLP-1 and PYY).
The resulting obesity is linked to insulin resistance. Epidemiological and physiological studies indicate that those who develop preferentially visceral obesity have a higher incidence of T2DM.
In sum, recent modifications in the human diet, i.e., overabundant and refined foods, have provoked enhanced absorption in the upper parts of the gut and a reduction in the distal parts, leading to duodenojejunal hyperactivity and ileal hypoactivity. A jejunectomy has the potential to attenuate both problems.
The EO studied here is an auxiliary surgical treatment of diabetic overweight or Grade I obese patients derived from the digestive adaptation used in the treatment of morbid obesity1 but without the use of a vertical gastrectomy. The final configuration of the gastrointestinal tract looks very normal because the stomach is untouched, and the small bowel retains a dimension that is considered normal among surgery-naïve humans. The transit through the duodenum and proximal jejunum is preserved, and there are no obstacles to food ingestion or nutrient absorption. Intestinal segments are not excluded; however, as food more rapidly reaches the ileum, the penetration of nutrients in the distal bowel increases, significantly raising GLP-1 and PYY. The maintenance of the duodenum and proximal jejunum in transit may avoid protein and micronutrient deficiencies without compromising the benefits achieved in the improvement in T2DM.
There are no reports in the literature regarding any kind of enteric insufficiency with a partial jejunectomy when the duodenum, ileum and colon are intact. Indeed, around one meter of small intestine is sufficient for adequate nutrition absorption if part of the ileum is preserved and the colon is present. The proposed resection maintains the bowel dimensions in a normal range, considering the variation in human small bowels. As expected, no patient had diarrhea, signs of poor absorption or malnutrition during the 36-month follow-up.
Unlike subcutaneous fat, visceral fat has a demonstrated special relationship with T2DM, insulin resistance, hypertension, dyslipidemia, thrombogenicity and vascular insults. As visceral fat is insulin resistant, insulin inhibits lipolysis and keeps releasing free fatty acids (FFA) to the portal system. It has been suggested that insulin resistance of the liver is due to the relative increase in the delivery of FFA from the omental fat depot to the liver.25 Thorne et al. found an improvement in the metabolism of patients by adding omentectomy to a common bariatric procedure (i.e., gastric banding).26
An omentectomy was added to the procedure to reduce visceral fat. Visceral adiposity is an important source of resistin,27 as is plasminogen activator inhibitor 1 (PAI-1)28. These two substances are linked to an enhanced peripheral resistance to insulin and atherothrombotic diseases related to the metabolic syndrome. In 1999, Barzilai et al. showed that visceral fat excision reduces insulin resistance; this result has been confirmed by another group.4
All surgical procedures designed to treat T2DM would probably benefit from the inclusion of an omentectomy because there is an impressive amount of information linking visceral fat to T2DM. In addition, there are no contraindications to the procedure. Indeed, omentectomy has been performed for many decades as part of the treatment of gastric and ovarian cancers, with no reports of complications.
An intense reduction in the triglyceride levels was observed after the entero-omentectomy, both fasting and postprandially, as shown in Figure 3. There are at least two good reasons for this reduction. First, GLP-1 is responsible for reducing the postprandial rise in triglycerides.29 Second, in insulin-resistant states, the gut overproduces lipoproteins.30 Reducing triglycerides is important in the treatment of diabetes once fat has inhibited insulin action (Randle's effect).31
The EO was also effective in improving systemic arterial hypertension. Among the eight patients with hypertension, only three still required drugs for control, and they required lower doses than before the operation. The remission or better control of hypertension in these patients may be attributed to reduced fat in the blood and to the correction of hyperinsulinemia, leading to a possible improvement in the sympathetic nervous system activity and a lower tubular absorption of sodium.32 Fat also inhibits the production of nitrite oxide, an important physiological vasodilator.33 These facts may explain the improvements in arterial hypertension.
The exact mechanisms by which the surgery proposed here improves T2DM are unknown. Some studies have demonstrated that pancreatic exocrine cells incubated with GLP-1 or exendin-4 differentiate into endocrine phenotype cells.34 Zhou and colleagues demonstrated that GLP-1 or exendin-4 induced the pancreatic cell lineage in mice to differentiate from the original cells into islets of Langerhans cells.35 These differentiated cells increased the expression of β-cells genes and the capacity to release insulin. The β-cells differentiation induced by GLP-1 may be an important factor in maintaining glucose control in patients who have undergone EO.
The secretion of intestinal PYY was also significantly greater postoperatively than preoperatively. PYY is primarily produced by L cells in the intestinal and colonic distal regions. GLP-1 and PYY release leads to multiple actions, such as the promotion of the ileal brake, increased release of glucose-dependent insulin, inhibition of the secretion of glucagon and increased growth of pancreatic β-cells.36 PYY also slows down gastric emptying and promotes the ileal brake activity, clearly contributing to the treatment of diabetes.
From a surgical standpoint, the EO proposed here is a simple and inexpensive procedure, as there is a single entero-anastomosis, with the maintenance of bowel linearity, physiology and nutrient absorption. The risk of hypoglycemia is low, as the satiation hormones of the distal intestine are released only as a response to food intake.
All of the patients submitted to EO experienced a significant improvement in glucose control. Improvements in glycemia levels occurred immediately following surgery and led to the suspension of NPH insulin among the six patients who previously made use of this medication. After discharge, all patients took oral hypoglycemia medication. Five patients benefited from monotherapy, whereas four patients used two drugs to control glycemia. At the 36-month follow-up, the improvement in fasting and postprandial glycemia was maintained (Tables 2 and 3). For glycemic control, five patients no longer used any medication, three used monotherapy, and only one used two drugs. These findings demonstrate that EO was efficient in improving T2DM.
Based on large epidemiological studies such as the Diabetes Control and Complications Trial,37 HbA1c is the most accepted indicator of glycemia as it best reflects the long-term control of glycemia and predicts co-morbidities. In the preoperative period, all patients were under clinical treatment for T2DM, but the control of A1c proved inadequate, with a mean value of 10.15±1.39%. At three, 18 and 36 months following surgery, the mean values were 6.76±0.85%, 6.82±0.65% and 6.49±0.68%, respectively, demonstrating a significant difference over time in relation to the preoperative period (Table 4).
These results are eloquent in terms of the physiopathology. They clearly indicate that a reduction of the jejunum and visceral fat improves diabetes through metabolic changes, without significant malabsorption. As it is extremely simple, EO might be considered as an auxiliary treatment to poorly controlled T2DM in some areas with no access to advanced medicine. Alternatively, EO may be added to other more complex surgical procedures to reliably cause complete remission of T2DM in the long term.
CONCLUSION
The metabolic effects of reducing the jejunum and visceral fat are beneficial in mildly obese, T2DM patients. There are no obstacles to food intake, no prosthesis, no excluded segment and no malabsorption. The general structure and functions of the digestive tract are maintained with this surgery. GLP-1 and PYY secretion increased, while fasting and postprandial glycemia, triglyceride levels, and HbA1c were significantly reduced. A slight weight loss was obtained with no need for nutritional support. Isolated EO has positive effects on the metabolic profile of metabolic syndrome patients. As it is simple, safe and inexpensive, the procedure may be considered as an auxiliary treatment for T2DM. Additionally, it may be considered in conjunction with other metabolic active procedures.
REFERENCES
- 1.Santoro S, Milleo FQ, Malzoni CE, Klajner S, Borges PC, Santo MA, et al. Enterohormonal changes after Digestive Adaptation: Five-year results of a surgical proposal to treat obesity and associated diseases. Obes Surg. 2008;18:17–26. doi: 10.1007/s11695-007-9371-0. 10.1007/s11695-007-9371-0 [DOI] [PubMed] [Google Scholar]
- 2.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. doi: 10.1210/jcem.87.6.8738. 10.1210/jc.87.6.2984 [DOI] [PubMed] [Google Scholar]
- 3.Gustavo PSM, João LMCA, Carlos GN, Cora LCBM, Elaine CV, Perseu SC. Glucose homeostasis and weight loss in morbidly obese patients undergoing banded sleev gastrectomy: a prospective clinical study. Clinics. 2009;64:1093–8. doi: 10.1590/S1807-59322009001100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, et al. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes. 1999;48:94–8. doi: 10.2337/diabetes.48.1.94. 10.2337/diabetes.48.1.94 [DOI] [PubMed] [Google Scholar]
- 5.Lottati M, Kolka CM, Stefanovski D, Kirkman EL, Bergman RN. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obesity. 2009;17:674–80. doi: 10.1038/oby.2008.642. 10.1038/oby.2008.642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–8. doi: 10.1381/096089206776944959. 10.1381/096089206776944959 [DOI] [PubMed] [Google Scholar]
- 7.Bojanowska E. Physiology and pathophysiology of glucagons–like peptide-1 (GLP-1): The role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Med Sci Monit. 2005;11:RA271–8. [PubMed] [Google Scholar]
- 8.Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence. Gut. 1996;38:916–9. doi: 10.1136/gut.38.6.916. 10.1136/gut.38.6.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lugari R, Dei Cas A, Ugolotti D, Finardi L, Barilli AL, Ognibene C, et al. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (non insulin-dependent) diabetes. Horm Metab Res. 2002;34:150–4. doi: 10.1055/s-2002-23199. 10.1055/s-2002-23199 [DOI] [PubMed] [Google Scholar]
- 10.Adkins RB, Jr, Davies J. Gross and microscopic anatomy of the stomach and small intestine. In: Scott HW Jr, Sawyers JL, editors. Surgery of the stomach, duodenum and small bowel. Boston: Blackwell Scientific Publications; 1987. [Google Scholar]
- 11.Ulshen MH, Hoyt EC, Fuller CR, Ghatei MA, Bloom SR, Lund PK. Increased ileal proglucagon expression after jejunectomy is not suppressed by inhibition of bowel growth. Dig Dis Sci. 1996;41:677–83. doi: 10.1007/BF02213122. 10.1007/BF02213122 [DOI] [PubMed] [Google Scholar]
- 12.Dowling RH. Glucagon-Like Peptide-2 and Intestinal Adaptation: An Historical and Clinical Perspective. J Nutr. 2003;133:3703–7. doi: 10.1093/jn/133.11.3703. [DOI] [PubMed] [Google Scholar]
- 13.Santoro S. Is the Metabolic Syndrome a Disease of the Foregut. Yes, Excessive Foregut. Ann Surg. 2008;247:1074–5. doi: 10.1097/SLA.0b013e3181758ddb. [DOI] [PubMed] [Google Scholar]
- 14.Le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. 10.1097/01.sla.0000183349.16877.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 16.Geloneze B, Repetto EM, Geloneze SR, Tambascia MA, Ermetice MN. The threshold value for insulin resistance (HOMA-IR) in an admixtured population IR in the Brazilian Metabolic Syndrome Study. Diabetes Res Clin Pract. 2006;72:219–20. doi: 10.1016/j.diabres.2005.10.017. 10.1016/j.diabres.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 17.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 18.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. 10.1097/01.sla.0000102989.54824.fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patriti A, Facchiano E, Donini A. Comments on: Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;240:388–9. doi: 10.1097/01.sla.0000134632.08789.df. 10.1097/01.sla.0000134632.08789.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organ CH, Jr, Cegielski MM, Grabner BJ, Keig HE, Saporta JA. Jejunoileal Bypass. Long-term Results. Ann Surg. 1980;192:38–43. doi: 10.1097/00000658-198007000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Näslund E, Grybäck P, Hellström PM, Jacobsson H, Holst JJ, Theodorsson E, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–92. doi: 10.1038/sj.ijo.0800418. 10.1038/sj.ijo.0800418 [DOI] [PubMed] [Google Scholar]
- 22.Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. 10.1210/jc.2002-021873 [DOI] [PubMed] [Google Scholar]
- 23.Irwin N, Flatt PR. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009;52:1724–31. doi: 10.1007/s00125-009-1422-8. 10.1007/s00125-009-1422-8 [DOI] [PubMed] [Google Scholar]
- 24.Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes. 2004;53(Suppl3):S190–6. doi: 10.2337/diabetes.53.suppl_3.s190. 10.2337/diabetes.53.suppl_3.S190 [DOI] [PubMed] [Google Scholar]
- 25.Bergman RN, Van Citters GW, Mittelman SD, Dea MK, Hamilton-Wessler M, Kim SP, et al. Central role of adipocyte in the metabolic syndrome. J Investig Med. 2001;49:119–26. doi: 10.2310/6650.2001.34108. 10.2310/6650.2001.34108 [DOI] [PubMed] [Google Scholar]
- 26.Thörne A, Lönnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–9. doi: 10.1038/sj.ijo.0801871. 10.1038/sj.ijo.0801871 [DOI] [PubMed] [Google Scholar]
- 27.McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–7. doi: 10.1016/s0140-6736(02)07281-1. 10.1016/S0140-6736(02)07281-1 [DOI] [PubMed] [Google Scholar]
- 28.van Hinsbergh VW, Kooistra T, Scheffer MA, Hajo van Bockel J, van Muijen GN. Characterization and fibrinolytic properties of human omental tissue mesothelial cells. Comparison with endothelial cells. Blood. 1990;75:1490–7. [PubMed] [Google Scholar]
- 29.Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006;49:452–8. doi: 10.1007/s00125-005-0126-y. 10.1007/s00125-005-0126-y [DOI] [PubMed] [Google Scholar]
- 30.Khosrow A, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Current Opinion in Lipidology. 2008;19:221–8. doi: 10.1097/MOL.0b013e3282ffaf82. 10.1097/MOL.0b013e3282ffaf82 [DOI] [PubMed] [Google Scholar]
- 31.Felber JP. Significance of the Randle-Mechanism in the etiology of diabetes type II. Horm Metab Res Suppl. 1990;22:11–7. [PubMed] [Google Scholar]
- 32.De Fronzo RA, Ferranini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. 10.2337/diacare.14.3.173 [DOI] [PubMed] [Google Scholar]
- 33.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. 10.1161/01.HYP.0000154312.87612.07 [DOI] [PubMed] [Google Scholar]
- 34.Paris M, Tourrel-Cuzin C, Plachot C, Ktorza A. Review: Pancreatic β-Cell Neogenesis Revisited. Experimental Diab Res. 2004;5:111–21. doi: 10.1080/15438600490455079. 10.1080/15438600490455079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Wang X, Pineyro MA. Glucagon-like peptide 1 and exendin-4 convert, pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358–66. doi: 10.2337/diabetes.48.12.2358. 10.2337/diabetes.48.12.2358 [DOI] [PubMed] [Google Scholar]
- 36.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. 10.1016/j.cmet.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 37.Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]