Abstract
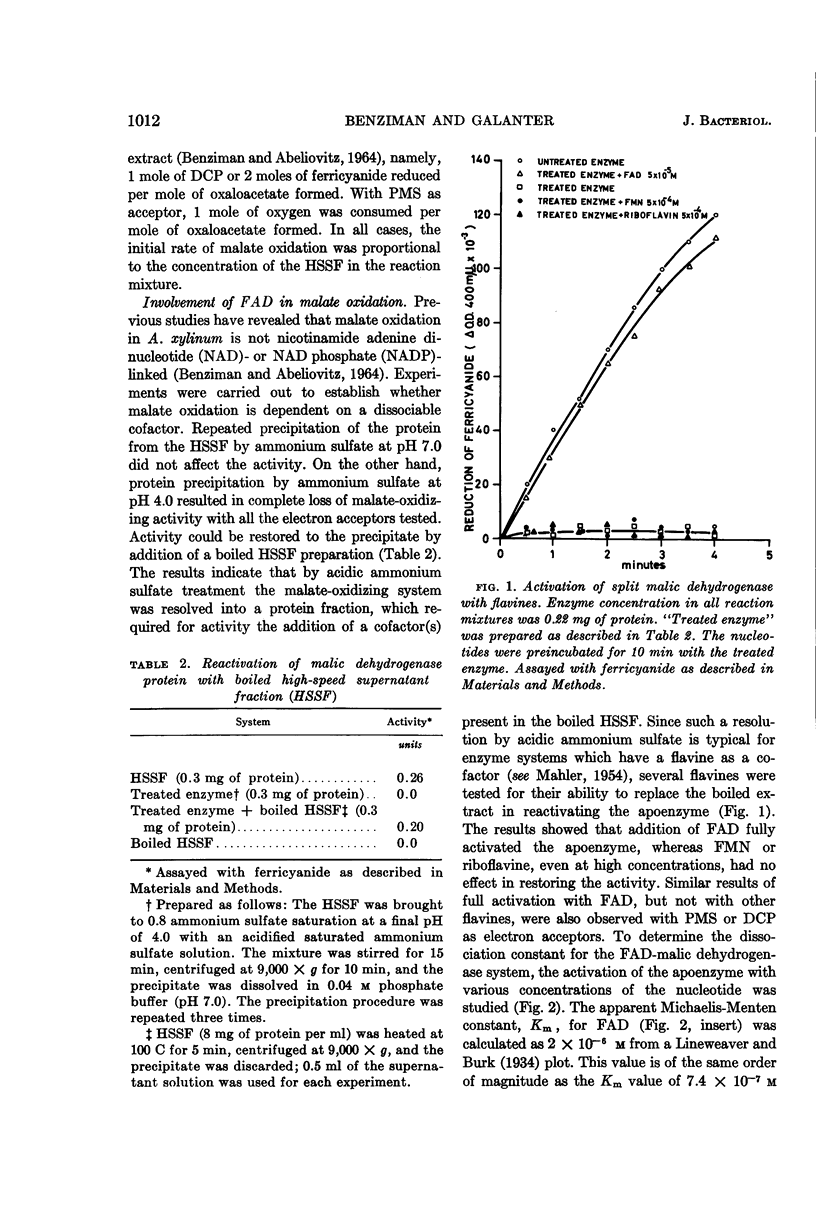
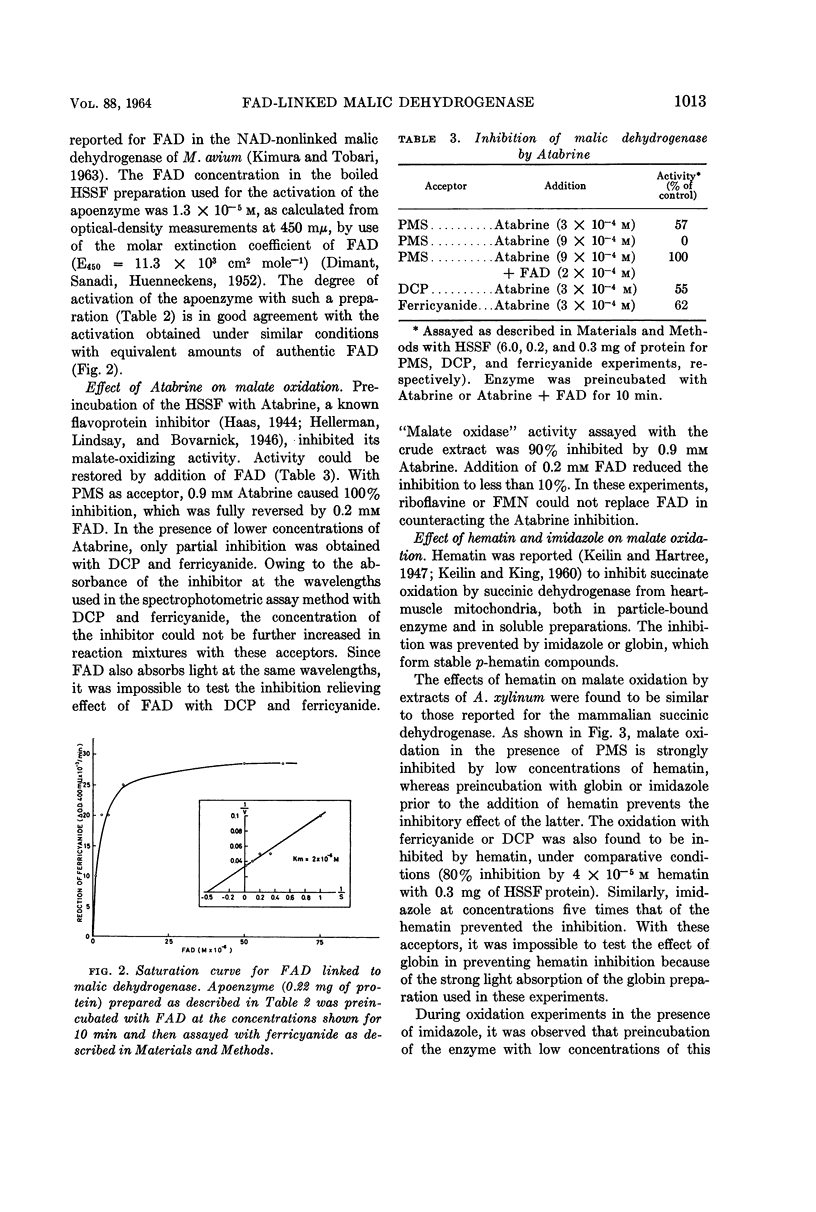
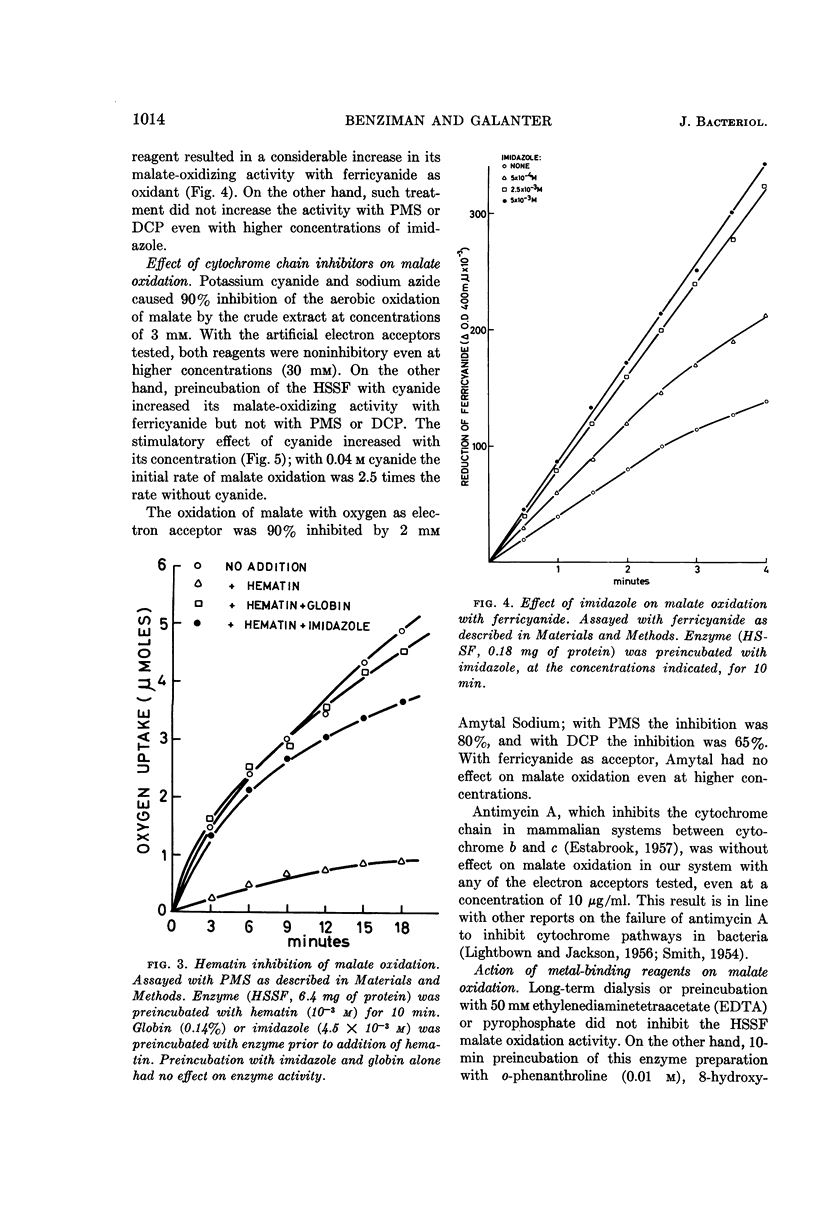
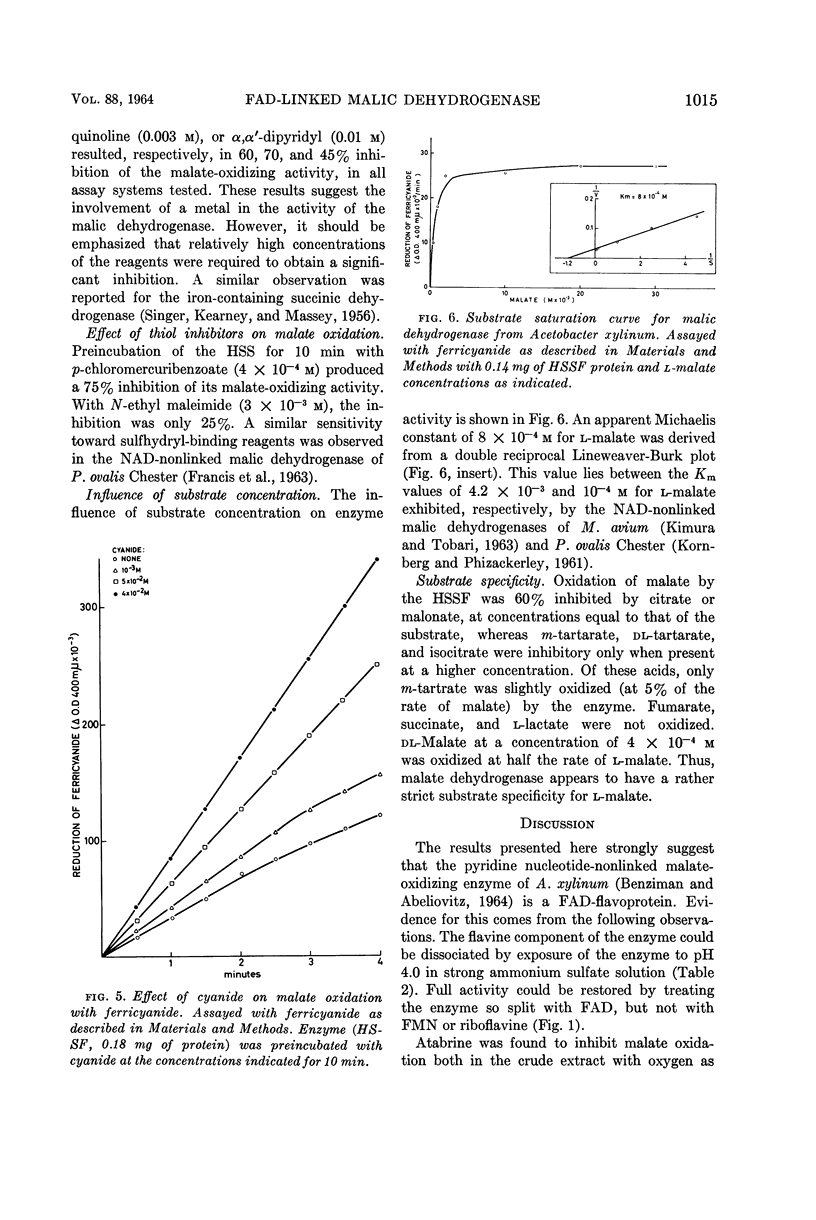
Benziman, Moshe (The Hebrew University of Jerusalem, Jerusalem, Israel), and Y. Galanter. Flavine adenine dinucleotide-linked malic dehydrogenase from Acetobacter xylinum. J. Bacteriol. 88:1010–1018. 1964.—The properties of the pyridine nucleotide-nonlinked malic dehydrogenase of Acetobacter xylinum were investigated in the supernatant fluid obtained by high-speed centrifugation of sonic extracts. Ferricyanide, phenazine methosulfate, and to a lesser extent dichlorophenolindophenol were active as oxidants for malate oxidation. After acid ammonium sulfate precipitation, the enzyme lost its malate-oxidizing activity. The enzyme was reactivated by low concentrations of flavine adenine dinucleotide (FAD) but not by flavine mononucleotide (FMN) or riboflavine. Atabrine inhibited the enzyme, and the inhibition was relieved by FAD but not by FMN or riboflavine. Malate-oxidizing activity was inhibited by hematin. The inhibition was prevented by imidazole or globin. o-Phenanthroline, 8-hydroxy quinoline, α,α'-dipyridyl, and p-chloromercuribenzoate inhibited malate oxidation. Amytal markedly inhibited oxidation of malate in the presence of oxygen, phenazine methosulfate, or dichlorophenolindophenol, but not in the presence of ferricyanide. The results suggest that the malic dehydrogenase of A. xylinum is a FAD enzyme, which contains an ironbinding site essential for its activity. Nonheme iron and sulfhydro groups are possibly involved in enzyme activity. The malic dehydrogenase is functionally linked to the cytochrome chain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZIMAN M., ABELIOVITZ A. METABOLISM OF DICARBOXYLIC ACIDS IN ACETOBACTER XYLINUM. J Bacteriol. 1964 Feb;87:270–277. doi: 10.1128/jb.87.2.270-277.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENZIMAN M., BURGER-RACHAMIMOV H. Synthesis of cellulose from pyruvate by succinate-grown cells of Acetobacter xylinum. J Bacteriol. 1962 Oct;84:625–630. doi: 10.1128/jb.84.4.625-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNATH P., KEARNEY E. B., SINGER T. P. Studies on succinic dehydrogenase. II. Isolation and properties of the dehydrogenase from beef heart. J Biol Chem. 1956 Dec;223(2):599–613. [PubMed] [Google Scholar]
- EICHEL H. J. Effects of atabrine and flavin mononucleotide on oxidation of succinic acid by Tetrahymena preparations. Biochim Biophys Acta. 1956 Dec;22(3):571–573. doi: 10.1016/0006-3002(56)90071-3. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. Kinetic properties of a reduced diphosphopyridine nucleotide cytochrome c reductase from heart muscle. J Biol Chem. 1957 Aug;227(2):1093–1108. [PubMed] [Google Scholar]
- FRANCIS M. J., HUGHES D. E., KORNBERG H. L., PHIZACKERLEY P. J. THE OXIDATION OF L-MALATE BY PSEUDOMONAS SP. Biochem J. 1963 Dec;89:430–438. doi: 10.1042/bj0890430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMEN M. D. Bacterial heme proteins. Bacteriol Rev. 1955 Dec;19(4):250–262. [PubMed] [Google Scholar]
- KEILIN D., KING T. E. Effect of inhibitors on the activity of soluble succinic dehydrogenase and on the reconstitution of the succinic dehydrogenase-cytochrome system from its components. Proc R Soc Lond B Biol Sci. 1960 May 17;152:163–187. doi: 10.1098/rspb.1960.0031. [DOI] [PubMed] [Google Scholar]
- KIMURA T., TOBARI J. PARTICIPATION OF FLAVIN-ADENINE DINUCLEOTIDE IN THE ACTIVITY OF MALATE DEHYDROGENASE FROM MYCOBACTERIUM AVIUM. Biochim Biophys Acta. 1963 Jul 9;73:399–405. doi: 10.1016/0006-3002(63)90441-4. [DOI] [PubMed] [Google Scholar]
- KORZENOVSKY M., AUDA B. V. Preparation and some properties of soluble choline dehydrogenase. Biochim Biophys Acta. 1958 Aug;29(2):463–464. doi: 10.1016/0006-3002(58)90227-0. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Effect of haematin on the oxidation of succinic acid by tissue preparations. Biochem J. 1947;41(4):503–506. doi: 10.1042/bj0410503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHTBOWN J. W., JACKSON F. L. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1956 May;63(1):130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAHLER H. R. Studies on the fatty acid oxidizing system of animal tissues. IV. The prosthetic group of butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):13–26. [PubMed] [Google Scholar]
- MAPSON L. W., BRESLOW E. Biological synthesis of ascorbic acid: L-galactono-gamma-lactone dehydrogenase. Biochem J. 1958 Mar;68(3):395–406. doi: 10.1042/bj0680395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINGLER R. L., MINAKAMI S., SINGER T. P. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. II. Isolation and molecular properties of the enzyme from beef heart. J Biol Chem. 1963 Feb;238:801–810. [PubMed] [Google Scholar]
- RINGLER R. L. Studies on the mitochondrial alpha-glycerophosphate dehydrogenase. II. Extraction and partial purification of the dehydrogenase from pig brain. J Biol Chem. 1961 Apr;236:1192–1198. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B., MASSEY V. Newer knowledge of succinic dehydrogenase. Adv Enzymol Relat Subj Biochem. 1957;18:65–111. doi: 10.1002/9780470122631.ch2. [DOI] [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes; difference spectra. Arch Biochem Biophys. 1954 Jun;50(2):299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]
- WARRINGA M. G., GIUDITTA A. Studies on succinic dehydrogenase. IX. Characterization of the enzyme from Micrococcus lactilyticus. J Biol Chem. 1958 Jan;230(1):111–123. [PubMed] [Google Scholar]



