Current approaches to dietary counseling for obesity are heavily rooted in the notion of “personal choice” Typically, patients receive education about dietary contributions to energy balance and are then encouraged to make dietary choices (e.g., food selections, portion sizes) consistent with weight loss. Yet even highly motivated and nutritionally-informed patients often struggle to refrain from highly palatable, energy-dense foods available in the modern environment, and ultimately, only a small percentage of individuals achieve sustained weight loss through dietary modification (1–4). Failed attempts at weight control are frustrating to patients and providers alike. Studies show that both parties frequently attribute obesity to poor “personal choices” or insufficient “willpower” on the part of the patient (5,6). For example, a sample of British dietitians ranked “lack of willpower” as more important to the development of obesity than genetic factors (7), even though adult body mass is 55–75% heritable (8,9). The suggestion that individuals become or remain obese due to their unhealthy personal choices, or a lack of willpower to make healthy choices, is stigmatizing to patients and unlikely to motivate patients to lose weight (10). De-emphasizing the role of personal choice in dietary counseling for obesity would reduce stigma, but doing so carries the risk of undermining patients’ perceived control over their weight loss success. The goal of this commentary is to help dietitians negotiate this dilemma by presenting a scientifically-informed framework that views the personal choices relevant to obesity counseling in terms of three neurobehavioral processes. We argue that applying this framework in dietary counseling can both minimize patient stigma and preserve patients’ sense of empowerment.
Personal choice: a problematic framework for obesity
The term “personal choice” implies that human behavior derives from conscious, volitional decisions, and connotes that humans have “free will” to decide between alternative courses of action - independent of biological and environmental forces. An implication of this definition of “personal choice” is that individuals can be considered causally, financially, and morally responsible for their behavior (11,12), a notion firmly embedded in the folk psychology of many cultures (13–15). The ethical and policy implications of personal choice in health have been discussed at length (11,16,17). In contrast to the notion of personal choice, some argue that human behavior is explained by neurobiological processes and their interaction with environmental stimuli (18). Supporting this deterministic1 model of “personal choice” are studies demonstrating that 1) future actions can be predicted by brain activation patterns up to 10 seconds before individuals become aware of having made a decision (19), 2) behavior is strongly influenced by processes outside of conscious awareness (20), and 3) individuals can be led to believe that they have caused actions outside of their control (21–24). Others dismiss the apparent conflict between free will and neurobiologically-based explanations of behavior (25–27). Whether human behavior is ultimately rooted in free will, neurobiology, or a combination of both will not be settled anytime soon. Yet, there is still considerable value for understanding how neurobehavioral processes interact with the environment to influence eating behavior for the purposes of understanding obesity’s etiology and reducing stigma.
As dietitians cannot control patients’ environments or their genetic vulnerabilities, and effective non-surgical weight loss treatments do not currently exist, it is understandable why many seek to instill responsibility for change in their patients. However, rather than engendering a sense of empowerment, the suggestion that sufficiently motivated patients can choose to engage in a healthier lifestyle can often lead to guilt and stigmatization by implying that individuals are responsible for failing to control their weight. Indeed, obese patients often feel stigmatized in healthcare settings, which can result in avoidance of the health care system, increased eating pathology, and even weight gain (10,28). Adopting a scientifically informed framework that clarifies how personal choice is affected by biological and environmental factors may reduce obesity stigma in healthcare settings (29) and empower patients by drawing attention to the environmental drivers of obesity that are within their control.
A scientific framework of personal choice in obesity
Building on emerging research, we propose that “personal choice” in obesity can be understood as a composite of neurobehavioral processes influenced by biological and environmental forces. Though a number of existing neurobiological models are potentially relevant to understanding personal choice in obesity [e.g., (20,30–32)], we focus on three neurobehavioral processes that have been most consistently implicated in obesity and overeating: food reward, inhibitory control, and time discounting.
Food reward
Obesity has been viewed almost exclusively as a disorder of energy homeostasis in which overeating results from insufficient satiety signaling or amplified hunger signaling (33,34). However, research conducted over the past decade has demonstrated that the sensory experience of palatable food can easily override homeostatic controls of energy balance, leading to overeating in the absence of true physiological hunger (35,36). It is also appreciated that the palatability of our food supply has been greatly enhanced by the food industry through the infusion of increasing amounts of sugar, fat, salt, and flavorings. This food engineering has been implicated as a key contributor to the obesity epidemic (37). Food reward includes both the experience of pleasure one receives from eating and the motivational drive to obtain and consume palatable food (38). Of these two aspects of reward, the motivational component may be more relevant to obesity since obese individuals do not report experiencing greater pleasure from palatable food than normal weight individuals (39,40). Of particular relevance to personal choice in obesity are findings that one’s sensitivity to food reward is grounded in genetics and neurobiology and is strongly linked to obesity (41).
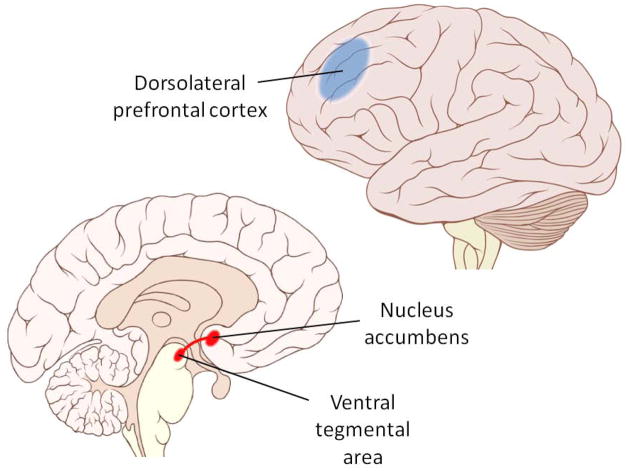
The neural processing of food reward has been traced to the mesolimbic system (Figure 1), the brain’s “reward circuit” which also mediates the motivation to engage in sex, gambling, and substance use (42). The hedonic pleasure associated with eating is linked to opioid neurotransmission in several small “hotspots” in the nucleus accumbens and other regions, whereas the motivational aspect of food reward is primarily mediated by dopamine pathways from the ventral tegmental area to the nucleus accumbens (38,42–46). Interestingly, blunted mesolimbic system neurotransmission (47), and biologic and genetic markers associated with diminished dopamine signaling (48–50) are linked to higher adiposity. The prevailing hypothesis is that this blunted mesolimbic signaling represents a deficiency in neural reward processing for which affected individuals compensate by overconsuming palatable food. In this way, deficient neural reward processing appears to equate with greater reward sensitivity at the level of behavior.
Figure 1.
Brain regions implicated in eating behavior. Food reward is largely mediated by the mesolimbic reward pathway (red), whereas inhibitory control of eating involves functions governed by the dorsolateral prefrontal cortex (blue). Delay discounting appears to be influenced by the functional interaction(s) between these two regions. Images modified from original productions by Patrick J. Lynch and C. Carl Jaffe, obtained under creative commons license.
Greater sensitivity to reward is linked to stronger food cravings (51), preferences for sweet and fatty foods (52), greater ad libitum food intake in laboratory studies(53), and higher body weight among adults and children (40,52,54,55). Sensitivity to reward is hypothesized to explain vulnerability to aspects of the “toxic food environment.” For example, living in areas with greater access to fast food outlets has been linked, albeit inconsistently, to an obesity-promoting diet and higher body mass (56,57), but these effects appears to be strongest among those most sensitive to reward (58). Essentially, high reward sensitivity combined with convenient access to highly palatable, energy-dense foods represents a biology-by-environment interaction that makes one extremely vulnerable to overeating and weight gain.
Inhibitory control
Food reward accounts for the “pull” towards palatable food that can drive overeating even in the absence of true physiological hunger. Beyond the intensity of food cravings, the question remains whether we can ignore or suppress such urges. After all, isn’t the ability to override our hedonic motivations the essence of “choice,” “self-control,” and “willpower”? The fact that we can refrain from eating palatable food (if only occasionally) while still finding food extremely tempting indicates that the capacity to refrain from eating is a distinct process from food reward. In other words, inhibiting our food intake is not simply a matter of reducing the motivation to eat; it involves actively controlling behavior despite a strong motivation to eat. Though exercising inhibitory control over eating has long been considered the central task in weight management, a scientific description of inhibitory control of eating at the behavioral and neurobiological levels is only now emerging.
We (59) and others (60) have proposed that inhibitory control over eating is supported by executive functions mediated by the prefrontal cortex (PFC). The PFC is considered critical for self-control, planning, and goal-directed behavior more generally (61,62), and the inhibition of eating can be considered a special class of behavior under its governance. There is ample evidence linking the functioning of prefrontal regions to performance on tasks measuring inhibitory control (31) and clinical syndromes characterized by impulsivity such as attention-deficit/hyperactivity disorder (ADHD) and drug addiction (63). In particular, the dorsolateral region of the PFC (Figure 1) has been implicated in the “decision” to engage inhibitory processes for the purpose of self-regulation (63–65). Several recent neuroimaging studies link differences in dorsolateral PFC function with the ability to inhibit eating. Hare et al (64) asked dieters to choose between pairs of 50 food items varying in taste and perceived healthiness. Dieters who consistently selected health over taste showed greater dorsolateral PFC activation when choosing the healthier options compared to those who more often selected taste over health. Further, there was evidence of functional connectivity between the dorsolateral PFC and brain areas associated with reward processing, consistent with the notion that the dorsolateral PFC inhibits the influence of reward on behavior. Other studies have shown that the dorsolateral PFC is activated following the ingestion of food (66,67), and that greater postmeal activation is associated with reduced adiposity (68,69), decreased food craving (70), and successful weight loss (71). Taken together, these findings indicate that the dorsolateral PFC supports active suppression of the motivation to eat palatable food. Unfortunately, life stress and other factors can easily disrupt inhibitory control (72) and lead to weight gain (73). Given this, it is not surprising that weight loss interventions, which largely rely upon persistent inhibitory control of eating, have meager long-term success rates (1–4).
Time discounting
A third factor that likely contributes to the low success rates of dietary interventions for obesity is the human tendency to devalue delayed rewards. Most of us would prefer to receive $200 today rather than $300 a year from now. This decision illustrates the fact that the brain discounts the value of money and other rewards over time, resulting in impulsive, short-sighted decision-making. Time discounting provides a framework for understanding why we sometimes knowingly make choices that are not in our best long-term interest [i.e., why the ‘will’ breaks down (74)]. For some individuals and not others, the immediate rewards of smoking, gambling, and drug use have a more potent influence on decision-making than the long-term social, financial, and physical costs of such behavior. Numerous studies have found that individuals who engage in these “addictive behaviors” assign disproportionate weight to the immediate pleasure derived from these activities compared to those who abstain (75–79). The link between time discounting and body weight is reflected neuroanatomically, with time discounting being governed by the same brain regions associated with food reward and inhibitory control. Time discounting is influenced by an impulsive, appetitive system that promotes pursuit of immediate rewards, as well as a reflective, deliberative system that seeks to maximize long-term gain. Neuroimaging studies indicate that these neural systems are composed of the mesolimbic dopamine system and its extensions, and the dorsolateral PFC, respectively (80–82). In fact, the reciprocal activation of these two brain regions predicts performance on time discounting tasks (83). The relevance of time discounting to obesity is substantial. In a very literal sense, weight loss requires consistent selection of delayed rewards [e.g., health benefits of weight loss (84)] over the immediate rewards associated with palatable food (85). In other words, the process of weight loss is directly at odds with the human tendency for time discounting. Consistent with this notion, several studies have linked higher body weight (86–88) and intake of palatable food (89) to greater time discounting on behavioral choice tasks.
Summary and Implications for Counseling
Thus far we have highlighted three neurobehavioral processes that promote overeating and obesity: 1) neurobiologically-based behavioral sensitivity to the rewarding properties of food, mediated by the mesolimbic dopamine system, 2) relative weakness in inhibitory control, mediated by the PFC (particularly dorsolateral regions), and 3) steeper discounting of the delayed rewards of weight loss relative to the immediate pleasure associated with eating, reflecting the interaction between the mesolimbic system and the PFC. There are at least three implications of this neurobehavioral model for dietary counseling for obesity.
First, the model explains eating behaviors which promote obesity without invoking character flaws (e.g., lack of willpower). By emphasizing genetically-influenced neurobiological processes that confer vulnerability to overeating in a toxic food environment, the model enables dietitians to more effectively address obesity (as discussed below) without promoting stigma.
Second, the neurobehavioral model preserves a sense of individual control. Though it may seem counter-intuitive, shifting the focus away from “personal choice” and towards the environmental and neurobehavioral processes involved in eating can encourage patients to take an active stance in their approach to weight management. We recommend that dietitians simultaneously convey two messages about weight control to their patients: 1) obesity is heavily influenced by genetic and environmental factors, and an epidemic of obesity is precisely what would be expected given the genetic heritage of our species and the omnipresence of palatable food in the environment; and 2) successful weight management can be achieved by taking active steps (such as those described below) to minimize the impact of the environment on eating behavior. The first message acknowledges that patients are working against potent genetic vulnerabilities and a toxic food environment, and normalizes patients’ (and dietitians’) frustration with failed attempts at weight control. The second message signals that patients can better control their weight through strategies focused on the interaction between the brain and the environment. For the majority of dietitians, this second message constitutes a shift in strategy from urging patients to make the “tough choices” required for weight control to helping patients minimize the number of tough choices they encounter. This differs from the traditional approach to obesity counseling, which by simply encouraging patients to eat fewer calories than they expend, ignores the very processes that make this advice so difficult to follow.
Finally, the framework presented above supports an increased emphasis on several behavioral strategies that have been considered adjuncts to dietary counseling for many years (90) (Table 1). Dietitians should assist patients in manipulating their environments to minimize exposure to palatable food cues, a step that is essential to reducing energy intake by preventing activation of the brain’s reward circuitry that generates the motivation to eat. For example, patients should remove tempting, high-calorie foods from their home and workplace. Of course, the decision to bring high-calorie foods into the home is made at the food store, and shopping from a grocery list or using online grocers (e.g., Peapod) can help reduce one’s susceptibility to the torrent of food cues at the supermarket (91). Another strategy involves learning to minimize exposure to stress and developing more effective stress reduction strategies, as stress promotes overeating and obesity by enhancing food reward processing (92,93) and disrupting inhibitory control (94,95). Dietitians may briefly review stress management techniques, encourage exercise as a stress reduction strategy, and refer patients to appropriate behavioral specialists. Finally, consideration of time discounting would suggest that increasing the delay to food rewards and decreasing the delay to weight loss rewards promotes better adherence to dietary goals. Consistent with this idea, patients should be encouraged to prepare healthy foods in advance to make them immediately accessible, keep tempting snacks out of the home (thus requiring a trip to the food store to obtain them), and focus on achieving short-term behavioral weight control goals (e.g., meeting a daily calorie goal) rather than focusing exclusively on long-term weight loss. The focus on short-term behavioral goals may be especially important considering that the rate of initial short-term weight loss is predictive of long-term weight loss outcomes (96), and that unrealistic long-term weight loss expectations are sometimes associated with poorer outcomes and higher attrition from weight loss treatment (97; also see 98). Focusing on achieving short-term behavioral goals would likely have the dual benefits of promoting early weight loss through behavior change and de-emphasizing any unrealistic weight loss expectations patients may have.
Table 1.
Summary of a neurobehavioral model of personal choice in obesity
| Behavioral process | Neural basis | Impact on personal choice | Clinical implication(s) |
|---|---|---|---|
| Food reward | Mesolimbic dopamine system | Increases motivation to consume palatable food Mechanism by which the highly engineered food supply overrides homeostatic controls of energy balance |
Removing palatable food cues from personal environments (e.g., home, workplace) reduces overeating by preventing activation of reward circuitry Limit the impact of reward on food choice by shopping with a grocery list, using online grocers, planning restaurant menu selections in advance |
| Inhibitory control | Prefrontal cortex, especially dorsolateral regions | Supports restraint from eating, which is a core component of weight management Inhibitory control can be disrupted by stress and demanding mental tasks, leading to overeating |
Avoid situations (e.g., buffets, restaurants) that challenge inhibitory control Counsel or refer for stress management Keep high-calorie foods out of reach where stress is anticipated |
| Time discounting | Interaction between mesolimbic system and prefrontal cortex | Immediate pleasure from eating has a greater impact eating has a greater impact on decision-making than delayed benefits of weight control | Focus on achievement of short-term goals (e.g., meeting a daily calorie goal) Advise patients to prepare healthy foods in advance to increase their accessibility relative to unhealthy convenience foods |
As the neurobehavioral basis of eating behavior advances, so will our understanding of obesity and weight control. However, enough progress has been made to enable dietitians to shift from a model of obesity counseling grounded in personal choice to one rooted in the brain processes that govern eating behavior in an obesity-promoting environment. In addition to providing nutrition education and encouragement, dietitians should more heavily focus on helping patients overcome the brain-based processes that make dietary modification so difficult, largely through strategies that have been considered “behavioral adjuncts” to dietary obesity counseling for many years. Dietary lapses or failures should be conceptualized as the result of brain systems interacting with a toxic food environment, and not as a reflection of poor personal choices or lack of willpower. Even if this approach is no more effective in producing weight loss than current practices, it is much less likely to elicit patient stigmatization.
Footnotes
Determinism is the view that behavior is caused by previous mechanistic processes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bradley M. Appelhans, Departments of Preventive Medicine and Behavioral Sciences, Rush University Medical Center, Chicago, IL, 60612, USA.
Matthew C. Whited, Email: matthew.whited@umassmed.edu, Department of Medicine, Division of Preventive and Behavioral Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA, 01655, USA.
Kristin L. Schneider, Email: kristin.schneider@umassmed.edu, Department of Medicine, Division of Preventive and Behavioral Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA, 01655, USA.
Sherry L. Pagoto, Email: sherry.pagoto@umassmed.edu, Department of Medicine, Division of Preventive and Behavioral Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA, 01655, USA.
References
- 1.Weiss EC, Galuska DA, Kettel Khan L, Gillespie C, Serdula MK. Weight regain in U.S. adults who experienced substantial weight loss, 1999–2002. Am J Prev Med. 2007;33:34–40. doi: 10.1016/j.amepre.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: The effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 3.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am Psychol. 2007;62:220–233. doi: 10.1037/0003-066X.62.3.220. [DOI] [PubMed] [Google Scholar]
- 5.Ogden J, Flanagan Z. Beliefs about the causes and solutions to obesity: A comparison of GPs and lay people. Patient Educ Couns. 2008;71:72–78. doi: 10.1016/j.pec.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 6.McArthur LH, Ross JK. Attitudes of registered dietitians toward personal overweight and overweight clients. J Am Diet Assoc. 1997;97:63–66. doi: 10.1016/S0002-8223(97)00021-7. [DOI] [PubMed] [Google Scholar]
- 7.Harvey EL, Summerbell CD, Kirk SF, Hill AJ. Dietitians’ views of overweight and obese people and reported management practices. J Hum Nutr Diet. 2002;15:331–347. doi: 10.1046/j.1365-277x.2002.00385.x. [DOI] [PubMed] [Google Scholar]
- 8.Schousboe K, Visscher PM, Erbas B, Kyvik KO, Hopper JL, Henriksen JE, Heitmann BL, Sorensen TI. Twin study of genetic and environmental influences on adult body size, shape, and composition. Int J Obes Relat Metab Disord. 2004;28:39–48. doi: 10.1038/sj.ijo.0802524. [DOI] [PubMed] [Google Scholar]
- 9.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256:51–54. [PubMed] [Google Scholar]
- 10.Puhl RM, Heuer CA. Obesity stigma: Important considerations for public health. Am J Public Health. 2010 doi: 10.2105/AJPH.2009.159491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikler D. Who should be blamed for being sick? Health Educ Q. 1987;14:11–25. doi: 10.1177/109019818701400104. [DOI] [PubMed] [Google Scholar]
- 12.Dworkin G. Voluntary health risks and public policy. Hastings Cent Rep. 1981;11:26–31. [PubMed] [Google Scholar]
- 13.Nichols S. The folk psychology of free will: Fits and starts. Mind & Language. 2004;19:473–502. [Google Scholar]
- 14.Nichols S, Knobe J. Moral responsibility and determinism: The cognitive science of folk intuitions. Nous. 2007;41:663–685. [Google Scholar]
- 15.Sarkissian H, Chatterjee A, De Brigard F, Knobe J, Nichols S, Sirker S. Is belief in free will a cultural universal? Mind & Language. 2010;25:346–358. [Google Scholar]
- 16.Schmidt H, Voigt K, Wikler D. Carrots, sticks, and health care reform--problems with wellness incentives. N Engl J Med. 2010;362:e3. doi: 10.1056/NEJMp0911552. [DOI] [PubMed] [Google Scholar]
- 17.Brownell KD, Kersh R, Ludwig DS, Post RC, Puhl RM, Schwartz MB, Willett WC. Personal responsibility and obesity: A constructive approach to a controversial issue. Health Aff (Millwood) 2010;29:379–387. doi: 10.1377/hlthaff.2009.0739. [DOI] [PubMed] [Google Scholar]
- 18.Montague PR. Free will. Curr Biol. 2008;18:R584–5. doi: 10.1016/j.cub.2008.04.053. [DOI] [PubMed] [Google Scholar]
- 19.Soon CS, Brass M, Heinze HJ, Haynes JD. Unconscious determinants of free decisions in the human brain. Nat Neurosci. 2008;11:543–545. doi: 10.1038/nn.2112. [DOI] [PubMed] [Google Scholar]
- 20.Suhler CL, Churchland PS. Control: Conscious and otherwise. Trends Cogn Sci. 2009;13:341–347. doi: 10.1016/j.tics.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Wegner DM, Wheatley T. Apparent mental causation. Sources of the experience of will. Am Psychol. 1999;54:480–492. doi: 10.1037//0003-066x.54.7.480. [DOI] [PubMed] [Google Scholar]
- 22.Pronin E, Wegner DM, McCarthy K, Rodriguez S. Everyday magical powers: The role of apparent mental causation in the overestimation of personal influence. J Pers Soc Psychol. 2006;91:218–231. doi: 10.1037/0022-3514.91.2.218. [DOI] [PubMed] [Google Scholar]
- 23.Ebert JP, Wegner DM. Time warp: Authorship shapes the perceived timing of actions and events. Conscious Cogn. 2010;19:481–489. doi: 10.1016/j.concog.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wegner DM. The Illusion of Conscious Will. Cambridge, MA: MIT Press; 2002. [Google Scholar]
- 25.Roskies A. Neuroscientific challenges to free will and responsibility. Trends Cogn Sci. 2006;10:419–423. doi: 10.1016/j.tics.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Baumeister RF. Free will in scientific psychology. Perspect Psychol Sci. 2008;3:14–19. doi: 10.1111/j.1745-6916.2008.00057.x. [DOI] [PubMed] [Google Scholar]
- 27.Vohs KD, Baumeister RF. Addiction and free will. Addict Res Theory. 2009;17:231–235. doi: 10.1080/16066350802567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greener J, Douglas F, van Teijlingen E. More of the same? Conflicting perspectives of obesity causation and intervention amongst overweight people, health professionals and policy makers. Soc Sci Med. 2010;70:1042–1049. doi: 10.1016/j.socscimed.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien KS, Puhl RM, Latner JD, Mir AS, Hunter JA. Reducing anti-fat prejudice in preservice health students: A randomized trial. Obesity (Silver Spring) 2010;18:2138–2144. doi: 10.1038/oby.2010.79. [DOI] [PubMed] [Google Scholar]
- 30.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn S, Haggard P, Brass M. Intentional inhibition: How the “veto-area” exerts control. Hum Brain Mapp. 2009;30:2834–2843. doi: 10.1002/hbm.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haggard P. Human volition: Towards a neuroscience of will. Nat Rev Neurosci. 2008;9:934–946. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- 33.Bray GA. History of obesity. In: Williams G, Fruhbeck G, editors. Obesity: Science to Practice. Chichester, West Sussex, UK: Wiley; 2009. [Google Scholar]
- 34.Stunkard AJ, Wolff HG. Pathogenesis in human obesity; function and disorder of a mechanism of satiety. Psychosom Med. 1958;20:17–29. doi: 10.1097/00006842-195801000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiol Behav. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: Reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33 (Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler DA. The End of Overeating. New York: Rodale; 2009. [Google Scholar]
- 38.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 39.Mela DJ. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite. 2006;47:10–17. doi: 10.1016/j.appet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Saelens BE, Epstein LH. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 41.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge KC, Ho CY, Richard JM, Difeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010 doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: Where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 45.Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- 46.Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–886. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 51.Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: A model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–19. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Epstein LH, Wright SM, Paluch RA, Leddy JJ, Hawk LW, Jr, Jaroni JL, Saad FG, Crystal-Mansour S, Shields PG, Lerman C. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- 54.Temple JL, Legierski CM, Giacomelli AM, Salvy SJ, Epstein LH. Overweight children find food more reinforcing and consume more energy than do nonoverweight children. Am J Clin Nutr. 2008;87:1121–1127. doi: 10.1093/ajcn/87.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis C, Fox J. Sensitivity to reward and body mass index (BMI): Evidence for a non-linear relationship. Appetite. 2008;50:43–49. doi: 10.1016/j.appet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Fleischhacker SE, Evenson KR, Rodriguez DA, Ammerman AS. A systematic review of fast food access studies. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00715.x. [DOI] [PubMed] [Google Scholar]
- 57.Mellor JM, Dolan CB, Rapoport RB. Child body mass index, obesity, and proximity to fast food restaurants. Int J Pediatr Obes. 2010 doi: 10.3109/17477161003777433. [DOI] [PubMed] [Google Scholar]
- 58.Paquet C, Daniel M, Knauper B, Gauvin L, Kestens Y, Dube L. Interactive effects of reward sensitivity and residential fast-food restaurant exposure on fast-food consumption. Am J Clin Nutr. 2010;91:771–776. doi: 10.3945/ajcn.2009.28648. [DOI] [PubMed] [Google Scholar]
- 59.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: Implications for dieting and obesity. Obesity (Silver Spring) 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 60.Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- 61.Mesulam MM. The human frontal lobes: Transcending the default mode through contingent encoding. In: Stuss DT, Knight RL, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. pp. 8–30. [Google Scholar]
- 62.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 63.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 65.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 66.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 67.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am J Clin Nutr. 2006;84:725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 69.Le DS, Pannacciulli N, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 71.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 72.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170:181–192. doi: 10.1093/aje/kwp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ainslie G. Breakdown of Will. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 75.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 76.Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 78.Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- 79.Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 81.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 82.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Brien K, Venn BJ, Perry T, Green TJ, Aitken W, Bradshaw A, Thomson R. Reasons for wanting to lose weight: Different strokes for different folks. Eat Behav. 2007;8:132–135. doi: 10.1016/j.eatbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 85.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54:208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 87.Weller RE, Cook EW, III, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–569. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 88.van den Bos R, de Ridder D. Evolved to satisfy our immediate needs: Self-control and the rewarding properties of food. Appetite. 2006;47:24–29. doi: 10.1016/j.appet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Rollins BY, Dearing KK, Epstein LH. Delay discounting moderates the effect of food reinforcement on energy intake among non-obese women. Appetite. doi: 10.1016/j.appet.2010.07.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phelan S, Liu T, Gorin A, Lowe M, Hogan J, Fava J, Wing RR. What distinguishes weight-loss maintainers from the treatment-seeking obese? Analysis of environmental, behavioral, and psychosocial variables in diverse populations. Ann Behav Med. 2009;38:94–104. doi: 10.1007/s12160-009-9135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gorin AA, Raynor HA, Niemeier HM, Wing RR. Home grocery delivery improves the household food environments of behavioral weight loss participants: Results of an 8–week pilot study. Int J Behav Nutr Phys Act. 2007;4:58. doi: 10.1186/1479-5868-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adam T, Epel E. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ward A, Mann T. Don’t mind if I do: Disinhibited eating under cognitive load. J Pers Soc Psychol. 2000;78:753–763. doi: 10.1037//0022-3514.78.4.753. [DOI] [PubMed] [Google Scholar]
- 95.Lattimore P, Maxwell L. Cognitive load, stress, and disinhibited eating. Eat Behav. 2004;5:315–324. doi: 10.1016/j.eatbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med. 2010;17:161–167. doi: 10.1007/s12529-010-9092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dalle Grave R, Calugi S, Molinari E, Petroni ML, Bondi M, Compare A, Marchesini G QUOVADIS Study Group. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13:1961–1969. doi: 10.1038/oby.2005.241. [DOI] [PubMed] [Google Scholar]
- 98.Fabricatore AN, Wadden TA, Womble LG, Sarwer DB, Berkowitz RI, Foster GD, Brock JR. The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 2007;31:1739–1745. doi: 10.1038/sj.ijo.0803649. [DOI] [PubMed] [Google Scholar]