Abstract
Introduction
We report pharmacokinetic (PK) data, evaluation of modifications for increased stability, evaluation for cellular uptake, and mediation of regression of breast cancer for the aptamer OPN-R3.
Methods
OPN-R3 aptamer was assessed for PK data in vivo with additional comparison of IV and SQ dosing. Five aptamer variants were generated by differential 2’-O-methylation for comparison with parent. OPN-R3-Cy3 was incubated with MDA-MB231 cells and cellular uptake evaluated under confocal microscopy. Mice were treated with OPN-R3, mutant, or saline three weeks after inoculation with MDA-MB231 cells and tumor size was evaluated.
Results
OPN-R3 PK data were: t1/2 7.76 hrs, Tmax 3 hrs, Cmax 13.2 mmol/L, MRT 9 hrs, AUC (0-t) 161.9 mmol-hr/L, Kd 57.2 nM. Half-life was higher when given IV versus SQ (E1/2 7.93 hrs vs 0.74 hrs). 2’ methylation of all available bases increased unmodified aptamer stability and affinity (t1/2 6.2 hrs, Kd 520 nM) but this did not improve on parent aptamer (t1/2 7.78 hours, Kd 18 nM). The aptamer remained extracellular. OPN-R3 caused regression of tumor to levels seen at 1 wk after tumor inoculation.
Conclusions
We show efficacy of OPN-R3 for reversing growth of breast cancer cells with adequate pharmacokinetic stability for clinical application.
Introduction
The development of targeted nucleotide sequences capable of binding small molecule or protein ligands was first described in 1990. The initial experiments involved targeting specific proteins or organic dyes with a small pool of known nucleotide sequences and observing improved affinity after selection, then termed Systematic Evolution of Ligands by Exponential enrichment (SELEX) [1, 2]. Ellington and Szostak were the first to use a completely random oligonucleotide library as the starting point for their iterative evolution of a targeting nucleotide sequence [3]. Also in 1990, Sullenger described the use of such a targeted sequence as a therapeutic agent targeting HIV replication [4]. Since that time, the field of RNA aptamers, a term coined to describe these single stranded oligonucleotides with secondary structure conferring ligand affinity, has quickly blossomed, so much so that at least nine aptamers are currently in clinical trials and many more being developed for future evaluation as potential therapeutic and diagnostic agents [5].
The purpose of this work was to characterize the pharmacokinetic and pharmacodynamic properties of an RNA aptamer against osteopontin (OPN), a bone sialoprotein implicated in a number of benign and malignant functions. Over the past decade, OPN has been increasingly associated with enhanced metastatic phenotypes in a variety of human cancers. It is thought to promote metastatic behavior by promoting extracellular matrix degradation and basement membrane degradation by MMPs (matrix metalloproteases) and uPA and acts via CD44 and αvβ3 cell surface receptors [6]. The aptamer described in this work was originally developed in 2009. Called OPN-R3, this aptamer was developed using eight rounds of SELEX and was further reduced in length from 80 to 40 nucleotides after no difference in affinity was found between original and truncated versions. In addition, a mutated aptamer with the same sequence was generated by mutating the proposed binding site of the functional aptamer based on secondary structure; this mutant aptamer was shown to have poor binding to OPN using gel-shift assays. Pharmacokinetic data was originally assessed for the OPN-R3 aptamer as reported previously, revealing a Kd of 18 nM, an in vitro half life of approximately 8 hours, and a half life in human serum of >24 hours [7]. Here, we tested several variants of the aptamer with differential 2’-O-methylation in an attempt to increase its half-life and binding affinity. We compared the aptamer’s half life in mice between intravenous and subcutaneous injection. We also sought to show that the aptamer was not taken up by target cells, instead maintaining its activity in the extracellular space. Finally, we sought to show that aptamer administration could ameliorate tumor growth in a murine model of human breast cancer.
Materials and Methods
Materials
The OPN-R3 aptamer, mutant, and all test variants were synthesized by Dharmaco, Lafayette, CO. Synthesized OPN-R3 and its mutant OPN-R3-2 with 2’-O-methylated purines, 5’ cholesterol and 3’-IDT modification were used in in-vivo experiments, xenograft model, and intracellular uptake assays. This aptamer has the usual stem-loop structure of other RNA aptamers. OPN-R3 had been previously tested for affinity and specificity for OPN using RNA electrophoretic mobility shift assays and its in vitro half-life and Kd previously reported [7]. Human OPN was obtained from R&D Systems, Minneapolis, MN. All mice were obtained from Jackson Laboratories, Bar Harbor, Maine.
In vivo PK data for OPN-R3
For this determination, 18 6 week old NOD scid mice were used. Mice were injected with 2.2 nM OPN-R3 via tail vein injection and sacrificed at 3, 6, 12, 24, and 48 hours. Blood was harvested and serum obtained by pelleting cellular constituents and recovery of supernatant in the presence of heparin. OPN-R3 concentration was then determined by quantitative RT-PCR.
In vivo comparison of IV versus SQ injection
Twenty-one 6 week old female NOD scid mice were used (3 mice per time point) for these experiments. OPN-R3 was injected into the tail vein or the subcutaneous tissue at the neck at an initial dose of 2.2 nM. Mice were sacrificed and blood harvested at 0, 3, 6, 12, 24, 48, and 72 hours. Serum was harvested by pelleting cellular constituents and recovery of supernatant in the presence of heparin. OPN-R3 concentration was determined by quantitative RT-PCR.
Evaluation of differential 2’-O-methylation
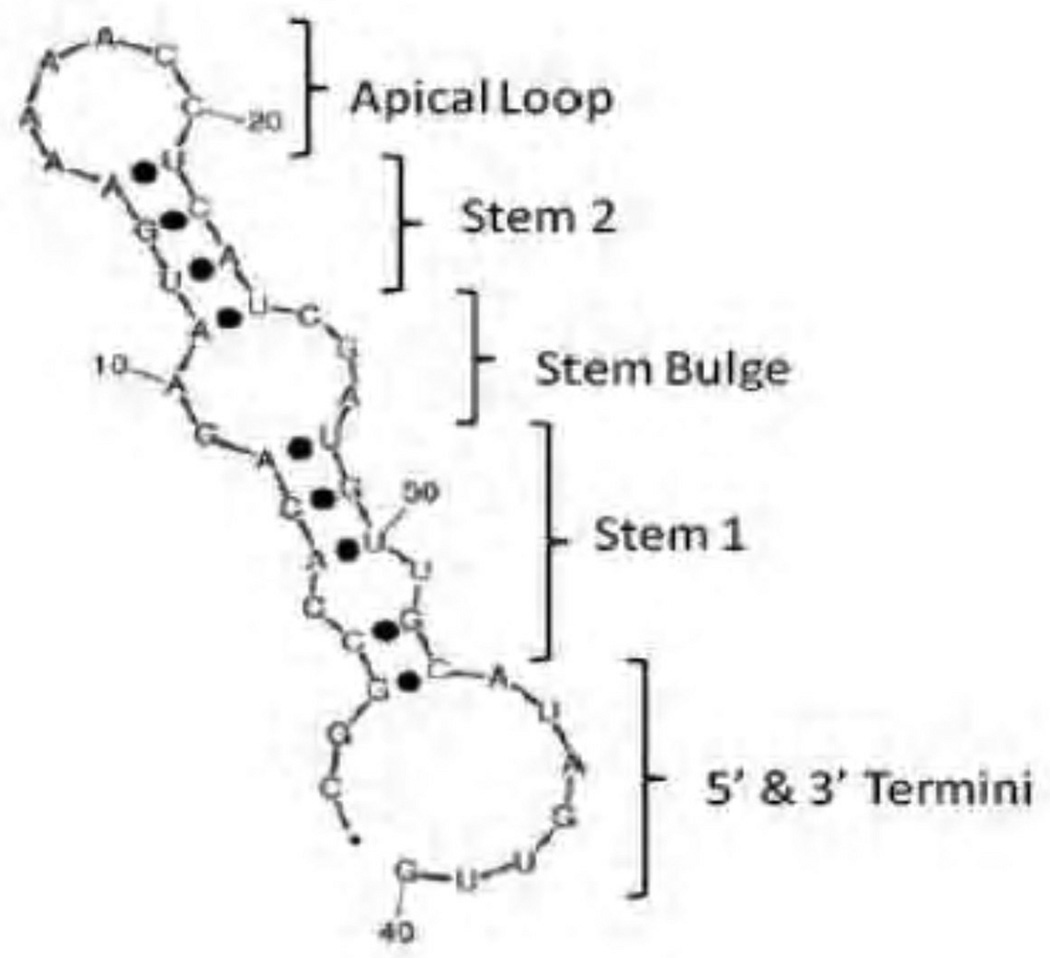
For in-vitro testing of modifications of OPN-R3, five different variants were constructed (Figure 1). Variant 1 was the previously described aptamer. Variant 2 was 2’-O-Me modification of all purines in the stem bulge and apical loop. Variant 3 was 2’-O-Me modification of all purines in only the stem bulge. Variant 4 was 2’-O-Me modification of all purines in only the apical loop. Variant 5 was 2’-O-Me modification of all available bases, purine and pyrimidine, throughout the structure of the aptamer. All five variants contained a cholesterol moiety at the 5’ end and 3’ IDT.
Figure 1. OPN-R3 secondary structure.
Shown are the secondary structure of the OPN-R3 aptamer. Purine 2’-O-methylation was performed in the shown regions as follows: Variant 1) unmodified throughout; variant 2) modified apical loop and stem bulge; variant 3) modification of stem bulge only; variant 4) modification of apical loop only; variant 5) modification of all bases available. All test aptamers contained a 5’ cholesterol moiety and 3’ IDT modification.
In vitro half life determination
Test aptamers were incubated in mouse plasma harvested from NOD scid mice. 0.5 picomoles of each aptamer was incubated with 50 uL of mouse plasma at 37 degrees Celcius in 5% CO2. Samples were taken of the coincubation mixtures at 0, 3, 6, 12, 24, and 48 hours after mixture and test aptamer concentration determined by quantitative RT-PCR.
Binding affinity assays
Test aptamer RNA-protein equilibrium dissociation constants (Kd) were determined by the double-filter nitrocellulose filter binding method [8]. For all binding assays, RNAs were dephosphorylated using bacterial alkaline phosphatase (Invitrogen, Carlsbad, CA) and 5’-end labeled using T4 polynucleotide kinase (New England Biolabs, Beverly, MA) and γ-32P-ATP (MP Biomedicals, Solon, OH). Direct binding assays were carried out by incubating 32P-labeled RNA at a concentration of less than 0.1 nM and target human OPN protein at concentrations ranging from 300 nM to 10 pM in selection buffer at 37°C. The fraction of RNA bound was quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Raw binding data were corrected for nonspecific background binding of radiolabeled RNA to the nitrocellulose filter.
Evaluation of intracellular uptake
MDA-MB231 cells were cultured in Dulbecco modified Eagle’s medium with 10% fetal bovine serum and 1% penicillin and streptomycin. Cultures were incubated at 37 degrees Celcius with 5% CO2. Cells were then washed three times with warmed PBS and harvested in 1% trypsin. 4 × 105 cells were then seeded onto each well of 12 well plates in antibiotic-free Dulbecco modified Eagle’s medium the day before transfection. For positive controls, 20 uM OPN-R3 and 4uL Lipofectamine 2000 (Invitrogen, Frederick, MD) diluted with OptiMEM medium were mixed gently and incubated with cells. Experimental cells were treated with 80uM OPN-R3 and OptiMEM medium only. After 6 hours of incubation, culture medium was changed to Dulbecco modified Eagle’s medium with 10% FBS and cultures were allowed to incubate for 24 hours. Images were taken at 24, 48, and 72 hours using a Leica SP2 confocal microscope.
Xenograft regression model
6 week old female NOD scid mice were obtained from Jackson Laboratories. 1 × 106 MDA-MB231 cells expressing luciferase were suspended in 50% Matrigel-Hank’s balanced salt solution and implanted into the R4 or L4 mammary fat pads. Four mice were used per group. Mice were anesthetized with intraperitoneal ketamine (75 mg/kg) and xylazine (10 mg/kg). Three weeks after inoculation, treatment was initiated with modified OPN-R3, mutant OPN-R3-2 with the same modifications, or saline control via tail vein injection every 2 days. Bioluminescence images were obtained at baseline and every 2 weeks thereafter. For bioluminescent imaging , mice were anesthetized using 3% ketamine to a MAC of 2.5 and treated with 150 mg/kg D-luciferin via intraperitoneal injection. The animals were then placed in a light-tight chamber under nose-cone anesthesia. Reference grayscale imaging was obtained under dim conditions. Ten minutes after luciferin treatment, the animals were imaged using an IVIS 100 Imaging system (Xenogen, Alameda, CA) following the manufacturer’s protocol. Photon expression was assessed over a sixty-second period and total photon counts as well as counts per second were obtained.
Pharmacokinetic and pharmacodynamic calculations were performed using Kinetica software (Adept Scientific). All animal handling and procedures were approved by the Duke University Animal Care and Use Committee.
Results
OPN-R3 In-Vivo Pharmacokinetic Parameters
When tested in vivo, the OPN-R3 aptamer was found to have a half-life of 7.76 hours in serum, a Tmax of 3 hours, a Cmax of 13.2 mmol/L, an MRT of 9 hours, an AUC (0 – t) of 161.9 mmol-hr/L, and a Kd of 57.2 nM (Figure 2). After receiving an original dose of 200ug, the aptamer concentration in mouse serum at 40 hours after injection was found to be 410 nM, which is twenty times the estimated average circulating OPN level in patients with metastatic breast cancer [9].
Figure 2. OPN-R3 in vivo PK parameters.
Mice were injected with OPN-R3 via tail vein injection and serum harvested for OPN-R3 concentration at 0, 3, 6, 12, 24, and 48 hours. Aptamer concentration was determined by RT-PCR. X-axis shows time after injection and Y axis picomolar concentration of OPN-R3. Data points represent average values of assays performed in triplicate.
We further tested the elimination of OPN-R3 when given intravenously as a tail vein injection as compared to subcutaneously through mammary fat pad injection. This revealed that when given IV, the aptamer had an elimination half-life of 7.93 hours, compared to 0.74 hours when injected subcutaneously (Figure 3).
Figure 3. IV versus SQ elimination time of OPN-R3.
PK curves are shown plotting serum OPN-R3 concentration versus time for IV and SQ dosing of OPN-R3. Mice were injected with 2.2 nM OPN-R3 and sacrificed for blood harvest at 0, 3, 6, 12, 24, 48, and 72 hours. IV administration resulted in an elimination half life of 7.93 hours versus 0.74 hours for SQ administration.
OPN-R3 Variants In-Vitro Pharmacokinetic Parameters
Testing of the five OPN-R3 variants in vitro using plasma incubation revealed their half-lives to be between 4.5 – 6.2 hours (Figure 2). Of the five variants, only 2’-O-methylation of all available base sites resulted in a significant increase in half-life from the 4.5 – 4.8 hour range to 6.2 hours.
The 2’-methylation of all available base pairs also resulted in a higher affinity for OPN of the modified aptamer (Kd = 520 nM) compared to the other modified variants (Kds ranging between 1251 and 2262 nM). However, the methylation did result in a lower Kd than the unmodified aptamer (kd = 18 nM).
Intracellular uptake assay
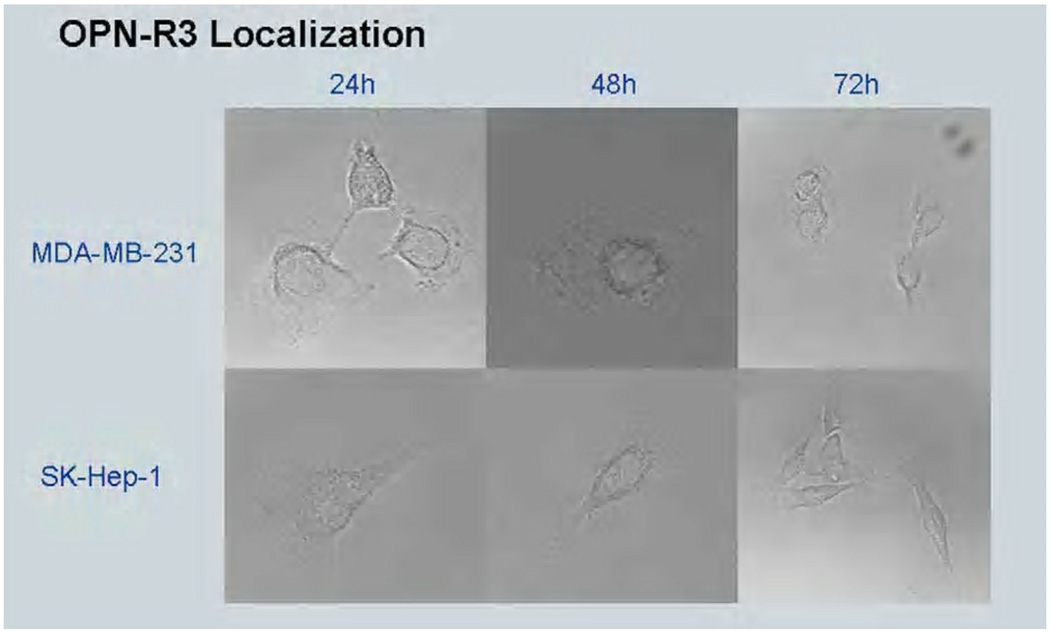
After modifying the variant 5 OPN-R3 aptamer with a Cy3 fluorophore, the aptamer was assessed for cellular uptake. After incubation with MDA-MB231 cells both in the presence of lipofectamine and without, only those cells incubated with OPN-R3 and lipofectamine showed fluorescence after 72 hours (Figure 4). Those cells treated with only the aptamer showed no intracellular fluorescence, indicating that the aptamer is not taken up by MDA-MB231 cells and exerts its effects in the extracellular environment.
Figure 4. In vitro PK data for variant aptamers differentially modified with purine 2’-O-methylation.
Variant aptamers were incubated in mouse serum harvested from NOD-scid mice. 0.5 picomoles of aptamer was incubated with 50 uL mouse serum for each assay. Data points represent assays repeated in triplicate. Corresponding Kds for the variants are: OPN-R3: 18 nM; Test 1: 2262 nM; Test 2: 2258 nM; Test 3: 1251 nM; Test 4: 1657 nM; Test 5: 520 nM.
In vivo breast cancer xenograft model
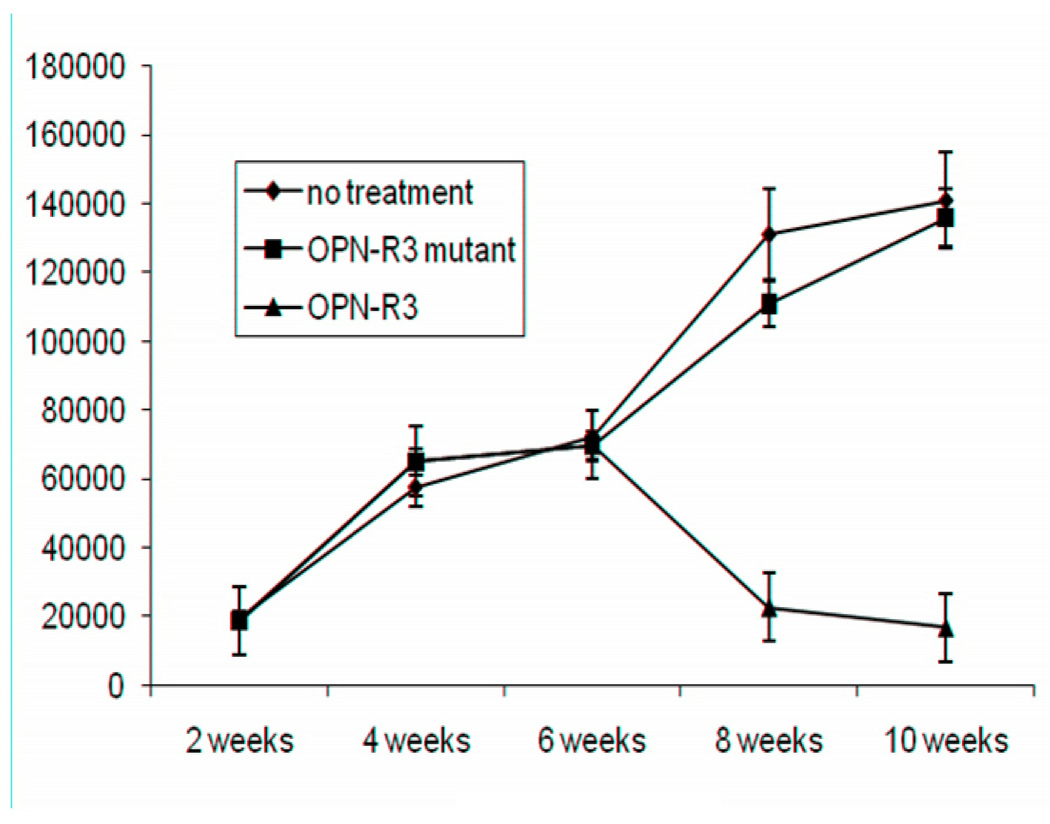
We used a xenograft model of MDA-MB231 breast cancer cells injected into the mammary fat pads of 6 week old NOD scid mice. The MDA-MB231 cells had been previously engineered to express luciferase. The modified OPN-R3 aptamer, mutant nonfunctional aptamer or saline control was given via tail vein injection every two days, beginning 3 weeks after tumor inoculation to allow establishment and growth. After two weeks of treatment, tumor size, as assessed by bioluminescence, had regressed to approximately one-fourth peak size in the OPN-R3 treated group, while the tumor size continued to increase in both the mutant-aptamer and saline treated control groups (Figure 5).
Figure 5. Demonstration of lack of intracellular uptake of OPN-R3.
Shown are MDA-MB231 breast cancer cells and SK-hep-1 hepatocellular carcinoma cells under confocal microscopy. Figure 5a: Positive controls were incubated with Cy3-tagged OPN-R3 in presence of lipofectamine. Figure 5b: Experimental cells were incubated with Cy3-tagged OPN-R3 alone. Cy3 fluorescent signal reveals intracellular uptake only in presence of lipofectamine.
Discussion
We have here demonstrated that the OPN aptamer OPN-R3 has an in vivo half life of 7.8 hours and Kd of 57.2 nM. The pharmacokinetic data presented here suggest that the unmethylated OPN-R3 aptamer in has a half life of 4.6 hours in vitro in mouse plasma, associated with a Kd of 2262 nM, which is at the higher end of the range for aptamer Kds that have previously been reported. After modification with 2’-O-methylation of all purine base pairs, addition of a 5’ cholesterol moiety, and addition of an inverted IDT at the 3’ end of the oligonucleotide, the aptamer has a 7.8 hour half life in mouse serum with a Kd of 57.2 nM, indicating improved stability and affinity for OPN. The modification of the aptamer with 2’-O-methylation appears to significantly increase the half life of the aptamer while maintaining acceptable aptamer affinity. However, any further or different modification in the pattern of base methylation appears to have a deleterious effect on the aptamer’s affinity and stability. Furthermore, these data suggest that the aptamer has a much higher elimination time when given via IV injection as opposed to SQ, with an elimination time nearly ten times longer in the IV treated mice. We demonstrate that the aptamer is not taken up by breast cancer cells, meaning that it only binds extracellular OPN and therefore must exert its effect by blockade of signaling rather than by intracellular mechanisms. We also show that treatment with the aptamer can induce regression in a xenograft model of human breast cancer. This has not been previously reported as a consequence of OPN blockade in breast cancer.
As previously discussed, OPN-induced extracellular matrix and basement membrane degradation appear to mediate its prometastatic behavior. We have shown that OPN blockade in addition changes protein expression in a number of signaling pathways related to apoptosis and proliferation in in-vivo studies. Downregulated pathways included VEGF, PDGF, IL-10, and anti-apoptotic signaling (β-catenin, BCL-2 like protein), while upregulated pathways included apoptosis (CAMK2A), GM-CSF, antiproliferative (BTG-3b) and antimetastatic (CD82) signaling [10]. This data provides a likely mechanism whereby OPN blockade reduces not only metastatic activity but reduces production of proteins involved in cellular growth and proliferation, providing a possible mechanism by which OPN blockade can reduce primary tumor growth.
Further determination of the aptamer’s pharmacokinetic and pharmacodynamic properties will be required in higher-order systems before its full potential as a therapeutic agent can be investigated. Unpublished data indicates a preliminary half life in human serum of greater than 24 hours for OPN-R3 [7]. We anticipate that serum half life will increase in accordance with size when the aptamer is tested in higher order animals and finally in humans. If this is the case, it may be possible to derive a favorable dosing interval of several days prior to initiation of phase I clinical evaluation.
Those aptamers currently in clinical trials or therapeutic use have been administered via IV, SQ, and intravitreal dosing. For our purposes, only the IV and SQ routes are relevant. Aptamers appear to be absorbed from the SQ space slowly and with low bioavailability [11], which may reduce their detection in plasma and explain the shorter elimination time seen in our work. As the transition is made to potential human testing, it will be useful to determine if further modifications (such as PEGylation) will result in a favorable half life when dosed subcutaneously, in order to facilitate the ease of use of the aptamer as a therapeutic agent. It may be possible to develop aptamer modification that allows dosing as a depot injection with slow release from the SQ space if adequate bioavailability can be achieved.
We have previously reported that treatment with this aptamer resulted in reduction of breast cancer growth and metastasis in a similar xenograft model to that used here [7]. These data also suggest that the aptamer may be useful in a number of settings in breast cancer therapy, including in adjuvant and neoadjuvant settings.
Nucleic acid aptamers, based on both RNA and DNA, have a number of characteristics making them attractive therapeutic and diagnostic agents for a number of diseases and applications. They are small, non-immunogenic molecules with a high affinity and specificity for target ligands, with dissociation constants ranging from the very low picomolar range to 500 nanomolar range with a few exceptions [12]. They are sensitive for ligands even in the nanomolar to picomolar concentration. They are synthesized chemically rather than biologically, making synthesis cheaper, faster, and more reliable than that of antibodies and other biologic therapies. In addition, they are easily modified during the process of synthesis. Because they are single stranded sequences, opposing sequences can be easily designed based on Watson-Crick base pairing, leading to highly effective and nontoxic antidotes for therapeutic aptamers. Their activity is also titratable based on concentration in the system of interest.
Aptamers are currently in development to fulfill a variety of functions. They have been extensively evaluated as the basis of new diagnostic modalities, including detection of serum metabolites, molecules, pathogens, and endogenous cell types; cell sorting and recovery for research and diagnostic purposes; cellular homing for imaging purposes; and as tools for new methods of protein and nucleic acid sequencing. In general these “aptasensors” and other modalities involve conjugation of the aptamer to an electrochemoluminescent reporter, nanoparticles, or magnetic cell sorting systems [13–15]. From a therapeutic standpoint, aptamers have been generated against a variety of human proteins for the treatment of both benign and malignant conditions. They have been used to prevent adhesion and aggregation of platelets for anticoagulation [16], diseased cells in sickle cell disease [17], and targeting of amyloid in Alzheimer’s [18] to name a few. Significant progress has been made in targeting factor IXa for specific and easily reversible anticoagulation using the aptamer/antidote pair REG1 [19]. Von Willebrand factor has also been successfully targeted [16]. For treatment of malignant processes, aptamers have been designed against tumor-expressed and associated proteins including PSMA [20], BCL [21], nucleolin [22], and E-selectin [23] to name only a few. In addition, these aptamers have been conjugated to therapeutic agents including chemotherapy drugs [24] and photodynamic cytocidal mechanisms [25] in order to deliver targeted tumoricidal activity. Several advances have already been made over the initially described SELEX procedure, including primer free synthesis to reduce noise in the oligonucleotide pool [26], development of protocols for performing SELEX in cell lysate [27], against live cells [28], or even in vivo for targeting specific tumors [29], and development of high-output, microfluidic, rapid synthesis methods that reduce the aptamer identification time from several weeks to less than one week [30]. These advances in our knowledge of aptamer function, therapeutic and diagnostic applications, and toxicology, as well as advances in manufacture, are likely to ensure that aptamers as diagnostic and therapeutic tools will be clinically relevant for the forseeable future.
Figure 6. Xenograft regression model of human breast cancer.
NOD-scid mice were inoculated with MDA-MB231 cells and tumors allowed to grow for three weeks. At that time, treatment with OPN-R3, mutant OPN-R3, or saline control was initiated on an every-other-day dosing schedule. Bioluminescence photographs were taken every 2 weeks subsequently. Data points represent average of luminescent signal for five mice per treatment group. X-axis represents time in weeks and Y-axis luminescent signal in photons per second. OPN-R3 treated mice showed regression in tumor size to approximately one-quarter the peak size to stabilize at sizes near that seen at 2 weeks after inoculation. Control groups showed no amelioration of tumor growth.
Acknowledgements
This work has been funded by the following NIH grants: R01-GM065113-05A2, T32-GM069331, and UL1-RR024128. In addition we would like to thank Mark Dewhirst, Duke University, NC, for his gift of luc-labelled MDA-MB231 breast cancer cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344(6265):467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 2.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 3.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 4.Sullenger BA, et al. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990;63(3):601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- 5.Bunka DH, Platonova O, Stockley PG. Development of aptamer therapeutics. Curr Opin Pharmacol. 2010;10(5):557–562. doi: 10.1016/j.coph.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27(1):103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 7.Mi Z, et al. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17(1):153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mi Z, et al. Thrombin-cleaved COOH(−) terminal osteopontin peptide binds with cyclophilin C to CD147 in murine breast cancer cells. Cancer Res. 2007;67(9):4088–4097. doi: 10.1158/0008-5472.CAN-06-4066. [DOI] [PubMed] [Google Scholar]
- 9.Bramwell VH, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12(11 Pt 1):3337–3343. doi: 10.1158/1078-0432.CCR-05-2354. [DOI] [PubMed] [Google Scholar]
- 10.Mi Z, Guo H, Kuo PC. Identification of osteopontin-dependent signaling pathways in a mouse model of human breast cancer. BMC Res Notes. 2009;2:119. doi: 10.1186/1756-0500-2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard PR, Hutabarat RM, Thompson KM. Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol. 2010;50:237–257. doi: 10.1146/annurev.pharmtox.010909.105547. [DOI] [PubMed] [Google Scholar]
- 12.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soontornworajit B, Wang Y. Nucleic acid aptamers for clinical diagnosis: cell detection and molecular imaging. Anal Bioanal Chem. 2010 doi: 10.1007/s00216-010-4559-x. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Yang X, Wang E. Review: Aptamers in microfluidic chips. Anal Chim Acta. 2010;683(1):12–20. doi: 10.1016/j.aca.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Battig MR, Wang Y. Aptamer-based molecular recognition for biosensor development. Anal Bional Chem. 2010;398(6):2471–2480. doi: 10.1007/s00216-010-3987-y. [DOI] [PubMed] [Google Scholar]
- 16.Povsic TJ, et al. Translating nucleic acid aptamers to antithrombotic drugs in cardiovascular medicine. J Cardiovasc Transl Res. 2010;3(6):704–716. doi: 10.1007/s12265-010-9230-6. [DOI] [PubMed] [Google Scholar]
- 17.Gutsaeva DR, et al. Inhibition of cell adhesion by anti-P-selectin aptamer: a new potential therapeutic agent for sickle cell disease. Blood. 2010 doi: 10.1182/blood-2010-05-285718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimi F, Bitan G. Selection of aptamers for amyloid beta-protein, the causative agent of Alzheimer's disease. J Vis Exp. 2010;(39) doi: 10.3791/1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen MG, et al. First clinical application of an actively reversible direct factor IXa inhibitor an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation. 2010;122(6):614–622. doi: 10.1161/CIRCULATIONAHA.109.927756. [DOI] [PubMed] [Google Scholar]
- 20.Min K, et al. Dual-aptamer-based delivery vehicle of doxorubicin to both PSMA (+) and PSMA (−) prostate cancers. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Mallikaratchy PR, et al. A multivalent DNA aptamer specific for the B-cell receptor on human lymphoma and leukemia. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe T, et al. Nucleolin on the cell surface as a new molecular target for gastric cancer treatment. Biol Pharm Bull. 2010;33(5):796–803. doi: 10.1248/bpb.33.796. [DOI] [PubMed] [Google Scholar]
- 23.Mann AP, et al. Identification of thioaptamer ligand against E-selectin: potential application for inflamed vasculature targeting. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taghdisi SM, et al. Reversible Targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur J Pharm Biopharm. 2010 doi: 10.1016/j.ejpb.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Stephanopoulos N, et al. Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano. 2010;4(10):6014–6020. doi: 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]
- 26.Pan W, et al. Primer-free aptamer selection using a random DNA library. J Vis Exp. 2010;(41) doi: 10.3791/2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanoatov M, Javaherian S, Krylov SN. Selection of aptamers for a non-DNA binding protein in the context of cell lysate. Anal Chim Acta. 2010;681(1–2):92–97. doi: 10.1016/j.aca.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi HP, Smiley RD, Jaykus LA. Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Appl Microbiol Biotechnol. 2010;87(6):2323–2334. doi: 10.1007/s00253-010-2728-7. [DOI] [PubMed] [Google Scholar]
- 29.Mi J, et al. In vivo selection of tumor-targeting RNA motifs. Nat Chem Biol. 2010;6(1):22–24. doi: 10.1038/nchembio.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho M, et al. Quantitative selection of DNA aptamers through microfluidic selection and high-throughput sequencing. Proc Natl Acad Sci U S A. 2010;107(35):15373–15378. doi: 10.1073/pnas.1009331107. [DOI] [PMC free article] [PubMed] [Google Scholar]