Abstract
Purpose
A 70-gene prognostic signature has prognostic value in patients with node-negative breast cancer in Europe. This diagnostic test known as "MammaPrint™ (70-gene prognostic signature)" was recently validated and implementation was feasible. Therefore, we assessed the 70-gene prognostic signature in Korean patients with breast cancer. We compared the risk predicted by the 70-gene prognostic signature with commonly used clinicopathological guidelines among Korean patients with breast cancer. We also analyzed the 70-gene prognostic signature and clinicopathological feature of the patients in comparison with a previous validation study.
Methods
Forty-eight eligible patients with breast cancer (clinical T1-2N0M0) were selected from four hospitals in Korea. Fresh tumor samples were analyzed with a customized microarray for the 70-gene prognostic signature. Concordance between the risk predicted by the 70-gene prognostic signature and risk predicted by commonly used clinicopathological guidelines (St. Gallen guidelines, National Institutes of Health [NIH] guideline, and Adjuvant! Online) was evaluated.
Results
Prognosis signatures were assessed in 36 patients. No significant differences were observed in the clinicopathological features of patients compared with previous studies. The 70-gene prognosis signature identified five (13.9%) patients with a low-risk prognosis signature and 31 (86.1%) patients with a high-risk prognosis signature. Clinical risk was concordant with the prognosis signature for 29 patients (80.6%) according to the St. Gallen guidelines; 30 patients (83.4%) according to the NIH guidelines; and 23 patients (63.8%) according to the Adjuvant! Online. Our results were different from previous validation studies in Europe with about a 40% low-risk prognosis and about a 60% high-risk prognosis. The high incidence in the high-risk group was consistent with data in Japan.
Conclusion
The results of 70-gene prognostic signature of Korean patients with breast cancer were somewhat different from those identified in Europe. This difference should be studied as whether there is a gene disparity between Asians and Europeans. Further large-scale studies with a follow-up evaluation are required to assess whether the use of the 70-gene prognostic signature can predict the prognosis of Korean patients with breast cancer.
Keywords: Breast neoplasms, Gene expression profiling, Korea
INTRODUCTION
Breast cancer is the most common fatal cancer in Korean women [1]. The recurrence rate of breast cancer is relatively high, at 20-30%, depending on stage, despite its relatively high 5-year survival rate [2]. Therefore, it is important to predict the risk of recurrence and administer adjuvant chemotherapy to high risk patients.
Adjuvant systemic treatment decreases the risk of developing distant metastases and death by about 30% in patients with node-negative breast cancer, but 70% of patients with node negative breast cancer will experience toxicity without benefit from such treatment [3,4]. Despite this observation, most of these patients are still considered candidates for chemotherapy. Several commonly used guidelines have been developed based on clinicopathological factors: lymph-node status, age, tumor size, histological grade, and estrogen receptor (ER) status. However, these factors do not accurately predict the exact clinical outcomes. In Korea, patients with node-negative breast cancer comprise about 65% of all patients with breast cancer [5]. Therefore, new prognostic markers are needed to reduce overtreatment with its unnecessary exposure to toxicity. One of the new prognostic markers is the 70-gene prognostic signature (MammaPrint™; Agendia, Amsterdam, Netherlands). Analysis of gene expression patterns has led to the discovery of this prognostic gene signature [6]. The 70-gene prognostic signature has been validated in a number of European patient series [7-10]. But, there is only one Japanese study [11] about a 70-gene prognostic signature in Asians and no Korean patients with breast cancer. Thus, our study is the first about the 70-gene prognostic signature in Korea.
We assessed the 70-gene prognostic signature in Korean patients with breast cancer. We compared the risks predicted by the 70-gene prognostic signature with commonly used clinicopathological guidelines among Korean patients with breast cancer. We also analyzed the 70-gene prognostic signature and clinicopathological features of patients compared with a previous validation study.
METHODS
Patients
This study was conducted using tumor samples and clinical data from 48 patients at four different hospitals in Korea: Ajou University Hospital, Suwon; Samsung Medical Center, Seoul; Asan Medical Center, Seoul; and Ewha Womans' University Mokdong Hospital, Seoul. The patients were selected according to the following criteria: they were diagnosed between March 2008 and September 2009, they had a clinically unilateral T1 or T2 (tumor size ≤5 cm) tumor that was node-negative, and they had not received neoadjuvant therapy. Patients with previous malignancies or with bilateral synchronous breast tumors were excluded. All patients were told about the 70-gene prognostic signature and voluntarily agreed to take the test. This retrospective study was approved by principal investigator's institutional review board (Accept No. AJIRB-MED-MDB-10-228). Memorandum of Understanding was concluded between other institutions. The clinicopathological data were collected from the medical records, histologic tumor grading was defined according to Elston and Ellis [12]. The ER and progesterone receptor (PR) status were determined by immunohistochemistry, which was interpreted as positive if more than 10% of the tumor cell nuclei stained positive. The erb-B2 receptor status was assessed by the intensity of the membrane staining using immunohistochemistry and fluorescent in situ hybridization. Tumors with a score of 3+ (strong homogeneous staining) were considered HER2-positive. In case of 2+ scores (moderate homogeneous staining) fluorescent in situ hybridization (FISH) was used to determine amplification [13].
Tumor samples
Because collecting tumor tissue from surgical specimens is not a standard procedure, patient permission and informed consents were obtained before surgery. Within 1 hour after surgery, a tumor sample was taken for a quality control check of the RNA. For this purpose, a punch biopsy device was used to obtain a tumor sample with a minimum thickness of 3 mm and a maximum thickness of 5 mm. The samples were stored in a container with RNA stabilizing solution (such as RNARetain; Asuragen, Austin, USA) directly after their removal, and the samples were sent to Agendia's laboratories for RNA extraction and microarray analysis, as previously described [6]. Samples were available for RNA extraction if they contained at least 30% tumor cells on the hematoxylin-eosin stained sections. Useful RNA could be extracted for hybridization and analysis from 75.0% of the tumor samples, so 38 samples were available for the analysis. Ten samples were rejected based on having less than 30% invasive tumor cells in the samples or an insufficient quality of tumor RNA.
Gene expression analysis
To assess the mRNA expression level of the 70 genes, the RNA was hybridized to a custom-designed array (MammaPrint™, FDA 510(k) cleared) at Agendia Laboratories, and the personnel were 'blinded' to the clinical data. The tumors were classified only according to a dichotomized value: a low-risk prognostic signature or a high-risk prognostic signature. A tumor was defined as having a low-risk prognostic signature if the Pearson's correlation coefficient for the expression of the 70-gene prognostic signature in that tumor with the previously established classifier was greater than 0.4, which is the cut-off point we used, as described previously [6,7,10,14]. The previously established classifier defined a good prognosis, as a more than 90% probability for 5-year distant metastasis-free survival for node-negative patients [6].
Clinical risk classification
According to the St. Gallen guideline, a low clinical risk was defined as possessing all of the following criteria: an ER positive or/and PR positive status, a tumor size of 2 cm or less, histological grade 1, and an age of 35-year or above [15]. The National Institutes of Health (NIH) guideline recommends that the low-risk group in the node negative group had a tumor size smaller than 1 cm and a favorable histological subtype such as tubular and mucinous cancer [16,17].
The Adjuvant! Online software version 8.0 (www.adjuvantonline.com) calculates the 10-year survival probability based on patient age, tumor size and grade, ER-status, nodal status, and co-morbidities [18,19]. The low-clinical risk group for this calculation was defined as patients with 10-year overall survival probabilities of at least 88% in ER-positive tumors and of at least 92% if ER expression was seen in ER-negative tumors [20].
Statistical analysis
Analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). The p-value was two-sided. Differences between the groups of interest were tested with the Pearson χ2 test or the Fisher's exact test. A p-value <0.05 was considered significant.
RESULTS
Of the 48 eligible patients, 12 were excluded because of sampling failure (n=10) or lymph node metastasis on permanent pathological findings (n=2). A prognostic signature was obtained for the remaining 36 patients.
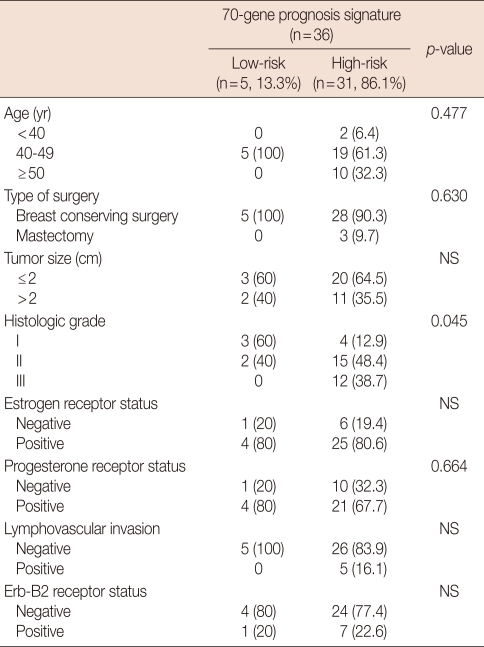
Five of the 36 (13.9%) patients had a low risk prognostic signature and 31 (86.1%) patients had a high-risk prognostic signature. The mean age of the eligible patients was 47 years (median, 45; range, 23-68), and the mean tumor diameter was 2.0 cm (median, 1.8; range, 0.3-5.1). No significant difference in age, operative method, tumor size, ER status, the PR status, lymphovascular invasion, and erb-B2 receptor status between the low-risk prognostic signature group and the high-risk prognostic signature group was observed. Only histological grade was significantly different between the low and high-risk prognostic signature groups (p=0.045). A low-risk signature was not found for tumors with lympovascular invasion, for erb-B2 receptor positivity, and those with a poor histological grade. The median tumor diameter was 1.9 cm (range, 0.3-4.5 cm) in the high-risk group and 1.6 cm (range, 0.5-5.1 cm) in the low-risk group (Table 1).
Table 1.
Characteristics of the patients and their tumor for the analysis of the 70-gene prognostic signature
NS=not significant.
Data are presented as number (%).
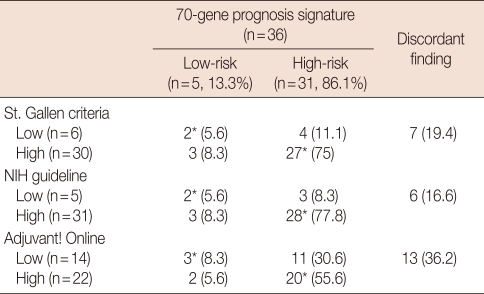
According to the St. Gallen criteria, 30 of 36 (83.3%) patients were at a high-risk, according to the NIH guideline, 31 of 36 (86.1%) patients were at a high-risk, and according to the Adjuvant! Online, 22 of 36 (61.2%) patients were at a high-risk. The clinical risk for seven patients (19.4%), six patients (16.6%) and 13 patients (36.2%) was discordant with using the prognostic signature according to the St. Gallen criteria, the NIH guideline and Adjuvant! Online, respectively (Table 2).
Table 2.
Concordances between the 70-gene prognostic signature and the risk assessment according to the other clinicopathological risk indexes
NIH=National Institute of Health.
Data are presented as number (%).
*These values were summed to obtain concordant findings.
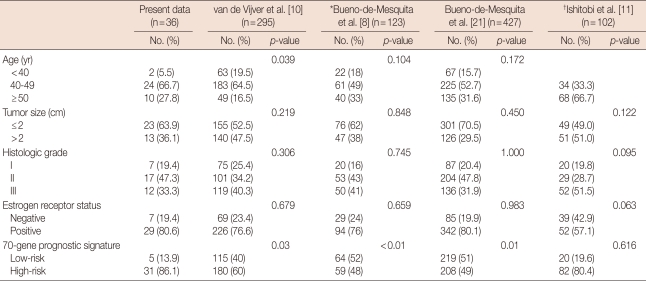
We compared our results with those of previous validation studies[7,8,10,13,21]. Table 3 shows the clinicopathological characteristics and results of the 70-gene prognostic signature derived from previously published studies. Except for patient age in one validation study and 70-gene prognostic signature results, the cinicopathological factors of patients were not significantly different between previous studies and our study.
Table 3.
Comparison of the clinicopathologic features of the previous validation studies
*This study classified age according to <40, 41-50, 51-60; †This study classified of age according to <50, ≥50.
The previous published studies in Europe, but not the data released in Japan, showed a similar pattern. In the European studies, 36.8-52% of the patients were classified in a low-risk group, and 49-63.2% were in the high-risk group. In a study published in Japan, 19.6% of the patients were in the low-risk group and 80.4% were in the high-risk group.
DISCUSSION
A 70-gene prognostic signature was identified by performing gene expression profiling on 78 patients with breast cancer at the Netherlands Cancer Institute [6]. Subsequently, the 70-gene signature was then applied to a large test set of 295 patients with breast cancer from the same institution, and the results confirmed that the signature could classify patients according to 10-years survival outcome [10]. This signature has been validated in many studies as a more powerful prognostic factor for distant metastasis and death than the current clinicopathological factors. These studies verified the molecularly identified patients with biologically less aggravated cancer [7,8,13,21]. Many recent studies have shown that this signature can be used to classify postmenopausal women (older age women), neoadjuvant chemotherapy patients and node-positive patients [9,22,23].
We studied the 70-gene prognostic signature in Korean patients with breast cancer who had a median age of 45-years with node-negative, clinical T1-T2 breast cancer. The 70-gene prognostic signature displayed a remarkably high-risk group in our patients (13.9% of the low-risk group, 86.1% of the high-risk group). No significant differences in age, tumor size, and ER status between the low-risk group and the high-risk group were found. Only histological grade was significantly different between the low and high-risk prognostic signature groups.
In our report, the proportion of tumors with a low-risk prognosis signature was 13.9%, which is less than the percentage (36.8-52%) reported in previous validation studies in Europe [8,10,13,21]. Yet the results released in Japan (19.6%) are similar to our results for the high-risk group [11]. No significant differences in tumor size, histological grade, and the ER status between our patients and the patients of the previous validation studies in Europe were found. Except for one validation study [10], patient age also was not significantly different. Nevertheless, our results are different from the previous validation studies in Europe showing that about 40% of the European patients had a low-risk prognosis and about 60% had a high-risk prognosis. In Europe, incidence rate of breast increase with age and the highest rates occur in the oldest age group but incidence rate of breast cancer in Korea is the highest in the 40s [1,5,24]. Furthermore, 70-gene prognostic signature is a prediction model developed by extracting candidate genes in 5,000 genes from Europeans [6]. So the causes for difference between our results and those of European data need further study, but we think that racial differences had some effects on the study cohort differences.
The clinical utility of the 70-gene prognostic signature depends on its potential value in addition to using traditional prognostic factors. Therefore, we compared the signature's risk assessment to the clinicopathological risk assessment using the St. Gallen criteria, the NIH guidelines, and Adjuvant! Online. Overall, as compared with that of the clinicopathological risk guidelines, about one fifth of the patients were discordant with the 70-gene prognostic signature. (Discordance of 19.4% compared to the St. Gallen criteria, 16.6% compared to the NIH guideline, and 36.2% compared to Adjuvant! Online) In a previous study, the discordant rate between the 70-gene prognostic signature and other clinicopathological guidelines was 28.3-39% [7,19]. A discordant rate is not mentioned in many of the previous validation studies, and a comparison between the 70-gene prognostic signature and the clinicopathological guidelines indicates that this signature is a more powerful predictor of disease outcome than the currently used clinicopathological guidelines [7,9,10,19]. Therefore, these discordant rates indicate that using this signature could result in a substantial reduction of the number of patients who would be recommended for chemotherapy. But, our results of a low incidence for the low-risk group indicate that there are fewer Korean patients that would benefit from this prognostic signature than those in Europe. Moreover, because our study did not assess the overall survival rate and recurrence rate, it is unclear whether the 70-gene prognostic signature is purely prognostic or predictive for treating this group in Korea.
This study had several limitations. First, our sample size was relatively small, suggesting that a larger study may be able to define whether or not the 70-gene prognostic signature can identify patients with node negative breast cancer and who have an excellent disease outcome in Korea. Second, no assessment was made after the follow-up period (this is a preliminary report involving patients who were diagnosed within 1 year), and a follow-up evaluation may be required to determine whether the 70-gene prognostic signature has prognostic value compared with the clinicopathological guidelines. If further prospective randomized trials confirm the prognostic and predictive values of the 70-gene prognostic signature in Korea as compared with that for the conventional clinicopathological guidelines, then this signature may become a powerful prognostic tool for Korean patients with breast cancer to administer chemotherapy to properly selected patients.
The results of the 70-gene prognostic signature of Korean patients with breast cancer were different from those from Europe. These differences should be studied as to whether the gene disparity between Asians and Europeans influenced the results. Further large-scale studies with a follow-up evaluation are required to assess whether the use of the 70-gene prognostic signature can predict a prognosis for Korean patients with breast cancer.
Footnotes
The authors declare that they have no competing interests.
References
- 1.National Cancer Center; Korea Central Cancer Registry. 2006 Annual Report of the Korea Central Cancer Registry. Seoul: Ministry of Health and Welfare; 2007. pp. 1–114. [Google Scholar]
- 2.Korea Breast Cancer Society. Breast Cancer Facts and Figures 2006-2008. Seoul: Korean Breast Cancer Society; 2008. [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 5.Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2004 using breast canser registration program. J Breast Cancer. 2006;9:151–161. [Google Scholar]
- 6.van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 7.Buyse M, Loi S, van't Veer L, Viale G, Delorenzi M, Glas AM, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 8.Bueno-de-Mesquita JM, Linn SC, Keijzer R, Wesseling J, Nuyten DS, van Krimpen C, et al. Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat. 2009;117:483–495. doi: 10.1007/s10549-008-0191-2. [DOI] [PubMed] [Google Scholar]
- 9.Mook S, Schmidt MK, Viale G, Pruneri G, Eekhout I, Floore A, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1-3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat. 2009;116:295–302. doi: 10.1007/s10549-008-0130-2. [DOI] [PubMed] [Google Scholar]
- 10.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 11.Ishitobi M, Goranova TE, Komoike Y, Motomura K, Koyama H, Glas AM, et al. Clinical utility of the 70-gene MammaPrint profile in a Japanese population. Jpn J Clin Oncol. 2010;40:508–512. doi: 10.1093/jjco/hyp195. [DOI] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 13.Hauser-Kronberger C, Dandachi N. Comparison of chromogenic in situ hybridization with other methodologies for HER2 status assessment in breast cancer. J Mol Histol. 2004;35:647–653. doi: 10.1007/s10735-004-2186-6. [DOI] [PubMed] [Google Scholar]
- 14.Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer. November 1-3, 2000. J Natl Cancer Inst Monogr. 2001;(30):5–15. [PubMed] [Google Scholar]
- 17.Hellekson KL. NIH statement on adjuvant therapy for breast cancer. Am Fam Physician. 2001;63:1857–1855. 1861. [PubMed] [Google Scholar]
- 18.Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 19.Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 21.Bueno-de-Mesquita JM, van Harten WH, Retel VP, van't Veer LJ, van Dam FS, Karsenberg K, et al. Use of 70-gene signature to predict prognosis of patients with node-negative breast cancer: a prospective community-based feasibility study (RASTER) Lancet Oncol. 2007;8:1079–1087. doi: 10.1016/S1470-2045(07)70346-7. [DOI] [PubMed] [Google Scholar]
- 22.Wittner BS, Sgroi DC, Ryan PD, Bruinsma TJ, Glas AM, Male A, et al. Analysis of the MammaPrint breast cancer assay in a predominantly postmenopausal cohort. Clin Cancer Res. 2008;14:2988–2993. doi: 10.1158/1078-0432.CCR-07-4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straver ME, Glas AM, Hannemann J, Wesseling J, van de Vijver MJ, Rutgers EJ, et al. The 70-gene signature as a response predictor for neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2010;119:551–558. doi: 10.1007/s10549-009-0333-1. [DOI] [PubMed] [Google Scholar]
- 24.Botha JL, Bray F, Sankila R, Parkin DM. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718–1729. doi: 10.1016/s0959-8049(03)00118-7. [DOI] [PubMed] [Google Scholar]