Abstract
Purpose
The aim of this retrospective study was to identify the reliable long term prognostic factors in patients with stage II/III breast cancer who were treated with an adjuvant extension of neoadjuvant chemotherapy (NC).
Methods
Women under the age of 70-years, with previously untreated clinical stage II and III breast cancer, were treated with NC, which was comprised of three cycles of FEC (5-FU, epirubicin, and cyclophosphamide every 3 weeks) or MMM (methotrexate, mitoxantrone, and mitomycin-C every 3 weeks) with an adjuvant extension of three cycles of the same regimen.
Results
Cumulative 10-years disease-free survival (DFS) was 87.3% for patients with a good response and 55.5% for patients with no response (p=0.032); 92.9% for node negative patients, 75.0% for 1-3 positive nodes, 50.0% for 4-9 positive nodes and no survival for 10 or more positive nodes (p<0.001). Cumulative 10-years overall survival (OS) was 89.1% for patients with good response and 55.5% for patients with no response (p=0.024); 95.2% for node negative patients, 80.0% for 1-3 positive nodes, 50.0% for 4-9 positive nodes and no survival for 10 or more positive nodes (p<0.001). No significant difference was observed in DFS and OS between the FEC and MMM treated groups.
Conclusion
Based on a review of data with a long follow-up, only the clinical response to NC and the absolute number of metastatic axillary lymph node identified at surgical staging were independent predictors of both DFS and OS in patients with stage II/III breast cancer patients treated with adjuvant extension of NC.
Keywords: Breast neoplasms, Neoadjuvant therapy, Prognosis
INTRODUCTION
Neoadjuvant chemotherapy (NC) has traditionally been regarded as the standard for care in patients with locally advanced breast cancer with the purpose of achieving both local and systemic control [1-4]. In recent years, with the advent of more effective chemotherapeutic drugs, NC is being administered to patients with operable breast cancer with the aim of breast conservation after shrinkage of the primary tumor [5,6]. Another advantage of NC is that it can be used as an in vivo chemosensitivity test, allowing for a rapid individual evaluation of a regimen [7]. Local clinical response to NC might also be a predictor of response to distant micrometastases and eventual outcome. Prognostic factors are important for clinical decision making and for tailoring treatment of individual patients with cancer. Axillary lymph node (ALN) status is the single most reliable prognostic indicator of early breast cancer, followed by primary tumor size. However, downstaging of both the primary tumor and ALN involvement by NC results in a loss of traditional prognostic factors. With increased use of NC, outcomes must be predicted to provide tailored treatment for patients treated with NC. Only a few studies have investigated concerning the prognostic factors related to long term survival in patients with operable breast cancer treated with NC. In 2000, the results of a phase II trial were published for 73 patients with stage II/III breast cancer, comparing FEC (5-FU, epirubicin, and cyclophosphamide) and MMM (mitoxantrone, methortexate, and mitomycin) in the Journal of the Korean Surgical Society [8]. The aim of this retrospective study was to identify the reliable long-term prognostic factors in patients with stage II/III breast cancer treated with an adjuvant extension of NC.
METHODS
Eligibility criteria
Women under the age of 70-years with previously untreated clinical stage II and III breast cancer, who had suspicious ALN involvement or tumors larger than 3 cm, were eligible for the study according to the American Joint Committee on Cancer 5th edition. The Institutional Review Board approved the study (IRB: 1002-19), and all patients provided written informed consent. Other eligibility criteria included adequate performance status (Eastern Cooperative Oncology Group [ECOG] performance status ≤1); adequate hematology (hemoglobin, ≥10 g/dL; absolute neutrophil count, ≥1.5×109/L; and platelets, ≥100×109/L), renal (serum creatinine, ≤1.5 upper normal limits), and liver (AST, ALT, and alkaline phosphatase all ≤1.5×upper limit of normal, and bilirubin within normal limits) function; and have no evidence of metastatic disease. Patients were excluded if there was any evidence of active cardiac disease or a history of malignancy at another site.
Treatment scheme
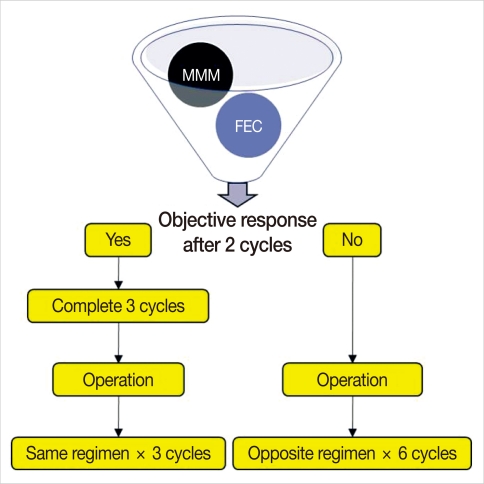
Patients were treated with NC comprised of three cycles of FEC (500 mg/m2 5-FU, 75 mg/m2 epirubicin, 500 mg/m2 cyclophosphamide every 3 weeks) or MMM (40 mg/m2 methotrexate, 8 mg/m2 mitoxantrone, 8 mg/m2 mitomycin-C every 3 weeks) with an adjuvant extension of three cycles of the same regimen. The regimen was chosen according to patient's own preference with regard to alopecia. The clinical response was assessed after two consecutive cycles of preoperative chemotherapy. An early operation was performed 2-3 weeks after discontinuing the chemotherapy for resectable tumors in cases of tumor progression after two cycles of preoperative chemotherapy. All patients underwent ALN dissection either with mastectomy or lumpectomy. After operative treatment, adjuvant chemotherapy was administered with three cycles of the same regimen for those who achieved objective clinical response, but six cycles of switching regimen, either FEC or MMM, were administered for those who had stable disease or progression (Figure 1). Radiation therapy began within 4 weeks after the last cycles of chemotherapy, if indicated. Tamoxifen was administered after the chemo-radiation for 5 years when the hormone receptor was positive.
Figure 1.
Treatment scheme of chemotherapy. FEC=5-FU+epirubicin+cyclophosphamide; MMM=methotrexate+mitoxantrone+mitomycin-C.
Evaluation of clinical response and toxicity
Clinical response was assessed by a physical examination and ultrasonography for bidimensionally measurable disease according to standard Union Internationale Contra le Cancer (UICC) criteria. During the physical examination, we used a transparent grid for mapping the extent of the tumor at the beginning and again after the two cycles of NC. A clinical complete response (cCR) was defined as the disappearance of all known disease and a clinical partial response (cPR) was defined as reduction of 50% or greater in the product of the two largest perpendicular dimensions of the primary lesion. Progressive disease (PD) was defined as an increase of more than 25% in the product of the two largest perpendicular dimensions of the primary tumor or the appearance of new lesions. Stable disease (SD) was represented by a less than 50% decrease or a less than 25% increase in the product of the two largest perpendicular dimensions of the primary tumor. A pathological complete response (pCR) was defined as no residual tumor cells in the breast and axilla based on histopathology of the operative specimens following NC. The number of involved ALN was recorded from the axillary dissection specimen by the pathologist. Toxicity was graded according to the ECOG common toxicity criteria.
Survival analysis
Overall survival (OS) and disease-free survival (DFS) were considered from the onset of NC and from the day of surgery, respectively. A statistical analysis was conducted using the SPSS version 12.0 (SPSS Inc., Chicago, USA). A univariate survival analysis was performed with the Kaplan-Meier method, and comparisons between curves were performed with the log-rank test. Factors were entered into a Cox multivariate regression model to analyze the potential simultaneous effect of the significant predictors of DFS and OS identified by the univariate analysis. Patient follow-up was performed in the clinic until either death or the patient's last visit on or before December 2007 in both the FEC and MMM groups. Survival data were provided by the Korea National Statistical Office for those unavailable for follow-up.
RESULTS
Patient characteristics
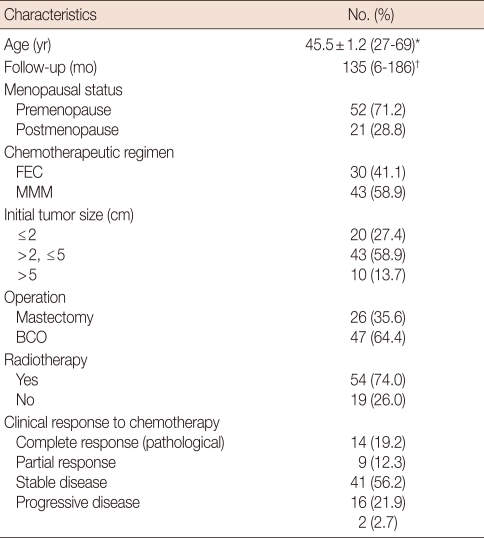
From February 1991 to October 1998, seventy three patients diagnosed with breast cancer, with a tumor size ≥3 cm or clinically axillary node positive, were enrolled for the phase II trial. Patients with known distant metastases were excluded. The mean age of patients was 45.5±1.2 years (range, 27-69). The median follow-up period was 135 months (range, 6-186). The clinical characteristics of the patients are shown in Table 1. No significant difference was observed with regard to clinicopathological parameters between the FEC and MMM groups.
Table 1.
Clinical characteristics of patients (n=73)
SE=standard error; FEC=fluorouracil+epirubicin+cyclophosphamide; MMM=mitoxantrone+methotrexate+mitomycin; BCO=breast conserving operation.
*Mean±SE (range); †Median (range).
Clinical response and toxicity
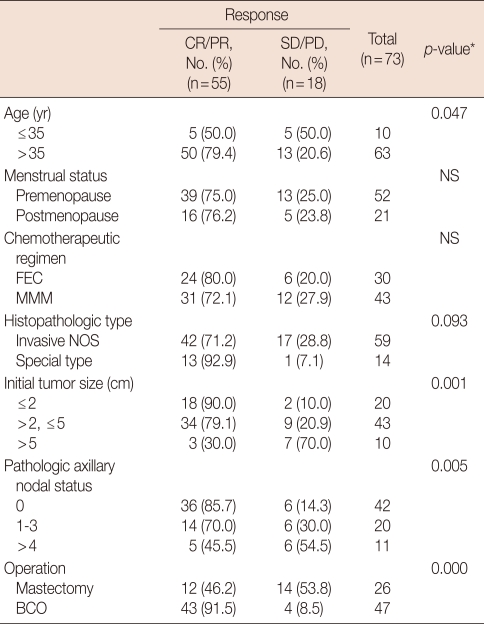
An overall objective clinical response was observed in 75.4% (cCR, 19.2%; cPR, 56.2%) of the patients including nine cases (12.3%) of pCR (Table 1). The remaining 24.6% showed SD (21.9%) and PD (2.7%), respectively. Pretreatment tumor size and age were significantly related to clinical response to NC (Table 2). Smaller tumors showed a better response to NC than larger tumors (p=0.001). Patients older than 35-years of age showed a better response to NC than younger patients (p=0.047). No significant difference was observed with regard to clinical response to NC between the FEC and MMM groups. According to the ECOG common toxicity criteria, no significant difference was observed with regard to grade 3/4 severe toxicities. Reversible alopecia and mild to moderate nausea were more common in the FEC group than in the MMM group.
Table 2.
Significance of various factors on clinical response to chemotherapy
CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease; NS=not significant; FEC=fluorouracil, epirubicin, cyclophosphamide; MMM=mitoxantrone, methotrexate, mitomycin; NOS=not otherwise specified; BCO=breast conserving operation.
*Linear-by-linear association.
Prognostic factors affecting DFS and OS
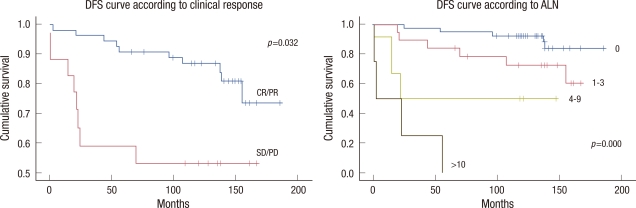
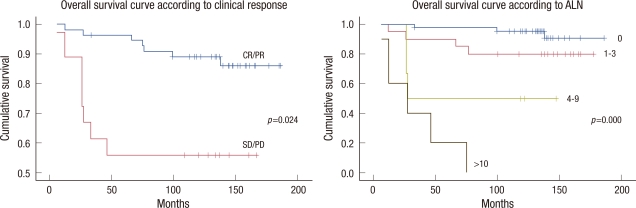
A univariate analysis showed that initial tumor stage, pathological ALN status, and clinical response were prognostic factors of DFS and OS. Additionally, pathological tumor stage was associated with OS in the univariate analysis. However, a multivariate analysis indicated that DFS and OS were significantly related to the clinical response and the number of involved ALNs (Table 3). Cumulative 10-year DFS was 87.3% for patients with CR/PR, 55.5% for patients with SD/PD (p=0.032), 92.9% for node negative patients, 75.0% for patients with 1-3 positive nodes, 50.0% for patients with 4-9 positive nodes, and no survival for patients with 10 or more positive nodes (p<0.001, Figure 2). Cumulative 10-year OS was 89.1% for patients with CR/PR, 55.5% for patients with SD/PD (p=0.024), 95.2% for node negative patients, 80.0% for patients with 1-3 positive nodes, 50.0% for patients with 4-9 positive nodes, and no survival for patients with 10 or more positive nodes (p<0.001, Figure 3). No significant difference was observed in DFS and OS between the FEC and MMM groups. Histopathological type, differentiation, hormone receptor, Ki-67, c-erbB2, and triple negative cancer were not predictive of DFS or OS. All of these pathological findings and biological markers (hormone receptor, Ki-67, c-erbB2) were examined in postoperative specimens.
Table 3.
Analysis of factors affecting disease-free survival rates (DFSR) and overall survival rates (OSR)
EC=fluorouracil+epirubicin+cyclophosphamide; MMM=mitoxantrone+methotrexate+mitomycin; BCO=breast conserving operation; NOS=not otherwise specified; ALN=axillary lymph node; NA=not available; CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease.
*Log-rank test; †Cox multivariate regression model.
Figure 2.
Disease-free survival (DFS) curves according to the clinical response to chemotherapy and pathological axillary lymph node (ALN) status using the Kaplan-Meier statistical method and the log-rank test.
CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease.
Figure 3.
Overall survival curves according to the clinical response to chemotherapy and pathological axillary lymph node (ALN) status using the Kaplan-Meier statistical method and the log-rank test.
CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease.
DISCUSSION
The clinical characteristics of the patients were remarkable in terms of the high proportion of young premenopausal patients. The median age of the studied patients was far below 50 years because breast cancer in Korean women tends to occur earlier than in Western countries, showing a peak incidence in the 40s [9]. In general, Korean women who have breast tumors larger than 3 cm are not candidates for breast conserving operations because they have smaller breasts than those of women from western countries. NC for patients with breast cancer is currently used with the main aim of achieving pathological complete remission and improving survival. Second, it is used to conserve the breast and axilla due to tumor downstaging. The main problem with NC is the loss of traditional prognostic markers such as tumor size or axillary LN status. pCR following NC is a well known powerful prognostic marker for longer survival. Unfortunately, the vast majority of patients treated with NC will not have a pCR, and no guidelines exist for these patients. This is the most important problem in the field of NC and needs to be resolved through large randomized clinical trials to identify a tailored treatment. Two large randomized trials, the EORTC 10902 and the NSABP B-18, reported that clinical and pathological responses were be long term prognostic factors following NC. In a study from Korea, initial clinical stage, estrogen receptor, bcl-2, and triple negative phenotype were statistically significant variables which impacted on OS [10]. In our study, based on a review of data with a long follow-up, the clinical or pathological response to neoadjuvant chemotherapy and the absolute number of metastatic ALNs identified at surgical staging were independent predictors of both DFS and OS. The clinical response to NC was related to tumor size and age: tumor response was more marked in smaller tumors and older patients ≥35 years (Table 2). However, most groups have reported no influence of age or menopausal status on the response to chemotherapy. Jacquillat et al. [11] reported that tumor response is inversely related to age. A cCR was achieved in 18% of patients less than 50 years of age compared to 37% of those who were over 50 years (p=0.007). It is well known that patients with large tumors are less likely to have a pCR compared to those with smaller tumors, because larger tumors tend to have decreased drug delivery by inefficient blood flow and relatively high intratumoral pressure [12]. Achieving cCR without pCR following NC may be associated with partial eradication of the micrometastasis. pCR following NC is infrequent: 12.3% in our series, 11% in the NSABP B-18 trial, and 4% in the EORTC trial 10902 [1,13,14]. In the current study, surgery was performed in all patients with cCR because of the reported significant increase in locoregional recurrences when only radiotherapy was administered without surgery in the case of cCR [15]. As observed, tumor progression during NC is rare: 2.7% in our series, 1.4% in the EORTC trial 10902, and 3% in the NSABP B-18 trial [1,13,14]. Results from NSABP B-18 show no statistically significant differences in DFS and OS regarding sequencing chemotherapy preoperatively or postoperatively between the neoadjuvant and adjuvant groups. However, there were trends in favor of preoperative chemotherapy for DFS and OS in women under 50 years (hazard ratio [HR], 0.85, p=0.090 for DFS; HR, 0.81, p=0.060 for OS). Five-year DFS also demonstrated a strong trend in favor of the neoadjuvant group (HR, 0.81, p=0.053) [13]. Considering the relatively younger age of the Korean patients with breast cancer as compared with women from Western countries, the benefit from NC might be more considerable for Korean patients with breast cancer than for women from Western countries. In the current study, chemotherapy was performed with an adjuvant extension of NC for those whom achieved an objective clinical response to NC. According to the results of the in vivo chemosensitivity test, these patients could have better clinical outcome by changing the resistant chemotherapeutic regimen to a non-cross-resistant chemotherapeutic regimen. However, in the MDACC trial, the change to a non-cross-resistant regimen in an adjuvant setting did not improve the outcome for the patients with a poor response to NC [16]. The NSABP B-27 trial did not identify any group of patients who would benefit from adding neoadjuvant or adjuvant docetaxel chemotherapy in patients who received four cycles of neoadjuvant doxorubicin and cyclophosphamide [4,13]. In the B-27 trial, treatment was not based on in vivo chemosensitivity test which was applied in the current study. Currently, the role of combining a neoadjuvant and adjuvant chemotherapy regimen is unclear. pCR rate, which is an indicator of good clinical outcome, increases by adding neoadjuvant or adjuvant sequential taxane, or by adding more cycles of NC or a combination of trastuzumab with NC [4,13,17-20]. However, it is unclear whether these results will translate into an improvement in survival. In the Nottingham experience, six cycles of preoperative FEC chemotherapy has resulted in a better response and a significantly better outcome to six cycles of preoperative MMM chemotherapy [21]. However, in our study, neither clinical response nor long term clinical outcome were different between the FEC and MMM groups. This was possibly due to the limitation of a smaller sample size and a different treatment schedule. Generally, ALN status is regarded as the most important surrogate marker for patients with breast cancer, and ALN dissection is an integral part of the accepted management of breast cancer. NC completely eradicates axillary metastatic disease in approximately 20% to 40% of patients before ALN dissection [4,13,22]. In the current study, negative ALN metastasis was significantly higher in the responsive group (36/55) than in the non-responsive group (6/18) (Table 2). One study from M.D. Anderson suggests that axillary irradiation without ALN dissection may provide adequate local control in patients with at least a partial response of the primary tumor to NC and a clinically negative axilla [23]. Daveau et al. [24] reported that omission of axillary irradiation in patients with no pathologic ALN involvement after NC resulted in no increase in the risk of distant metastasis, locoregional recurrence, or death. Furthermore, ALN dissection can be avoided through sentinel LN biopsy after NC in virtually all patients and accurately selected patients who required complete level I and II ALN dissection [25]. In terms of axillary treatment, the responsive group could have had more chance to avoid ALN dissection or irradiation than the non-responsive group. There has been some debate on the role of ALN status following NC as a prognostic factor [10,26]. Based on a review of data with a long follow-up, ALN status after NC was still the most powerful prognostic indicator followed by clinical response in the current study. Thus, patients with partial or minor response following NC can be further stratified with respect to DFS and OS by the number of involved ALNs.
Footnotes
This work was supported by the 2010 Chungnam National University Hospital research grant.
The authors declare that they have no competing interests.
References
- 1.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Blumenschein GR, Spanos W, Montague ED, Buzdar AU, Yap HY, et al. Multimodal treatment of locoregionally advanced breast cancer. Cancer. 1983;51:763–768. doi: 10.1002/1097-0142(19830301)51:5<763::aid-cncr2820510502>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 4.Mamounas EP. NSABP Protocol B-27. Preoperative doxorubicin plus cyclophosphamide followed by preoperative or postoperative docetaxel. Oncology (Williston Park) 1997;11(6 Suppl 6):37–40. [PubMed] [Google Scholar]
- 5.Kimura M, Sano M, Fujimori M, Nakagomi H, Negishi T, Yanagita Y, et al. Neoadjuvant paclitaxel for operable breast cancer: multicenter phase II trial with clinical outcomes. Anticancer Res. 2008;28:1239–1244. [PubMed] [Google Scholar]
- 6.Kaufmann P, Dauphine CE, Vargas MP, Burla ML, Isaac NM, Gonzalez KD, et al. Success of neoadjuvant chemotherapy in conversion of mastectomy to breast conservation surgery. Am Surg. 2006;72:935–938. [PubMed] [Google Scholar]
- 7.Forrest AP, Levack PA, Chetty U, Hawkins RA, Miller WR, Smyth JF, et al. A human tumour model. Lancet. 1986;2:840–842. doi: 10.1016/s0140-6736(86)92872-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim JR, Chang ES. Prognostic factors in breast cancer patients following neoadjuvant chemotherapy. J Korean Surg Soc. 2000;59:729–737. [Google Scholar]
- 9.The Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2002. J Korean Breast Cancer Soc. 2004;7:72–83. [Google Scholar]
- 10.Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Prognostic impact of clinicopathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer. 2007;7:203. doi: 10.1186/1471-2407-7-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacquillat C, Weil M, Baillet F, Borel C, Auclerc G, de Maublanc MA, et al. Results of neoadjuvant chemotherapy and radiation therapy in the breast-conserving treatment of 250 patients with all stages of infiltrative breast cancer. Cancer. 1990;66:119–129. doi: 10.1002/1097-0142(19900701)66:1<119::aid-cncr2820660122>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Sanchez M, Gamboa-Dominguez A, Uribe N, Garcia-Ulloa AC, Flores-Estrada D, Candelaria M, et al. Clinical and pathological predictors of the response to neoadjuvant anthracycline chemotherapy in locally advanced breast cancer. Med Oncol. 2006;23:171–183. doi: 10.1385/MO:23:2:171. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 14.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 15.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 16.Knauer M, Haid A, Schneider Y, Köberle-Wührer R, Lang A, Winder T, et al. Adjuvant extension of chemotherapy after neoadjuvant therapy may not improve outcome in early-stage breast cancer. Eur J Surg Oncol. 2009;35:798–804. doi: 10.1016/j.ejso.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Kim J, Lee J, Chang E, Gwak G, Cho H, et al. Comparison of 6 cycles versus 4 cycles of neoadjuvant epirubicin plus docetaxel chemotherapy in stages II and III breast cancer. Eur J Surg Oncol. 2009;35:583–587. doi: 10.1016/j.ejso.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Steger GG, Galid A, Gnant M, Mlineritsch B, Lang A, Tausch C, et al. Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG-14. J Clin Oncol. 2007;25:2012–2018. doi: 10.1200/JCO.2006.09.1777. [DOI] [PubMed] [Google Scholar]
- 19.Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 20.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 21.Mathew J, Asgeirsson KS, Agrawal A, Mukherjee A, Ellis IO, Cheung KL, et al. Neoadjuvant chemotherapy in locally advanced primary breast cancers: the Nottingham experience. Eur J Surg Oncol. 2007;33:972–976. doi: 10.1016/j.ejso.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. doi: 10.1200/JCO.2007.15.3510. [DOI] [PubMed] [Google Scholar]
- 23.Singletary SE. Neoadjuvant chemotherapy in the treatment of stage II and III breast cancer. Am J Surg. 2001;182:341–346. doi: 10.1016/s0002-9610(01)00724-3. [DOI] [PubMed] [Google Scholar]
- 24.Daveau C, Stevens D, Brain E, Berges O, Villette S, Moisson P, et al. Is regional lymph node irradiation necessary in stage II to III breast cancer patients with negative pathologic node status after neoadjuvant chemotherapy? Int J Radiat Oncol Biol Phys. 2010;78:337–342. doi: 10.1016/j.ijrobp.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GF, Tannebaum JE, Jernigan AM, Palazzo JP. Axillary sentinel lymph node biopsy after neoadjuvant chemotherapy for carcinoma of the breast. Cancer. 2010;116:1243–1251. doi: 10.1002/cncr.24887. [DOI] [PubMed] [Google Scholar]
- 26.Pierga JY, Mouret E, Laurence V, Dieras V, Savigioni A, Beuzeboc P, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer. the role of clinical response. Eur J Cancer. 2003;39:1089–1096. doi: 10.1016/s0959-8049(03)00069-8. [DOI] [PubMed] [Google Scholar]