Abstract
The common sites of metastasis of breast cancer are bone, lung, and liver, but gastrointestinal metastasis from breast cancer is rare. We experienced a case of solitary ileal metastasis from breast cancer. A 45-years-old woman presented with melena for several weeks. She showed no other abdominal symptoms. Colonoscopy findings showed an ulcerative mucosal lesion in the terminal ileum, and biopsy was performed. Pathologic examination revealed metastatic carcinoma, originated from breast. The tumor cells were positive for estrogen receptor and negative for Cdx-2. She had had a previous medical history of bilateral breast cancer and undergone breast conserving surgery with sentinel lymph node biopsy for both breasts. The torso positron emission tomography scan at 19 months after surgery showed mildly increased uptake in the terminal ileum which was considered as inflammation. Finally, she was diagnosed with solitary ileal metastasis from breast cancer at 22 months after surgery.
Keywords: Breast, Gastrointestinal tract, Neoplasm metastasis
INTRODUCTION
Breast cancer is the second most common malignancy in Korean women, metastasis occurs in 20-30% of these patients [1]. The common sites of metastasis of breast cancer are bone, lung, liver, and brain, but solitary gastrointestinal metastasis from breast cancer is extremely rare [2]. We experienced a case of solitary ileal metastasis from breast cancer.
CASE REPORT
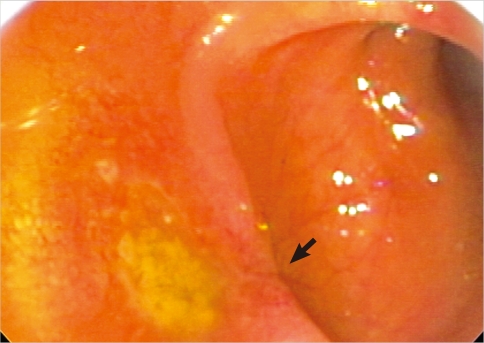
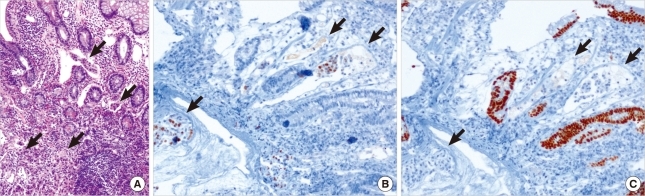
A 45-years-old woman presented with melena for several weeks. She showed no other abdominal or constitutional symptoms. The physical examination revealed mild abdominal distension with an alert mentality and stable vital signs. The laboratory data was within the normal range. Abdominal radiograph showed nonspecific multiple small bowel gases. Colonoscopy showed an ulcerative mucosal lesion in the terminal ileum (Figure 1), and biopsy was performed. Microscopic examination showed focal infiltration of neoplastic cells in intestinal wall. In Hematoxylin and Eosin stain, mucosal surface of epithelium was intact, but some tumor emboli infiltrations were seen in lamina propria, which is the typical feature of metastatic carcinoma (Figure 2A). In immunohistochemistry (IHC), the tumor cells were positive for estrogen receptor (ER) (Figure 2B) and negative for Cdx-2 (Figure 2C), which indicates metastatic carcinoma from breast primary.
Figure 1.
Colonoscopy showed ulcerative mucosa (arrow) in the terminal ileum.
Figure 2.
(A) Microscopic findings of terminal ileal wall showed tumor emboli infiltrations (arrows) in lamina propria lymphatic spaces, but intact mucosal epithelium (H&E stain, ×100). (B) The tumor cells (arrows) were positive for estrogen receptor; (C) negative for Cdx-2, a critical nuclear transcription factor for intestinal development (immunohistochemical staining, ×100).
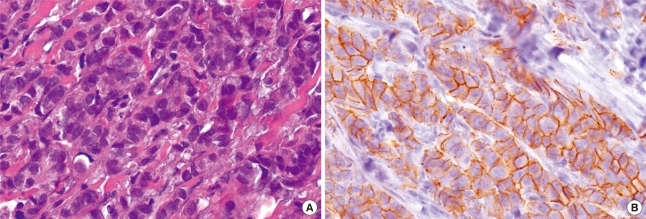
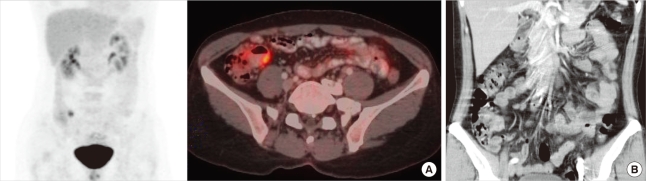
The woman had had a previous medical history of bilateral breast cancer and she had undergone breast conserving surgery with sentinel lymph node biopsy for both breasts. Both breast cancers were invasive ductal carcinoma (Figure 3A) and positive for E-cadherin (Figure 3B). The largest tumor was 1.9 cm at the greatest diameter and both sentinel lymph nodes were free of malignancy. The IHC stains for ER and progesterone receptor (PR) were positive in most of tumor cells and HER2 staining was negative. She then received adjuvant hormonal therapy with tamoxifen and GnRH agonist (Goserelin, Zoladex®; AstraZeneca, Macclesfiled, UK) on and radiation therapy. The postoperative follow-up evaluation was done with breast sonography and mammography at 6 months intervals and with bone scanning at 1-year intervals. The torso positron emission tomography scan was performed at 19 months after surgery and mildly increased uptake was detected in the terminal ileum (SUV max=5.7) (Figure 4A, B). We considered this originated from inflammation, and planned short-term follow up.
Figure 3.
(A) Microscopic findings of both breast revealed infiltration of malignant ductal cells. The primary invasive ductal carcinomas of breast shows well differentiation and no lymphovascular invasions (H&E stain, ×200). (B) The malignant cells are positive for E-cadherin (immunohistochemical staining, ×200).
Figure 4.
(A) The torso positron emission tomography scan showed mild uptake in the terminal ileum (SUV max=5.7). (B) The retroperitoneal computed tomography showed thickening of the wall of terminal ileum and multiple lymph node enlargement around them.
She was diagnosed with solitary ileal metastasis from breast cancer by colonoscopic biopsy at 22 months after surgery. We checked her whole bowels by capsule endoscope and could not find any other lesions. Retroperitoneal computed tomography (CT) showed thickening of the wall of terminal ileum and enlargement of multiple lymph nodes around them (Figure 4C). She wanted to have an operation in other hospital and was transferred. We heard that she underwent hand-assisted laparoscopic ileocecectomy, and then received chemotherapy.
DISCUSSION
Small bowel malignancies make up only 1.1-2.4% of all gastrointestinal malignancies, and primary adenocarcinoma is the most common of all the small bowel malignancies [3]. Most small bowel metastases are from primary tumors such as malignant melanoma, colon cancer and cervical cancer, and metastasis from breast cancer is uncommon [4]. However, autopsy of patients with breast cancer revealed 15-16% of metastasis was found in gastrointestinal tract and the most common site of gastrointestinal metastasis was the stomach followed by the large bowel and small bowel [4]. Almost all the signs and symptoms of gastrointestinal diseases, including abdominal pain, diarrhea, gastrointestinal bleeding, intestinal obstruction, intussusceptions and more, can be present in patients with metastatic small bowel malignancies [5-7]. This creates problems for making the differential diagnosis from other benign gastrointestinal diseases. A delayed diagnosis makes the treatment of metastatic cancer more difficult. In our case, if further examination, such as endoscopy, was performed when PET showed mild uptake in terminal ileum, more immediately, we may be get better result, now. If there are suspicions of breast origin in diagnosis, breast specific IHC stains such as ER or PR and intestinal specific IHC stains such as Cdx-2 can be helpful.
In our case, primary breast cancer was invasive ductal carcinoma. But lobular carcinoma rather than ductal carcinoma and tumor with PR positivity rather than ER positivity has a propensity for gastrointestinal metastasis in patients with breast cancer [8,9]. The treatment options for small bowel metastasis from breast cancer are surgery, chemotherapy, hormonal therapy and radiation therapy depending on the cancer status.
In patients with breast cancer, metastasis to gastrointestinal tract is uncommon and its clinical manifestations are non-specific, which makes it hard to diagnosis correctly in one swoop. Yet having a suspicion of gastrointestinal metastasis from breast cancer and making an early diagnosis are the most important keys for the prognosis of metastatic breast cancer. Although it is uncommon for breast cancer to metastasize to the gastrointestinal tract, we should always keep in mind the possibility of such metastasis in patients with a previous history of breast cancer.
References
- 1.Harris JR. Diseases of the Breast. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1101–1159. [Google Scholar]
- 2.Oyasiji T, Shoemake P, Bakhos C, Mansourian V. Small bowel obstruction from metastatic breast cancer masquerading as an obstructed incisional hernia. Conn Med. 2009;73:403–406. [PubMed] [Google Scholar]
- 3.Braasch JW, Denbo HE. Tumors of the small intestine. Surg Clin North Am. 1964;44:791–809. [PubMed] [Google Scholar]
- 4.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637–641. [PubMed] [Google Scholar]
- 5.Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol. 2006;12:6219–6224. doi: 10.3748/wjg.v12.i38.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen RM, Lewis JD, Janjan NA, Komorowski RA. Occult carcinoma of the breast masquerading as primary adenocarcinoma of the small intestine. A case report. J Clin Gastroenterol. 1988;10:213–217. doi: 10.1097/00004836-198804000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Chang SF, Burrell MI, Brand MH, Garsten JJ. The protean gastrointestinal manifestations of metastatic breast carcinoma. Radiology. 1978;126:611–617. doi: 10.1148/126.3.611. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz RE, Klimstra DS, Turnbull AD. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93:111–114. doi: 10.1111/j.1572-0241.1998.111_c.x. [DOI] [PubMed] [Google Scholar]
- 9.de la Monte SM, Hutchins GM, Moore GW. Estrogen and progesterone receptors in prediction of metastatic behavior of breast carcinoma. Am J Med. 1984;76:11–17. doi: 10.1016/0002-9343(84)90738-1. [DOI] [PubMed] [Google Scholar]