Abstract
An automated point-of-care (POC) immunodetection system for immunological detection of Staphylococcal enterotoxin B (SEB) was designed, fabricated, and tested. The system combines several elements: (1) ELISA-Lab-on-a-chip (ELISA-LOC) with fluidics, (2) a CCD camera detector, (3) pumps and valves for fluid delivery to the ELISA-LOC, (4) a computer interface board, and (5) a computer for controlling the fluidics, logging and data analysis of the CCD data. The ELISA-LOC integrates a simple microfluidics system into a miniature ninety-six well sample plate, allowing the user to carry out immunological assays without a laboratory. The analyte is measured in a sandwich ELISA assay format combined with a sensitive Electrochemiluminescence (ECL) detection method. Using the POC system, SEB, a major foodborne toxin, was detected at concentrations as low as 0.1 ng/ml. This is similar to the reported sensitivity of conventional ELISA. The open platform with simple modular fluid delivery automation design described here is interchangeable between detection systems and because of its versatility it can be also used to automate many other LOC systems, simplifying LOC development. This new point-of-care system is useful for carrying out various immunological and other complex medical assays without a laboratory and can easily be adapted for high throughput biological screening in remote and resource poor areas.
Keywords: Point of care, ELISA, lab on a chip, point of care, Lamination, charge-coupled device, open platform, CCD, microfabrication, micromachining, PMMA, polycarbonate, microfluidics, Staphylococcal enterotoxins, enhanced chemiluminescence, carbon nanotubes, point-of-care-testing, food safety
Introduction
Point-of-care (POC) diagnostics devices (i.e. a diagnostic test performed near the patient without needing a clinical lab) are generally integrated devices that do not require dedicated laboratories and complex equipment, and are thus well suited for medical diagnostics in remote settings with minimal medical infrastructure [1; 2; 3]. Although many detection systems have been described over the past several years, very few have been used for clinical or analytical applications in resource-poor settings because of their expense and complexity. Simple, low-cost sensors are needed to exploit the potential of the technology and bring it into wide-scale use in developing countries.
Enzyme-linked immunosorbent assays (ELISA) [4] [5] are widely used in medical diagnostic and research applications to detect proteins based on their binding to immobilized antibodies. It is a very popular assay method because it is simple, low cost and many samples can be assayed simultaneously. However, dedicated instruments are needed to automate the assay, including robotic pipetters, plate washers, and optical colorimetric detectors. In addition, the current standard 96-well ELISA format requires large volumes of samples and reagents, and must be performed in a lab.
Several Lab-on-a-chip (LOC) devices have been developed in recent years as alternatives to ELISA that are more compact and have better immunoassay performance. The LOC technologies incorporate new detection and fluid-handling technologies and are often based on optical-based immunodetection [6; 7; 8; 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19]. However, other devices use alternative detection methods, including electrochemical detection in combination with nanoparticles [20], superparamagnetic beads [21], a microcantilever transducer combined with an impedance analyzer [22], or a micro-channel with integrated electrodes, functionalized with antibody-coated polystyrene beads specific to the target. The limitation of these LOC devices is that they are often relatively complex and expensive, and few can simultaneously test as many samples as the “classical” 96-well ELISA.
To combine the advantages of the miniaturization and sophistication of LOC detection and the multi-sample capabilities of ELISA, a miniaturized 96-well ELISA plate requiring as little as 5 μL of sample [23; 24; 25; 26; 27] were developed to carry out low-cost immunodetection without a laboratory. However such devises requires manual addition of the various assay reagents. More recently [28; 29], an ELISA-LOC which incorporates microfluidics into a miniaturized ELISA plate to eliminate the need for manual fluid handling. The system can be operated by syringe and can be used without electrical power or with a battery for operation. ELISA-LOC utilizes the large surface area of carbon nanotubes (CNT) to improve the sensitivity of ELISA assays[30], and combines such assays with enhanced chemiluminescence (ECL) [24; 26] using detection with a CCD [31; 32]. Although simple, and suitable for resource-poor settings, the manual operation requires several steps and is not simple to use, limiting the practical usefulness of the system. Automation of the ELISA-LOC will make the system more practical to use.
Open platform describes a technical system which is based on open standards, such as published and fully documented information that allows using the system to function in other ways than the original developer intended, without requiring great modification of the source protocol. The open platform concept is used successfully in the computer world (e.g. Linux operating system) but not in biosensors development. However the concept has potential for the simplifying and speeding the development of diagnostic technology. Current detection technologies do not use “open platforms” enabling using the system to function in other ways than the original, instead, each detection system utilizes “custom” technology specific for an application. An "open platform" will simplify and speed up the development of medical diagnostics, reduce cost and enable the deployment of diagnostics technologies for global health.
For system automation, open-platform modular design for biosensors is a new concept to replace current non-modular, assay-specific, proprietary biosensors incompatible with each other. Modular design of compatible “generic” components (transducers, fluidics and ligands) will permit versatility in assembling new biosensors for specific applications, increase analytical capabilities, reduce development costs, speed development, simplify the FDA review process and broaden access to valuable technologies. Developing open platform requires building in access capability, such as a six way valve to handle six reagents or two pumps instead of one. This makes the open platform versatile enough to be used for broad array of applications ranging from analysis of 6 samples to analysis of 96 samples, and using as many as six reagents.
To demonstrate this approach, here we present a modular automated Point-of-care optical detection ELISA detection system that utilizes some of the fluidics modules developed for electrical percolation-based biosensors [33]
The modular system integrates several elements (1) ELISA-LOC fluidics, (2) a CCD camera as detector, (3) pumps and valves for fluid delivery to the ELISA-LOC, (4) a computer interface board for controlling fluid delivery, and (5) a computer for controlling the fluidics, logging and data analysis of the CCD data. The simple modular fluid deliver automation design described here can be also used to automate other LOC systems. To demonstrate the integrated Point-of-care system, we used it for the detection of Staphylococcal enterotoxins (SEs).
SEs are a family of structurally related twenty two (known) heat labile toxins implicated in several illnesses including food-borne diseases [34; 35; 36; 37; 38] causing various gastrointestinal symptoms such as vomiting, nausea, and diarrhea even at low levels of exposure (e.g. total intake of SEA of approx. 20–100 ng per person) [39]. In addition, SEs are also potent stimulator of T cells and have also been implicated in other diseases such as atopic eczema [40; 41; 42], chronic rhinosinusitis with nasal polyposis and chronic severe inflammatory disease of the upper airways [43], rheumatoid arthritis [44; 45], and toxic shock syndrome [46], and are also recognized as potential bioweapons [47; 48; 49; 50]
The results present demonstrate that the integrated Point-of-care system can be used for carrying out various immunological assays and other complex medical assays without a laboratory, and that the open platform Thus this device may be useful for high throughput biological screening in remote and resource poor areas.
Material and methods
Reagents and materials
Reagents
Single-walled Carbon Nanotubes (CNTs) were obtained from Carbon Solutions Inc (Riverside, CA). Poly(diallyldimethylammonium chloride) polymer (PDDA) and o-Phenylenediamine dihydrochloride (OPD) were purchased from Sigma-Aldrich (St. Louis, MO). The Immun-Star HRP Chemiluminescence Kit was obtained from Bio-Rad (Hercules, CA). Staphylococcal enterotoxin B (SEB) and rabbit anti-SEB affinity purified IgG were purchased from Toxin Technology (Sarasota, FL). All other reagents were of analytical grade and de-ionized water was used throughout.
Materials for the fabrication of LOC
Clear 0.25 mm polycarbonate film and 1/8 inch acrylic were obtained from Piedmont Plastics (Beltsville, MD).
Electronic components
For the open platform modular design, six way valve was purchased from Cole-Parmer (Vernon Hills, IL). The analog to digital/digital to analog converter (Multifunction I/O 779051–01, NI USB-6008) with 8 analog inputs (12-bit) and 2 analog outputs (12-bit) and Labview software was obtained from National Instruments (Austin, TX). The WPX-1 peristaltic pump was obtained from Welco (Japan), “Silver Liquid” from Electron Microscopy Sciences (Hatfield, PA) and the Digital Multimeter from Agilent Technologies (Santa Clara CA).
Preparation Equipment
A sonicator (FS-14) was obtained from Fisher Scientific (Pittsburgh, PA), and a mini-centrifuge from Beckman (Fullerton, CA).
Preparation of CNT for ELISA-LOC
Carbon nanotube preparation
Fabrication of ELISA-LOC
The ELISA-LOC described in our previous work [28; 29] the polymers micro-machined using a computer controlled Epilog Legend CO2 65W laser cutter (Epilog, Golden, CO), as described in previous work [23; 25; 27]. To prepare the plate assay, 15 μL of antibody functionalized CNT solution was dropped into the wells of the 96-well sample chip, which was then dried and rinsed with 2 ml washing buffer (20 mM phosphate buffer, pH 7.4) for 5 minutes to eliminate any loose or partially immobilized CNTs. The CNT-antibody modified wells were then blocked with 1% BSA from Sigma-Aldrich (St. Louis, MO) for 30 minutes.
CCD based detector
The CCD detector described in previous work[23; 25; 27] consists of several parts: an enclosure, a custom LED based light box with exchangeable filters for illumination, and an SXVF-M7 camera (Adirondack Video Astronomy, Hudson Falls, NY) equipped with a 752x582 pixel Sony ICX-429ALL CCD and with a Tamron manual zoom CCTV 4–12 mm, f1.2 lens (Spytown, Utopia, NY). Since the ECL assay used does not require illumination, the LED based light box developed for this detector was not used for this assay.
ELISA-LOC SEB detection assays
The primary antibody bound to carbon nanotubes was introduced to the LOC during LOC fabrication (see above). Various concentrations of SEB in phosphate buffer solution (20 mM, pH 8.0) were added to the ELISA-LOC wells containing the primary antibody-CNT complex. After adding the SEB samples (15 μl), the interchangeable fluid delivery system was bonded to the plate and the entire assembly was incubated for 45 min at room temperature and then washed automatically by activation of pump A. The valve incorporate into the system was set to draw from the phosphate buffer reservoir; 2 ml of phosphate buffer were used for washing. The buffer was removed via the fluid outlet system, bonded to the bottom of the plate, connected to pump B. Following another round of washing, 2 ml of HRP conjugated anti-rabbit IgG (0.01 mg/mL) in buffer were injected via the fluid delivery system by switching the valve to draw from the of HRP conjugated anti-rabbit IgG reservoir. The plate was then incubated for 1 h, and washed three more times with 2 ml of phosphate buffer as described above. The ECL assay was performed by adding 2 ml of ECL buffer (mixing the two solutions from Chemiluminescent Kit in a 1:1 volume ratio) and measuring ECL intensity. In the presence of HRP (bound to the secondary antibody), luminol in the ECL reagents was reduced by Hydrogen Peroxide, which emits light as it returns to its basal state, and is detectable using a CCD.
Data analysis
The CCD image intensities were analyzed using ImageJ software, developed and distributed freely by NIH (http://rsb.info.nih.gov/ij/download.html). The signal for the individual wells was calculated as the average of the intensity values of the respective pixels. The value obtained with concentration of 0 ng/mL SEB was defined as background. The ratio of the signal to noise ratio (SNR) was further used to quantify the SEB concentration.
Results
A prototype of a modular Point-of-care automated ELISA-LOC fluid delivery system open platform was adapted to simplify the use of ELISA-LOC for carrying out many different immunological assays in non-laboratory settings.
In term of design approach, while current trend in LOC development is to pack many functions on the disposable chip and increase the LOC’s complexity and cost, our design approach was to keep many functions, such as valves, off the chip. This simplifies the chip and reduces fabrication cost.
The ELISA-LOC described in our previous work [28; 29] incorporates microfluidics into the ELISA plate, so that an ELISA can be carried out without a washer. As shown in Figure 1, the ELISA-LOC brings together three elements: 1) an interchangeable fluid delivery system (Figure 2-I-A) which functions as the top of the plate; 2) a miniature 96-well plate (Figure 2-I-B) where the assays are carried out and detected; and 3) a fluid outlet system used to remove reagents from the wells (Figure 1-I-C) and attached to the bottom of the plate.
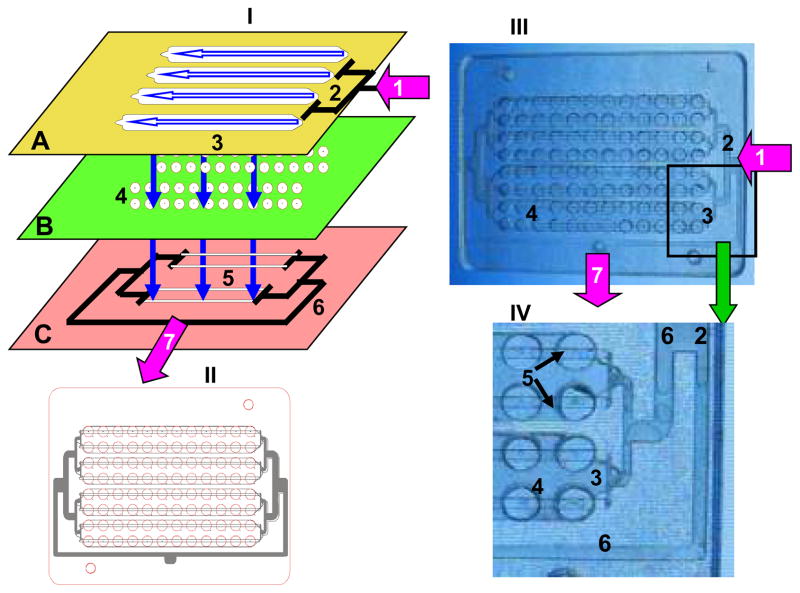
Figure 1. A simplified scheme of the ELISA-LOC.
I. the 3D design of the 96 well ELISA-LOC; II. The complete design overlay of the assembled 96 well device; III. Photo of the actual device, IV enlarged section of the main elements. The arrows show the fluidic path through the ELISA-LOC. In the expanded diagram (I), the three layers of the LOC are visible: fluid delivery layer (A), which serves as the cover for the device, sample wells layer (B) for assay incubation and detection, and the outlet system layer (C) for fluid removal. The main elements of the ELISA-LOC are: fluid inlet port (1) connected to pump A (or a syringe) for reagent delivery, input fluid distribution splitter (2) to distribute reagents to the four nodes (a group of 24 wells); and four fluid loading chambers (3). The assay plate (B) contains 96 wells for assays (4). Each well is fabricated with small holes in the bottom which serve as fluid outlets (shown as bright spots in IV). The outlet system (C) with two outlet channels per node (5) is placed directly under the well holes. All the outlet channels are interconnected via negative pressure distribution splitters (6), which are connected to the outlet port (7) and then to pump B (or syringe). The assembled schematic of the 96 well LOC chip (shown in II) includes the four nodes, each with 2 rows of twelve wells. A clear PMMA LOC device (III) was photographed with illumination to increase contrast. Finally, IV shows an enlarged section to make the fine details visible.
Figure 2. Automatic fluidics system for LOC.
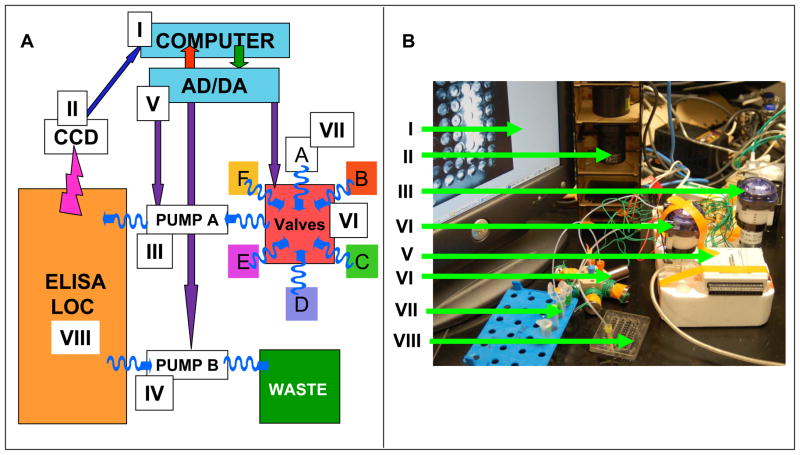
A. Schematic of the system and B the actual photo of the LOC LOC prototype. The system consists of: Computer (I), CCD digital camera (II), input pump (III), output pump (IV), analog to digital/digital to analog converter (V), six-way valve (VI) with the valves marked as A,B,C,D,E and F, reagents reservoirs (VII) and ELISA-LOC (VIII). Straight arrows indicate electric connections; wavy arrows indicate fluidics connections.
The overall ELISA-LOC automation system for fluid delivery which is based on our previous design[33] is shown in Figure 2A. The system is composed of four basic modules: (1) the ELISA-LOC chip, (2) an electronics and computer control system, (3) a fluid delivery system and (4) a CCD-based detector. When combined, the system delivers fluids (wash buffer, secondary antibody and ECL reagent) to the ELISA-LOC chip, carries out the ELISA, detects the signal and provides initial data analysis. Because the open platform access capability and modular design it can easily configured for new application.
Fluid flow in the ELISA-LOC
To better understand our fluidics automation approach for ELISA-LOC, we will first describe in more details the fluid flow in the ELISA-LOC. As discussed above ELISA-LOC incorporates three main modules: 1) an interchangeable fluid delivery module (Figure 1-I-A), 2) a miniature 96-well plate (Figure 1-I-B) where the assays are carried out and detected; and 3) a fluid outlet module used to remove reagents from the wells (Figure 1-I-C).
The design overlay of the LOC-ELISA (Figure 1-II) and the actual device (Figure 1-III) fabricated from clear PMMA, which allows the main elements to be seen clearly. In figure 1, we enlarged part of the ELISA-LOC image (shown in Figure 1-IV). The main elements of the interchangeable fluid delivery module (Figure 1-I-A) are fluid inlet port (#1 in Figure 1-I-A), input fluid distribution splitter (#2 Figure 1-I-A) to distribute reagents to the four nodes (a group of 24 wells); four fluid loading chambers (#3 in Figure 1-I-A). The assay plate (Figure 1-I-B) contains 96 wells for assays (#4 in Figure 1-I-B), each well is fabricated with small holes in the bottom which serve as fluid outlets (shown as bright spots in figure 2-IV). The outlet system (Figure-I-C) with two outlet channels per node (#5 in Figure 1-I-C) is placed directly under the well holes. All the outlet channels are interconnected via negative pressure distribution splitters (#6 in Figure 1-I-C), which are connected to the outlet port (#7 in Figure 1-I-C) and then to pump or syringe. The anti-SEB primary antibody for binding the analyte is conjugated onto a single wall carbon nanotube (SWNT) surface which is immobilized into each well during ELISA-LOC fabrication.
In term of the fluid flow in the chip, fluids move horizontally within module and vertically between modules. The fluids enter through the fluid inlet port (#1), distributes horizontally to the four nodes via distribution splitter (#2) and transfer vertically to the wells which functions as a “flow cell”, the samples to be analyzed flow through small volume (~5 ul) wells. To create the flow cell, a PMMA layer with holes for input fluid delivery for each of the 96 wells is placed under the wells. The fluids are kept in the wells by surface tension using a simple ‘surface tension’ valve was created by drilling a ~0.2 mm hole in the bottom of the well. When a vacuum is applied at the outlet (#7), fluid flows vertically through the holes and empties the wells into the outlet channels (#5) and transfer horizontally via the output distribution splitters (#6) to the outlet (#7). The wells with the outlet holes (the spots in the center of the wells) and the output distribution splitters (#6) are visible in the enlarged section of figure 1-IV. Although the figure shows the device fabricated with clear PMMA, for the purposes of visualization, black PMMA is typically used in actual devices to minimize light scattering and optical crosstalk between wells [27].
Computer control and fluid delivery systems for ELISA-LOC
While the ELISA-LOC module is the platform for carrying out the actual assay, a support system of other modules is needed for reagent delivery to the ELISA-LOC, measurement of assay output, and analysis of the output. Figure 2A shows a schematic of the open platform system for computer control and fluid delivery is based on our previous design[33]. Figure 2B shows the actual system which includes hardware and software.
Hardware for LOC computer control and fluid delivery
There are several components of the LOC computer control and fluid delivery modules shown in Figure 2 (the Roman numerals correspond to the component in the figure):
A computer for image analysis of the CCD measurements and for control of the fluidics system (pumps and valves)
A digital CCD camera controlled by the computer and used for imaging and assay measurements.
A fluid delivery peristaltic pump (A) for, moving fluids from fluid reservoirs through the valve and to the ELISA-LOC.
A second peristaltic pump (B) used for fluid removal from the ELISA-LOC to the waste.
A 12-channel ADC/DAC, an Analog to Digital and Digital to Analog converter which is the interface between the LOC, the pumps, the valve and the computer. The ADC/DAC converts a digital code to analog signals (current, voltage, or electric charge) needed for device operation. Because of the low output power of the ADC/DAC used in this work, a signal amplifier connected to the ADC/DAC is used to operate the pump and the valve.
A six way valve allowing fluid switches, so that reagents such as buffers and antibodies can be delivered at the right time and in the right order to the LOC.
Reservoirs for reagents (e.g. buffer, secondary antibody, ECL solutions etc) and fluids to be delivered to the ELISA-LOC.
The ELISA-LOC as a platform for the actual assay performance and measurement
For the actual prototype (Figure 2B) a desktop computer was used and the components were assembled without permanent electric wiring or fluid lines, making it easy to change the system’s configuration. Power is supplied by a 12 V power supply, although a 12V battery can also be used.
Computer control of the fluidic system
The automated ELISA-LOC is controlled by a computer via Labview control software (National Instruments). The interface between the computer and the device is a USB-6008 multifunction ADC/DAC activates the different devices including the two pumps, and the six-way valve. Because the low power of ADC/DAC, a signal amplifier was used to provides proper voltage and current for valve and the pumps.
The system’s fixed speed pumps used can be programmed to deliver fluids at a variable flow rate by selectively activating the appropriate ports using the 6-way valve. Each pump can be independently programmed to work at a different flow rate. The flow rate of each pump is determined by two user-defined variables: (a) the duration of each pump activation period, and (b) the duration of each flow pulse. Low flow rates are achieved by utilizing pulse width modulation (PWM) to vary the flow rate. Higher flow frequencies allow for a higher flow rate at the cost of more fluid micropulsations. The duration of the fluid pulse is measured as a percentage of each pulse, e.g. percentage duration of 100% is the equivalent of continuous, full speed fluid pumping. Zero percent duration effectively shuts off flow fluid flow, regardless of the frequency setting.
Operation of ELISA-LOC automated system
The six-way valve, also controlled by Labview, is designed to allow one to six different fluids to flow in response to computer input, and is used in conjunction with the pumps. Each fluid change requires extra time to clear fluid that is present in the pump tubing (dead volume) before delivering the new fluid. To minimize the dead volume, the pump is temporarily stopped while switching to a different valve, then restarted after the switch is complete.
The Labview software is designed run continuously, and is capable of timed operation, although that feature was not used in this experiment. The controlling functions for each valve and pump are independent of another, and can be started and stopped by the user as long as power is supplied. The final piece of hardware, the CCD, is connected to the computer directly via a USB port for data transmission and for the operation of the device.
Operation of ELISA-LOC
Reagents delivered from pump A enter through the inlet of the fluid delivery system (marked as arrow #1 in Figure 1-A), distribute to four nodes via a distribution splitter (Figure 1A # 2) and flow in two directions. The reagents flow horizontally within the four nodes of the fluid delivery system (shown as a horizontal arrow marked #3 in Figure 1A), and also flow vertically between the layers’ fluid delivery system (shown as vertical arrows) to the 96 well plate (Figure 1-I-B #4). Finally, the reagents flow through holes in the wells into the outlet system (Figure 1-I-C) with two outlet channels per node (Figure 1-I-C #5) placed directly under the well holes. The outlet channels are interconnected via two negative pressure distribution splitters (Figure 1-I-C #6) connected to the outlet port (Figure 1-I-C #7), which is connected to pump B.
Samples are loaded manually into each well and, after incubation and washing, HRP labeled secondary antibody is added, followed by additional washing. Finally, ECL reagent is used to generate the signal that is measured by CCD camera.
Staphylococcal enterotoxin B (SEB) detection using automated ELISA-LOC
Staphylococcal enterotoxins (SEs) have been traditionally assayed immunologically using Enzyme-Linked Immunosorbent assays (ELISA)[51], using labeled antibodies and optical detection, and taking several hours to complete. In previous work we used manual ELOSA-LOC to detect Staphylococcal enterotoxin B (SEB) [28; 29], To test the automated ELISA-LOC, we applied a similar a similar Staphylococcal Enterotoxin immunological assay used in previous work in order to compare the automated ELISA-LOC describe in this work to the manual device described in previous work. As discussed above, anti-SEB primary antibody was conjugated onto a SWNT surface which was immobilized into each of the 96 wells during ELISA-LOC fabrication. As we have shown previously[24; 26; 30], the large surface area of SWNT increases the sensitivity of immunoassays approximately six-fold. A serial dilution of the toxin SEB (0, 0.01, 0.1, 0.5, 1, 5, 10 and 50 ng/mL) were loaded manually into the wells of the plate (Figure 3-I, columns A-H), in 12 replicas (rows 1–12). Then, the cover containing the fluid delivery system (Figure 1-I-A) was bonded to the assay plate using the double sided tape bonded to the fluid delivery system during fabrication. The plate inlet (Figure 1-A-1) was connected to pump A (Figure 2-A-III), and the plate outlet (Figure 1-C-7) was connected to pump B (Figure 2-A-IV) and the entire apparatus was incubated for 60 minutes. After incubation, pump B was activated, using Labview, to remove the samples from the wells. The valve (Figure 2-A-VI) was switched to the buffer reservoir, and then both pumps were operated to perform the washing. Because the same reagents (washing buffer, secondary antibody, ECL reagents) are used for all samples, cross mixing and diffusion among channels were not factors. The binding of SEB to the primary antibody was detected using ECL, and the image was captured by a CCD camera (Figure 2-A-II).
Figure 3. ELISA-LOC detection of Staphylococcal enterotoxin B (SEB).
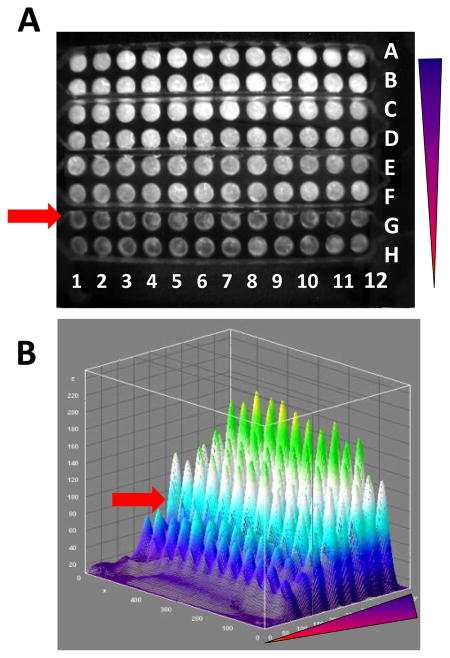
Various concentrations of SEB were tested in a four node ELISA-LOC using ECL detection. A. The initial CCD image, B a 3D imageJ analysis of A. The experiment was arranged so that each of the eight concentrations of SEB (0, 0.01, 0.1, 0.5, 1, 5, 10 and 50 ng/mL) were loaded in each row (represented by letters), from highest to lowest starting in row A. The triangle shows the range of SEB concentrations. Each of the eight concentrations (rows) were loaded as 12 replica columns (the numbers). The arrow indicates the limit of detection of the system (0.1 ng/ml). The 3D imageJ analysis shown in B enable better visualization of A.
A CCD image of an assay is shown in figure 3-A. Visual inspection suggests that the there is a difference between the intensity of the signal of row F (0.1 ng/ml) and the signal of row H (no-SEB control). Row G with 10-fold lower SEB concentration (0.01 ng/ml) also appears lighter by eye, and is marked with an arrow.
The signal intensities of figure 3-A were quantified using the ImageJ software and a 3D ImageJ analysis of figure 3-A is shown in figure 3-B in which the signal level for each well corresponds to the concentration of SEB. These results clearly show an increase in fluorescence intensity as the concentration of the toxin increases and signal (row G) is readily distinguishable from background (row H). The limit of detection (LOD) represents a measured concentration that generates a signal three times the standard deviation above background (no SEB). For SEB measured with the ELISA-LOC and detected using a CCD camera, the LOD is 0.1 ng/ml (row F).
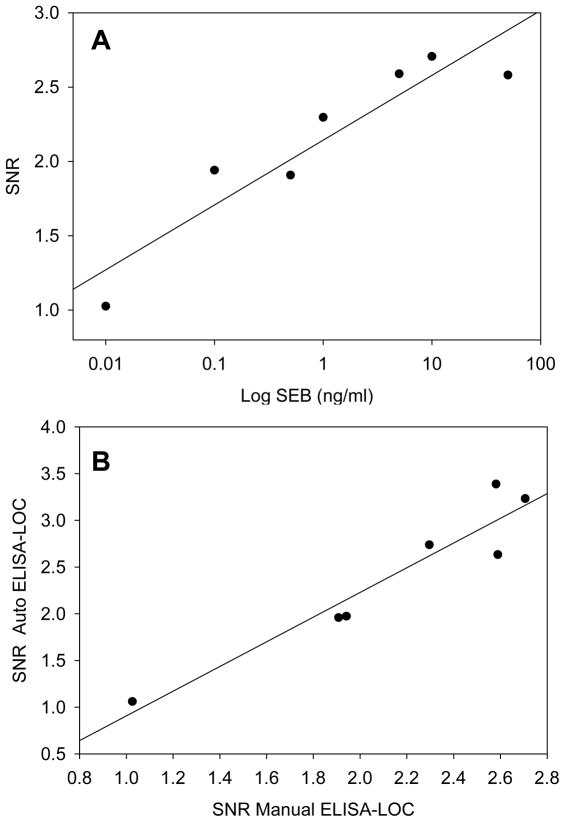
A plot of the data from figure 3-A is shown in figure 4-A, which indicates that there is a high correlation (r2 = 0.87) between the concentration of SEB and the signal to noise ratio (SNR). The level of SEB in figure 4-A is plotted in log scale.
Figure 4. Comparison of automated ELISA-LOC with the non-automated ELISA-LOC.
The signal intensities of various concentrations of SEB from figure 3-A were quantified using the ImageJ software and the calculated ratio of the signal to noise ratio (SNR) used to quantify the signal was plotted (A). The SNR values from the automated ELISA-LOC were plotted against values from non-automated ELISA-LOC (B)
We compared the automated POC with ECL detection to the non-automated ELISA-LOC. The LOD is shown for both assays in figure 4-B. The methods are very similar in terms of their linearity, and are highly correlated (r2=0.90) suggesting that the detection methods are comparable. For the standard ELISA assays of SEs, the reported LOD ranges from 0.1 10 ng/mL [51]. These data suggest that automated ELISA-LOC is as sensitive as a regular ELISA and non-automated ELISA-LOC.
Discussion
The original ELISA-LOC [28; 29], which integrated a washing system with an ELISA plate, had several features intended to make it a portable and suitable for use in field or point-of-care settings. These included operation by a syringe, rather than with battery or electric power, and capacity to analyze 96 samples simultaneously without a lab. However, manual operation limited the use of the device for point-of-care high throughput applications where many samples have to be analyzed rapidly.
The combination of ELISA-LOC and computerized fluid delivery open platform adapted from previous work [33], described here, enables automation and simplifies the operation of ELISA-LOC and broadens potential point-of-care applications for the technology. If this system is combined with more automated image analysis and image transfer technology, it will allow automated multi-analyte detection and high thoughput immunological detection providing results that can be used in resource-poor settings to support telemedicine.
In this point-of-care we used a cooled CCD was used to measure ECL light signal. The captured image can be quantitated and transmitted, allowing use in practically any setting. However, any low cost camera can be used, which may further reduce the cost of the detector.
As shown here, the point-of-care system detected SEB concentrations as low as 0.1 ng/ml, we also tested visual detection, using silver staining with the ELISA-LOC [29] and were able to measure SEB at concentrations as low as 0.5 ng/ml without using a CCD or other detector. However such visual simple detection which does not requires any equipment is only semiquantitative, at best and the CCD detection used in this work is more sensitive and quantitative.
The simple “generic” open platform modular fluid delivery automation design used here is based on off-the-shelf inexpensive components and can also be used to automate other LOC systems. We demonstrate that the open platform modular design for fluidics can be easily adapted to a broad array of detection technologies ranging from electronic sensors to optical sensors and from six channels of detection [33] to 96 channel of detection. Such versatility was possible because access capability was built in by including six-way valves to enable the performance of complex assays with six different reagents or a two-pump configuration. While this access capability was not used in the previous application (only two reagents) or for this detection modality (utilizing four reagents) it has the potential to be adapted for more complex assays in the future.
For Global health application the cost of a system is an issue, as discussed above, keeping many functions, such as valves, off the chip simplifies the chip and reduces fabrication cost. In term of actual costs, the reagents (primary and secondary antibodies+ ECL reagents) for SEB detection using a regular ELISA plate (e.g. 100ul microtiter plate) are about $1 per assay. Using our 20ul miniature plate for the same ECL assay the price per assay is approximately 20 cents per assay. The cost of the plate, if mass produced, is approximately $0.25. Thu, the new assay is significantly cheaper, both because a lower volume of reagent is used and because the cost of the actual reagents is lower. Moreover, the instrumentation cost for the assay has been reduced dramatically, replacing a costly (e.g. $3,000–$20,000) plate reader with ~$100 CCD camera or scanner, and replacing the washer (~$2500–$10,000) with a lower cost fluidic system. The cost of hardware (pumps, valves and AD/DA board), excluding the computer, is ~$700.
The new detection system may have many biomedical applications including screening for microbial pathogens and their toxins, containment of epidemics such as flu, HIV screening, and food safety testing in which rapid immunological detection of large number of sample in remote area is needed. It is also well suited for use in resource-poor settings.
References
- 1.Hay Burgess DC, Wasserman J, Dahl CA. Global health diagnostics. Nature. 2006;444(Suppl 1):1–2. doi: 10.1038/nature05440. [DOI] [PubMed] [Google Scholar]
- 2.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G, Hay Burgess DC. Requirements for high impact diagnostics in the developing world. Nature. 2006;444(Suppl 1):73–9. doi: 10.1038/nature05448. [DOI] [PubMed] [Google Scholar]
- 3.Yager P, Domingo GJ, Gerdes J. Point-of-care diagnostics for global health. Annu Rev Biomed Eng. 2008;10:107–44. doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 4.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–4. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 5.Van Weemen BK, Schuurs AH. Immunoassay using antigen-enzyme conjugates. FEBS Lett. 1971;15:232–236. doi: 10.1016/0014-5793(71)80319-8. [DOI] [PubMed] [Google Scholar]
- 6.Ihara M, Yoshikawa A, Wu Y, Takahashi H, Mawatari K, Shimura K, Sato K, Kitamori T, Ueda H. [In Process Citation] Lab Chip. 10:92–100. doi: 10.1039/b915516c. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Sherman PM, Sun Y, Li D. Multiplexed high-throughput electrokinetically-controlled immunoassay for the detection of specific bacterial antibodies in human serum. Anal Chim Acta. 2008;606:98–107. doi: 10.1016/j.aca.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 8.Kong J, Jiang L, Su X, Qin J, Du Y, Lin B. Integrated microfluidic immunoassay for the rapid determination of clenbuterol. Lab Chip. 2009;9:1541–7. doi: 10.1039/b818430e. [DOI] [PubMed] [Google Scholar]
- 9.Tseng YT, Yang CS, Tseng FG. A perfusion-based micro opto-fluidic system (PMOFS) for continuously in-situ immune sensing. Lab Chip. 2009;9:2673–82. doi: 10.1039/b823449c. [DOI] [PubMed] [Google Scholar]
- 10.Lee BS, Lee JN, Park JM, Lee JG, Kim S, Cho YK, Ko C. A fully automated immunoassay from whole blood on a disc. Lab Chip. 2009;9:1548–55. doi: 10.1039/b820321k. [DOI] [PubMed] [Google Scholar]
- 11.Javanmard M, Talasaz AH, Nemat-Gorgani M, Pease F, Ronaghi M, Davis RW. Electrical detection of protein biomarkers using bioactivated microfluidic channels. Lab Chip. 2009;9:1429–34. doi: 10.1039/b818872f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tachi T, Kaji N, Tokeshi M, Baba Y. Microchip-based homogeneous immunoassay using fluorescence polarization spectroscopy. Lab Chip. 2009;9:966–71. doi: 10.1039/b813640h. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Qiu X, Ongagna S, Chen D, Chen Z, Abrams WR, Malamud D, Corstjens PL, Bau HH. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip. 2009;9:768–76. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meagher RJ, Hatch AV, Renzi RF, Singh AK. An integrated microfluidic platform for sensitive and rapid detection of biological toxins. Lab Chip. 2008;8:2046–53. doi: 10.1039/b815152k. [DOI] [PubMed] [Google Scholar]
- 15.Reichmuth DS, Wang SK, Barrett LM, Throckmorton DJ, Einfeld W, Singh AK. Rapid microchip-based electrophoretic immunoassays for the detection of swine influenza virus. Lab Chip. 2008;8:1319–24. doi: 10.1039/b801396a. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Yu F, Zare RN. Microfluidic device for immunoassays based on surface plasmon resonance imaging. Lab Chip. 2008;8:694–700. doi: 10.1039/b800606g. [DOI] [PubMed] [Google Scholar]
- 17.Choi Y, Kang T, Lee LP. Plasmon resonance energy transfer (PRET)-based molecular imaging of cytochrome c in living cells. Nano Lett. 2009;9:85–90. doi: 10.1021/nl802511z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas LJ, Chesler JN, Yoon JY. Lab-on-a-chip immunoassay for multiple antibodies using microsphere light scattering and quantum dot emission. Biosens Bioelectron. 2007;23:675–81. doi: 10.1016/j.bios.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Stevens DY, Petri CR, Osborn JL, Spicar-Mihalic P, McKenzie KG, Yager P. Enabling a microfluidic immunoassay for the developing world by integration of on-card dry reagent storage. Lab Chip. 2008;8:2038–45. doi: 10.1039/b811158h. [DOI] [PubMed] [Google Scholar]
- 20.Tang D, Tang J, Su B, Ren J, Chen G. Simultaneous determination of five-type hepatitis virus antigens in 5min using an integrated automatic electrochemical immunosensor array. Biosens Bioelectron. 2009 doi: 10.1016/j.bios.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Mujika M, Arana S, Castano E, Tijero M, Vilares R, Ruano-Lopez JM, Cruz A, Sainz L, Berganza J. Magnetoresistive immunosensor for the detection of Escherichia coli O157:H7 including a microfluidic network. Biosens Bioelectron. 2009;24:1253–8. doi: 10.1016/j.bios.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Lee SM, Hwang KS, Yoon HJ, Yoon DS, Kim SK, Lee YS, Kim TS. Sensitivity enhancement of a dynamic mode microcantilever by stress inducer and mass inducer to detect PSA at low picogram levels. Lab Chip. 2009;9:2683–90. doi: 10.1039/b902922b. [DOI] [PubMed] [Google Scholar]
- 23.Sapsford KE, Francis J, Sun S, Kostov Y, Rasooly A. Miniaturized 96-well ELISA chips for staphylococcal enterotoxin B detection using portable colorimetric detector. Anal Bioanal Chem. 2009;394:499–505. doi: 10.1007/s00216-009-2730-z. [DOI] [PubMed] [Google Scholar]
- 24.Yang M, Kostov Y, Bruck HA, Rasooly A. Carbon nanotubes with enhanced chemiluminescence immunoassay for CCD-based detection of Staphylococcal enterotoxin B in food. Anal Chem. 2008;80:8532–7. doi: 10.1021/ac801418n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sapsford KE, Sun S, Francis J, Sharma S, Kostov Y, Rasooly A. A fluorescence detection platform using spatial electroluminescent excitation for measuring botulinum neurotoxin A activity. Biosens Bioelectron. 2008;24:618–25. doi: 10.1016/j.bios.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Kostov Y, Bruck HA, Rasooly A. Gold nanoparticle-based enhanced chemiluminescence immunosensor for detection of Staphylococcal Enterotoxin B (SEB) in food. Int J Food Microbiol. 2009;133:265–71. doi: 10.1016/j.ijfoodmicro.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun S, Ossandon M, Kostov Y, Rasooly A. Lab-on-a-chip for botulinum neurotoxin a (BoNT-A) activity analysis. Lab Chip. 2009;9:3275–81. doi: 10.1039/b912097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun S, Yang M, Kostov Y, Rasooly A. ELISA-LOC: lab-on-a-chip for enzyme-linked immunodetection. Lab Chip. 2010;10:2093–100. doi: 10.1039/c003994b. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Sun S, Kostov Y, Rasooly A. A simple 96 well microfluidic chip combined with visual and densitometry detection for resource-poor point of care testing. Sens Actuators B Chem. 2011 doi: 10.1016/j.snb.2010.10.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang M, Kostov Y, Rasooly A. Carbon nanotubes based optical immunodetection of Staphylococcal Enterotoxin B (SEB) in food. Int J Food Microbiol. 2008;127:78–83. doi: 10.1016/j.ijfoodmicro.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Hu D, Han H, Zhou R, Dong F, Bei W, Jia F, Chen H. Gold(III) enhanced chemiluminescence immunoassay for detection of antibody against ApxIV of Actinobacillus pleuropneumoniae. Analyst. 2008;133:768–73. doi: 10.1039/b715476c. [DOI] [PubMed] [Google Scholar]
- 32.Rubtsova M, Kovba GV, Egorov AM. Chemiluminescent biosensors based on porous supports with immobilized peroxidase. Biosens Bioelectron. 1998;13:75–85. doi: 10.1016/s0956-5663(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Sun S, Bruck HA, Kostov Y, Rasooly A. Lab-on-a-chip for label free biological semiconductor analysis of staphylococcal enterotoxin B. Lab Chip. 2010;10:2534–40. doi: 10.1039/c005141a. [DOI] [PubMed] [Google Scholar]
- 34.Archer DL, Young FE. Contemporary issues: diseases with a food vector. Clin Microbiol Rev. 1988;1:377–98. doi: 10.1128/cmr.1.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsen SJ, MacKinnon LC, Goulding JS, Bean NH, Slutsker L. Surveillance for foodborne-disease outbreaks--United States, 1993–1997. Mor Mortal Wkly Rep CDC Surveill Summ. 2000;49:1–62. [PubMed] [Google Scholar]
- 36.Bean NH, Goulding JS, Lao C, Angulo FJ. Surveillance for foodborne-disease outbreaks--United States, 1988–1992. Mor Mortal Wkly Rep CDC Surveill Summ. 1996;45:1–66. [PubMed] [Google Scholar]
- 37.Bunning VK, Lindsay JA, Archer DL. Chronic health effects of microbial foodborne disease. World Health Stat Q. 1997;50:51–6. [PubMed] [Google Scholar]
- 38.Garthright WE, Archer DL, Kvenberg JE. Estimates of incidence and costs of intestinal infectious diseases in the United States. Public Health Rep. 1988;103:107–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Asao T, Kumeda Y, Kawai T, Shibata T, Oda H, Haruki K, Nakazawa H, Kozaki S. An extensive outbreak of staphylococcal food poisoning due to low-fat milk in Japan: estimation of enterotoxin A in the incriminated milk and powdered skim milk. Epidemiol Infect. 2003;130:33–40. doi: 10.1017/s0950268802007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breuer K, Wittmann M, Bosche B, Kapp A, Werfel T. Severe atopic dermatitis is associated with sensitization to staphylococcal enterotoxin B (SEB) Allergy. 2000;55:551–5. doi: 10.1034/j.1398-9995.2000.00432.x. [DOI] [PubMed] [Google Scholar]
- 41.Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, Renz H. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999;103:119–24. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- 42.Mempel M, Lina G, Hojka M, Schnopp C, Seidl HP, Schafer T, Ring J, Vandenesch F, Abeck D. High prevalence of superantigens associated with the egc locus in Staphylococcus aureus isolates from patients with atopic eczema. Eur J Clin Microbiol Infect Dis. 2003;22:306–9. doi: 10.1007/s10096-003-0928-0. [DOI] [PubMed] [Google Scholar]
- 43.Ryan MW, Davis LS. T cells in chronic rhinosinusitis with nasal polyposis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:200–5. doi: 10.1097/MOO.0b013e3283382082. [DOI] [PubMed] [Google Scholar]
- 44.Howell MD, Diveley JP, Lundeen KA, Esty A, Winters ST, Carlo DJ, Brostoff SW. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991;88:10921–5. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uematsu Y, Wege H, Straus A, Ott M, Bannwarth W, Lanchbury J, Panayi G, Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991;88:8534–8. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herz U, Bunikowski R, Mielke M, Renz H. Contribution of bacterial superantigens to atopic dermatitis. Int Arch Allergy Immunol. 1999;118:240–1. doi: 10.1159/000024085. [DOI] [PubMed] [Google Scholar]
- 47.Wiener SL. Strategies for the prevention of a successful biological warfare aerosol attack. Mil Med. 1996;161:251–6. [PubMed] [Google Scholar]
- 48.Ler SG, Lee FK, Gopalakrishnakone P. Trends in detection of warfare agents. Detection methods for ricin, staphylococcal enterotoxin B and T-2 toxin. J Chromatogr A. 2006;1133:1–12. doi: 10.1016/j.chroma.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 49.Henghold WB., 2nd Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin. 2004;22:257–62. v. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbloom M, Leikin JB, Vogel SN, Chaudry ZA. Biological and chemical agents: a brief synopsis. Am J Ther. 2002;9:5–14. doi: 10.1097/00045391-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Bennett RW. Staphylococcal enterotoxin and its rapid identification in foods by enzyme-linked immunosorbent assay-based methodology. J Food Prot. 2005;68:1264–70. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]