Abstract
The stephacidin and notoamide natural products belong to a group of prenylated indole alkaloids containing a bicyclo[2.2.2]diazaoctane core. Biosynthetically, this bicyclic core is believed to be the product of an intermolecular Diels- Alder (IMDA) cycloaddition of an achiral azadiene. Since all of the natural products in this family have been isolated in enantiomerically pure form to date, it is believed that an elusive Diels-Alderase enzyme mediates the IMDA reaction. Adding further intrigue to this biosynthetic puzzle is the fact that several related Aspergillus fungi produce a number of metabolites with the opposite absolute configuration, implying that these fungi have evolved enantiomerically distinct Diels-Alderases. We have undertaken a program to identify every step in the biogenesis of the stephacidins and notoamides, and by combining the techniques of chemical synthesis and biochemical analysis we have been able to identify the two prenyltransferases involved in the early stages of the stephacidin and notoamide biosyntheses. This has allowed us to propose a modified biosynthesis for stephacidin A, and has brought us closer to our goal of finding evidence for, or against, the presence of a Diels-Alderase in this biosynthetic pathway.
Keywords: Aspergillus, biosynthesis, cycloaddition, Diels–Alder, indoles
1. Introduction
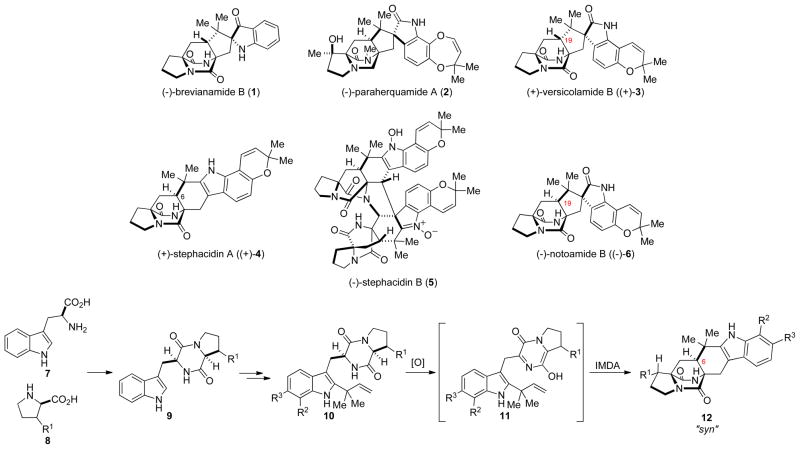
Fungal-derived natural products are a diverse class of compounds with interesting structural features and biological activities that have allowed for them to account for a significant number of clinical therapeutics.[1] For this reason, an increased effort has been placed on the isolation of new members of this class of natural products, and in screening these secondary metabolites for useful biological activities. The diverse class of prenylated indole alkaloids isolated from the Aspergillus and Penicillium genera of fungi have received increasing levels of attention do to their interesting chemical architectures and biological activities. Structurally, the paraherquamide,[2] brevianamide,[3] stephacidin,[4] and notoamide[5] natural products are unique in that many of them contain a bicyclo[2.2.2]diazaoctane core structure (Scheme 1). These families of alkaloids have attracted biomedical attention and have been reported to display antitumor,[4] insecticidal,[6] antihelmintic,[2d] calmodulin-inhibititory,[7] and antibacterial properties.[4] These intriguing structural and biological properties have lead to a number of synthetic endeavours towards this class of natural products.[8]
Scheme 1.
Representative prenylated alkaloids and their proposed biosynthesis.
The pioneering work from Sammes[9] and Birch,[10] along with more recent work from our laboratories,[11] has shown that these natural products are derived from L-tryptophan, a cyclic amino acid residue such as proline, β-methylproline, or pipecolic acid, and one or two isoprene units. Furthermore, the current experimental evidence suggests that the bicyclo[2.2.2]diazaoctane ring system arises from an intramolecular hetero Diels-Alder reaction (IMDA) of an achiral 5-hydroxypyrazin-2(1H)-one.[11, 12] Since all of the members of this family of natural products have been isolated in entiomerically pure form, the putative biosynthetic IMDA reaction must proceed in a stereo- and enantio-controlled fashion, implying that this cycloaddition is most likely enzyme-mediated. Although enzyme-mediated Diels-Alder reactions have been proposed in the biosyntheses of a number of natural products, the existence of a Diels-Alderase remains controversial.[13] In providing corroborating support for this biosynthetic proposal, we have shown that the IMDA is an effective approach for the laboratory synthesis of the bicyclo[2.2.2]diazaoctane core, and have applied this methodology to the biomimetic syntheses of a number of natural products.[14–18]
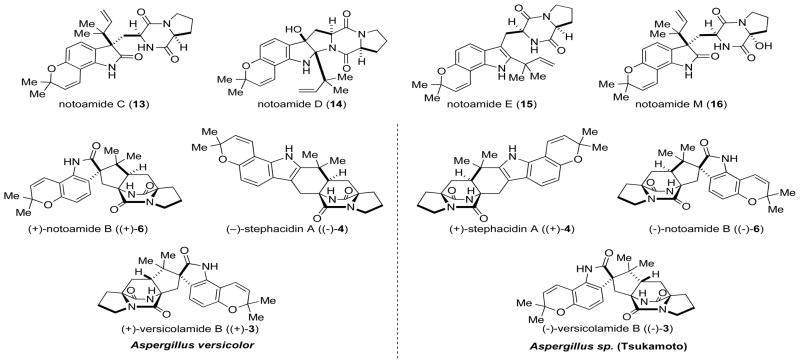
Since the isolation of (+)-stephacidin A ((+)-4) and B (5) in 2002 by Bristol-Meyers-Squib from Aspergillus ochraceus,[10] our group has taken an interest in both the synthesis[14, 19] and biosynthesis of this secondary metabolite. Our interest in the biosynthesis of 4 grew further when Tsukamoto and co-workers isolated the structurally related notamide natural products, as well as (+)-stephacidin A and (−)-versicolamide B ((−)-3), from a marine derived Aspergillus sp.[5] (Figure 1). Whereas the notoamide alkaloids are of interest because they may provide clues to the biogenesis of stephacidin A, the isolation of veriscolamide B was of particular interest due to its stereochemical relationship to the other stephacidin and notoamide metabolites. The majority of the members of the paraherquamides, stephacidins, and notoamides (ie. 2, 4, and 6) have a bridgehead hydrogen (C6 in stephacidin A) with a syn- relative configuration, while in veriscolamide B (C19 in vesicolamide B) and the brevianamides (ie. 1) the bridgehead hydrogen is in an anti- configuration. This difference in the relative stereochemistry about the bicyclo[2.2.2]diazaoctane core poses a number of fascinating questions relating to the biosynthesis of both 3 and 4.
Figure 1.
Representative notoamide alkaloids and the enantiomeric forms of stephacin A, notoamide B, and veriscolamide B.
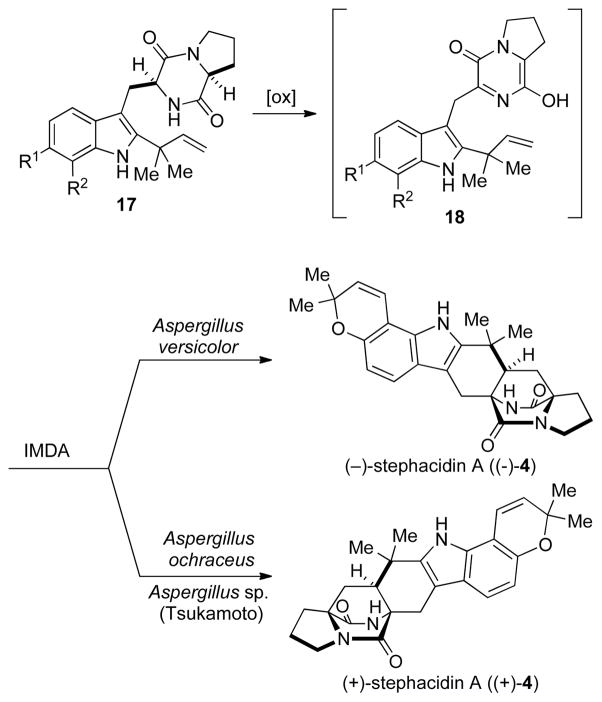
Adding further intrigue to the biogenesis of the stephacidins and notoamides is the fact that in 2006 the enantiomers of stephacidin A ((−)-4), notoamide B ((+)- 6), and veriscolamide B ((+)-3) were isolated from the terrestrial fungus Aspergillus versicolor.[20] Although examples of antipodal natural products are known to be produced by Nature, given the vast number of naturally occurring secondary metabolites, examples of this phenomena are exceedingly rare. In terms of the biosynthesis of 4, the isolation of the antipodal forms of stephacidin A implies that the different species of Aspergillus have evolved enantiomerically distinct genes for the biosyntheses of the stephacidin and notoamide natural products. Moreover, because the chirality of the bicyclo[2.2.2]diazaoctane core is believed to be formed from an achiral aza-diene via the putative IMDA, the implication is that the Aspergillus species may have evolved enantiomerically distinct Diels-Alderases (Scheme 2). Thus, stephacidin A would arise from an appropriate reverse-prenylated cyclo-L-typtophan-L-proline, which would undergo oxidation to give the achiral azadiene 18, which would undergo an IMDA to give the two enantiomers of stephacidin A in each specific species, respectively.
Scheme 2.
The proposed biogenesis of the both enantiomers of stephacidin A.
To answer these intriguing questions about the biosynthesis of the stephacidins and notoamides our research group has embarked on a program to elucidate every stage of their biosynthetic constructions. The goal of this project is to both identify every intermediate in the biosynthesis, as well as to identify and assign function to every gene in the notoamide gene cluster. To do this a number of chemical and biochemical techniques have been utilized, and our current efforts to determining the complete biosynthetic pathway are summarized herein.
2. Investigations Into the Biosynthesis of the Stephacidin and Notoamide Alkaloids
2.1. Originally Proposed Biosynthesis of Stephacidin A
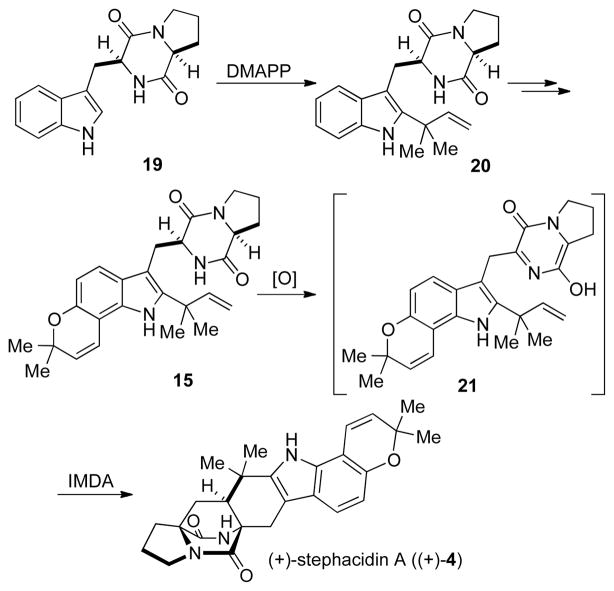
In 2009, our laboratory had inadvertently synthesized a prenylated indole alkaloid that would later be assigned the name notoamide E (15). We provided an authentic sample of this substance to the Tsukamoto laboratory wherein this synthetic material was used to isolate and identify this short-lived metabolite (notoamide E, 15) from a marine-derived Aspergillus sp.[5b] This led to our first proposed biogenesis of stephacidin A, which is outlined in Scheme 3. Starting from brevianamide F (19), reverse prenylation would give deoxybrevianamide E (20), a known metabolite in stephacidin-producing organisms.[5a] Further prenylation and oxidation could then provide notoamide E. Oxidation of 15 would provide the transient achiral azadiene (21), which could undergo the IMDA cyclization to give stephacidin A. In order to determine if notoamide E was indeed the precursor to 4 we under took the synthesis of both un 15 and doubly 13C 15 for use in biosynthetic incorporation studies.
Scheme 3.
Originally proposed biosynthetic route to stephacidin A via notamide E.
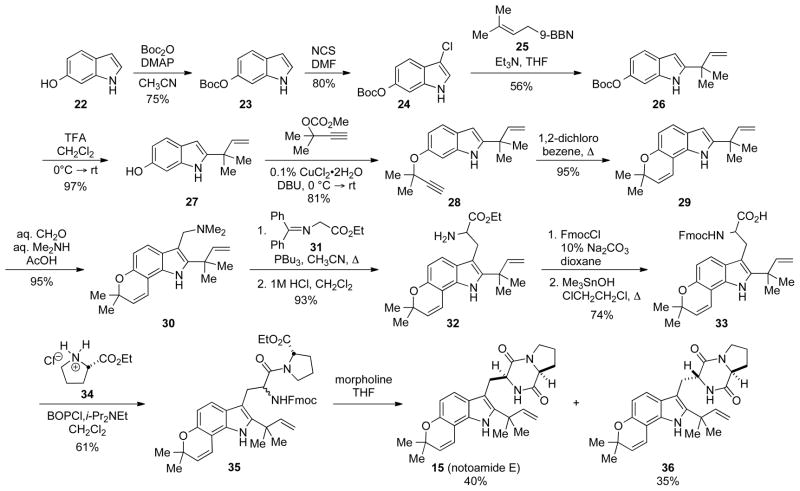
The synthesis of notoamide E[21] started from 6-hydroxyindole, which was O-protected to give 23 (Scheme 4). Subsequent chlorination and reverse prenylation following conditions first reported by Danishefsky[22] provided the C2-reverse-prenylated 26. The Boc-group was then removed with TFA, and the resulting phenol was propargylated to give 28 in good yield. Heating 28 in 1,2-dichlorobenzene resulted in the formation of the pyran ring. Tryptophan 32 was then readily obtained via a three step procedure involving formation of gramine 30, Somei-Kametani[23] coupling with the glycine benzophenone imine 31, and subsequent hydrolysis. The free amine was then Fmoc-protected, and Me3SnOH mediated hydrolysis of the ethyl ester provided the racemic acid 33.
Scheme 4.
Synthesis of notoamide E.
The acid 33 was then coupled with proline ethyl ester to give a mixture of diasteriomeric dipetides 35. Treatment of 35 with morpholine resulted in removal of the Fmoc-group and subsequent cyclization to provide notoamide E and epi-notoamide E in 40% and 35% yields, respectively. Thus, the total synthesis of notoamide E was completed in 13 steps from commercially available 6-hydroxyindole. This route also provided access to doubly 13C notoamide E (37), which was synthesized for use in biosynthetic incorporation studies.
2.1.2. Notoamide E Incorporation Studies
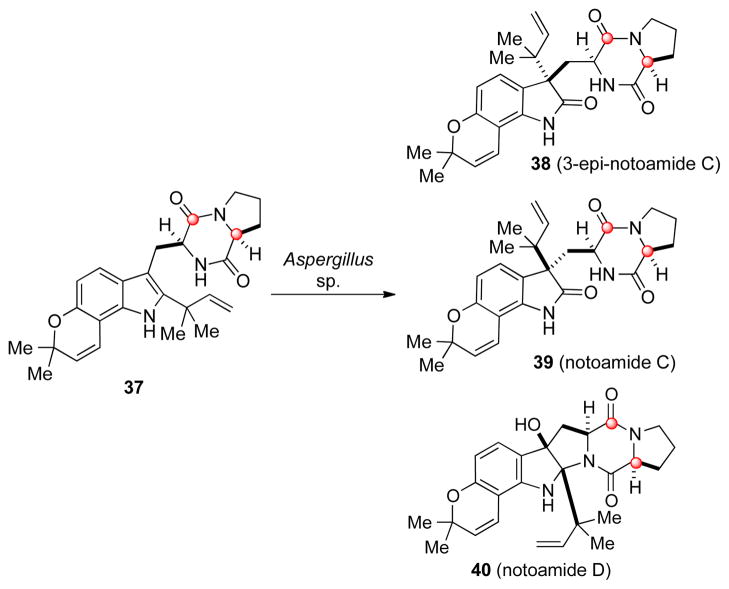
The synthetic doubly 13C-labeled 37 was incubated with the marine derived Aspergillus sp., and the fungal extracts were analyzed for the presence of 13C labelled metabolites. If notoamide E was indeed the precursor to stephacidin A, then the stephacidin A isolated from this experiment should exhibit some level of 13C incorporation. However, upon analyzing the fungal abstracts, the major metabolites found containing 13C enrichment were notoamides C, 3-epi-notoamide C, and notoamide D. To our surprise, no 13C labeled 3, 4, or 6 could be detected (Scheme 5).[5b]
Scheme 5.
Incorporation studies with doubly 13C-labeled notoamide E.
These results suggested that notoamide E is not the precursor to stephacidin A, and that our originally proposed biogenesis was incorrect. If notoamide E is not the precursor to stephacidin A, and if the bicyclo[2.2.2]diazaoctane ring does indeed arise from an IMDA reaction, then the order of steps in our original biosynthetic proposal needed to be re-evaluated. One plausible alternative, envisioned that the formation of the bicyclo[2.2.2]diazaoctane core occurred before the formation of the pyran ring. With this in mind, we set out to develop and test a new biogenetic hypothesis.
2.2. Alternative Biogenesis of Stephacidin A
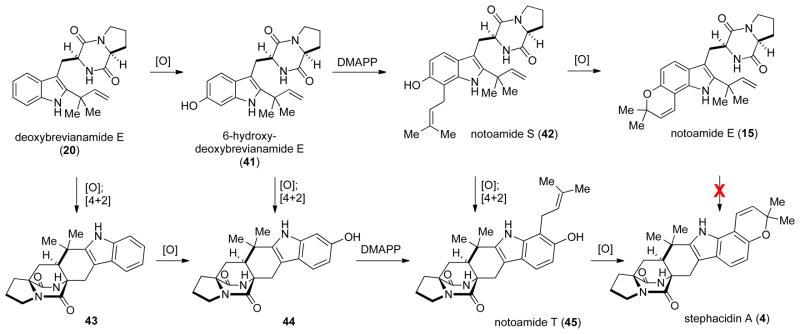
The formation notoamide E from 20 requires three distinct steps, namely, an oxidation, a prenylation, and a final oxidation/cyclization to form the pyran ring, with the order of the initial oxidation and prenylation stages being unknown. The IMDA could potentially occur before each of these steps, which leads to a number of biosynthetic possibilities that are outlined in Scheme 6.
Scheme 6.
Potential biosynthetic pathways to stephacidin A.
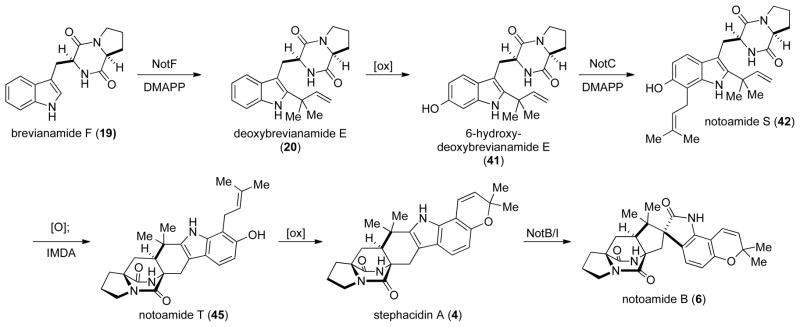
Deoxybrevianamide E (20) can undergo either oxidation to 41, or oxidation and IMDA cyclization to give 43. If 20 undergoes an oxidation and IMDA reaction to give 43, then a straightforward oxidation/prenylation/cyclization (43→44→45→4) pathway would lead to stephacidin A. However, if 20 undergoes oxidization to 41, two pathways to stephacidin A are possible. 6-Hydroxydeoxy-brevianamide E (41) could be prenylated to give 42, which must then undergo the IMDA to give 45. Alternatively, 41 could undergo the IMDA reaction to give hexacycle 44, which could then be prenylated to give 45. Additionally, since the exact order of oxidation and prenylation steps is unknown, it is possible that prenylation of 20 or 43 happens prior to the azadiene oxidation. Regardless of the pathway, compound 45, which we have named notoamide T, would be the last [stable] intermediate in the biosynthesis of stephacidin A.
In order to ascertain which of these pathways is operable in the biosynthesis of stephacidin A, we set out to synthesize all of the remaining potential biosynthetic intermediates for use as authentic samples in identifying hitherto unknown, trace or short-lived metabolites. We additionally required that access to 13C-labeled isotopomers of these substances for incorporation studies be readily adaptable from the synthetic routes. In addition to this, we have initiated efforts into the genome-based characterization of the producing Aspergillus sp. to help us fully elucidate the biogenesis of the notoamide and stephacidin alkaloids.
2.3. Syntheses of the Proposed Biosynthetic Intermediates to Stephacidin A
2.3.1. Synthesis of 6-Hydroxydeoxybrevianamide E
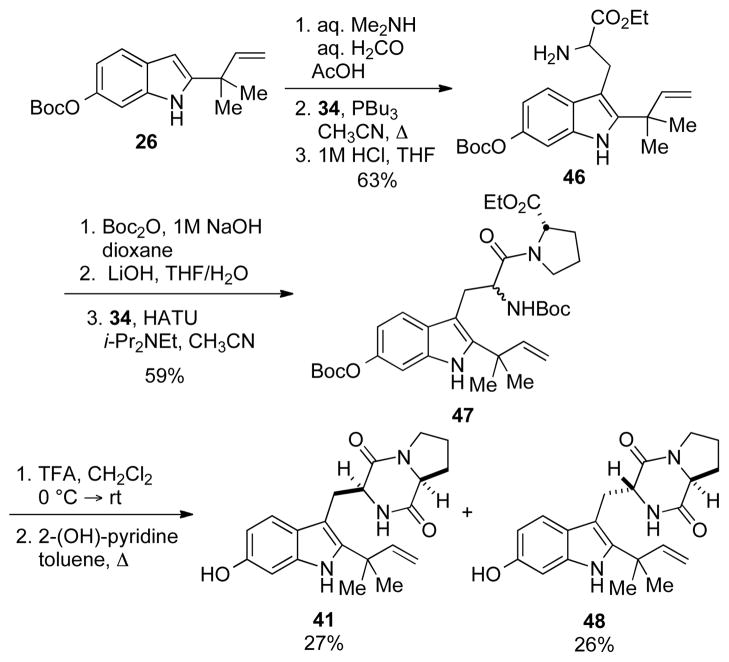
In addition to being a potential intermediate in the biosynthesis of stephacidin A, 6-hydroxydeoxy-brevianamide E is also believed the be the precusor to notoamide J, and it was in this context that the synthesis of 41 was developed.[24] The synthesis of 41 began from the reverse prenylated 6-hydroxyindole 26 and followed a route similar to that described for the synthesis of notoamide E (Scheme 7). Thus, indole 26 was converted to the corresponding gramine derivative and reacted in a Somei-Kametani coupling to provide tryptophan 46 in good yield. Subsequent re-functionalization of the amino acid and coupling with (S)-proline ethyl ester gave the dipeptide 47. The Boc-groups were then removed with TFA, and the resultant crude amine was heated in toluene to provide 41 in 27% yield along with an equal amount of the trans-diketopiperazine (DKP).
Scheme 7.
The synthesis of 6-hydroxydeoxybrevianamide E (41).
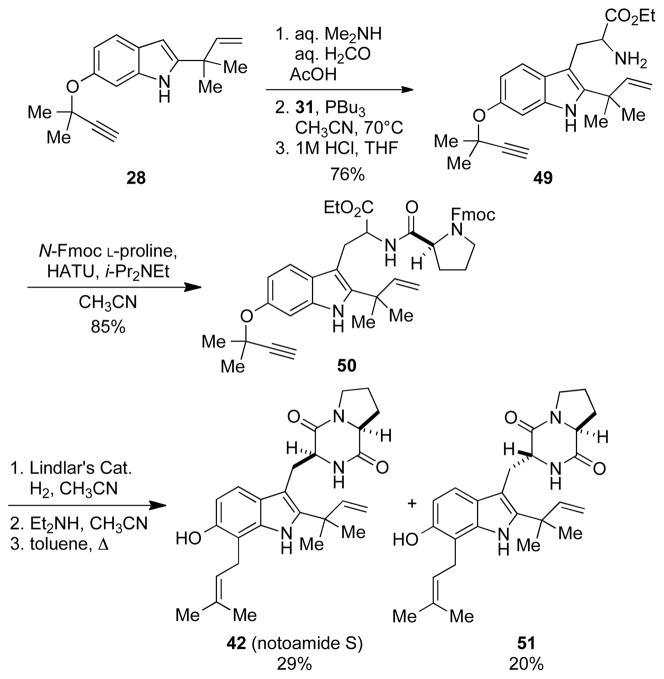
2.3.2. Total Synthesis of Notoamide S
The synthesis of notoamide S[25] was initiated from the propargylated indole 28, which was elaborated into tryptophan 49 as previously described (Scheme 8). Tryptophan 49 was then coupled with N-Fmoc proline to give 50 in high yield. Subsequent Lindlar’s reduction and removal of the Fmoc-group provided the free amine, which upon heating in toluene underwent an aromatic Claisen rearrangement and cyclization to give 42 in 29% yield over the three steps. Thus, the synthesis of notoamide S was completed in twelve steps from commercially available 6-hydroxyindole.
Scheme 8.
The total synthesis of notoamide S (42).
Although at the outset of the synthesis, the natural origin of notoamide S was unknown, the authentic sample was later used in help to prove the existence of 42 as an intermediate in the biosynthesis of stephacidin A. In addition, this route is currently being utilized for the synthesis of double 13C labelled notoamide S needed for future incorporation studies.
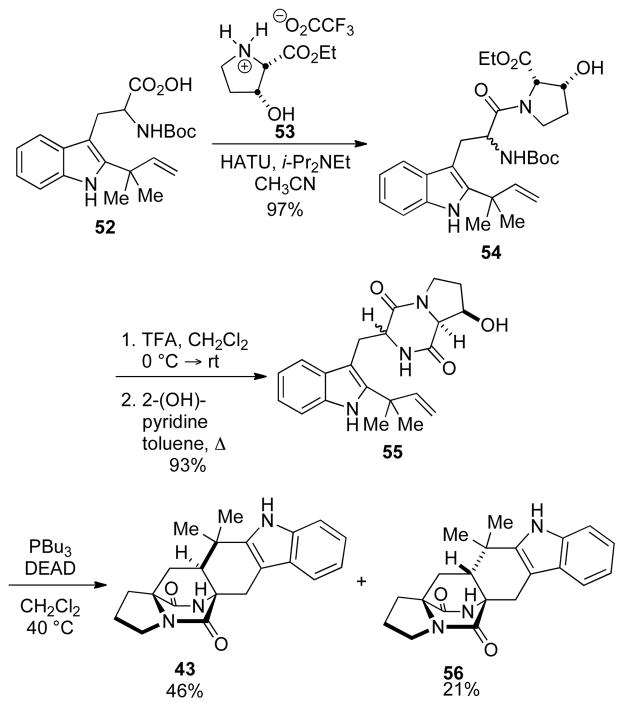
2.3.3. Synthesis of Substrate 43
The simple hexacycle 43 represents the earliest possible bicylo[2.2.2]diazaoctane-containing compound in the biosynthesis of stephacidin A. This substance (also named ketopremalbranchiamide) was first synthesized in our laboratory some twenty years ago,[26] and more recently has been employed as an intermediate in the synthesis of 13C-labeled compounds that were utilized in the interrogation of the biogenesis of the structurally related malbranchiamides.[27, 28]
Our biomimetic synthesis[14b] of 43 began with the coupling of the reverse-prenylated tryptophan 52[16a] with hydroxyproline 53 to give a diastereomeric mixture of dipeptides 54 (Scheme 9). Subsequent removal of the Boc-group and cyclization to the DKP provided 55. The reaction of 55 under Mitsunobu conditions in warm CH2Cl2 provided the cycloadducts 43 and 56 in 46% and 21% yields, respectively, thus providing us with ample amounts of 43 for further biological studies.
Scheme 9.
The synthesis of substrate 43.
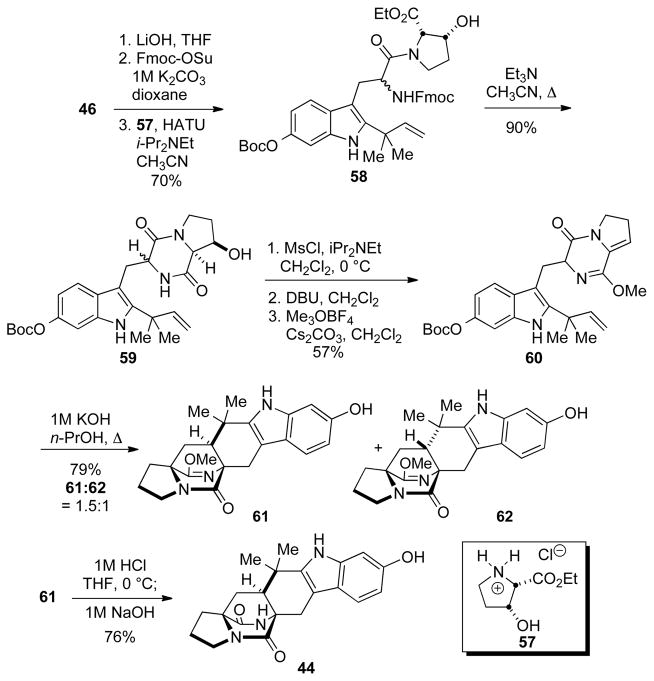
2.3.4. Synthesis of Substrate 44
The synthesis of substrate 44[29] was modeled after our first reported biomimetic synthesis of stephacidin A.[14a] Thus, the tryptophan 46, was re-functionalized and coupled with hydroxyproline derivative 57 to give 58. Treatment of 58 with Et3N in refluxing acetonitrile resulted in cleavage of the Fmoc-group and cyclization to the diketopiperazine. Subseqent elimination of the alcohol moiety in 59, and conversion of the resulting enamide to its lactim ether provided 60. Upon treatment with aqueous KOH 60 underwent the desired isomerization and cyclization to give a separable mixture of the syn- and anti- products 61 and 62. Final hydrolysis of the lactim ether in 61 provide the desired substrate 44.
With the syntheses of 41, 42, 43, and 44 we have accomplished the synthesis of a majority of the potential plausible intermediates fort he biosynthesis of stephacidin A. As seen in the next section, these authentic samples proved crucial in determining the early steps of the biosynthetic pathway.
2.4. Genome-Based Characterization of the Early Steps in the Stephacidin A Biosynthesis
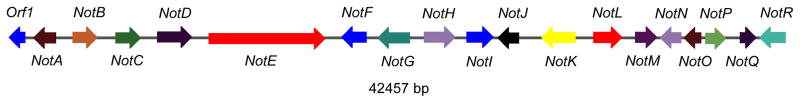
While we were in the process of synthesizing all of the potential intermediates on the stephacidin A biosynthetic pathway, we also under took a genome-based characterization of the notoamide and stephacidin biosynthetic pathways. To this end, the genome of the marine derived Aspergillus sp. was sequenced and the notoamide (not) gene cluster was identified (Figure 3).[29]
Bioinformatics analysis helped to predict the function of the majority of the Not gene products, and this led to the identification of two predicted aromatic prenyltransferases, NotC and NotF. These two prenyltransferases would presumably catalyze the two key prenylation reactions in the biosynthesis of stephacidin, and further analysis of their function led to the elucidation of the early stages of the pathway.
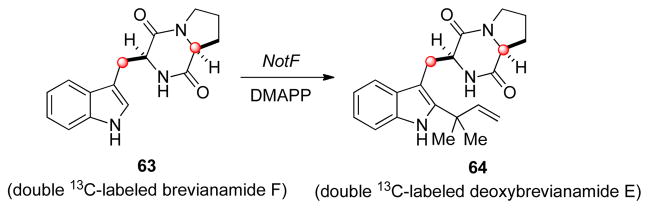
2.4.1. Identification of NotF as the Deoxybrevianamide E Synthase
NotF was first identified as a prenyltransferase due to it showing a high identity (40%) to a putative dimethylallyl tryptophan synthase (EER24759) found in Coccidioides posadasii. The NotF gene was expressed in E. coli and the purified enzyme was first tested with doubly 13C-labeled brevianamide F (63). The results of this experiment revealed that NotF was effective in converting brevianamide F into deoxybrevianamide E (Scheme 11), thus confirming the role of NotF as a reverse prenyltransferase.
Scheme 11.
Reaction of 13C-labeled brevianamide F with NotF.
The substrate specificity was then tested by reacting NotF with tryptophan, cyclo-(L-Phe-L-Pro), cyclo-(L-Trp-L-Trp), and cyclo-(L-Trp-L-Tyr). In each of these examples, no prenylated products were detected. These indicate that NotF shows selectivity for 19 and that the reverse prenylation must be an early step in the biosynthetic pathway as initially assumed.
2.4.2. Identification of NotC as the Notamide S Synthase
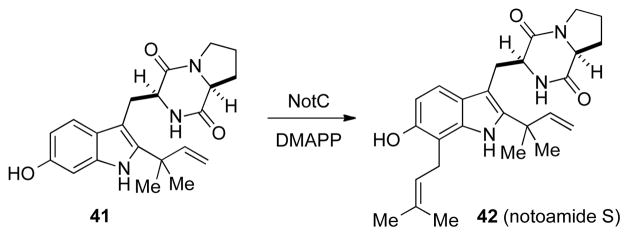
The role of NotC as a prenyltransferase was also first predicted because of its sequence identity (50%) to another known prenyltransferase, FtmH in A. fumigatus.[30] To determine the function of NotC, it was similarly expressed in E. coli and the recombinant protein was isolated and purified. The activity of NotC was then tested with four putative substrates, 20, 41, 43, and 44. In the cases of 20, 43, and 44 no prenylated products could be detected. However, when 41 was subjected to NotC, notoamide S was cleanly produced, indicating that NotC catalyzes a normal prenyltransferase at C7 of the indole ring. Similar to NotF, NotC is also highly substrate-selective, and this specificity has allowed us to identify the early stages of stephacidin biogenesis.
2.5. Revised Biogenesis of Stephacidin A
By combining the data garnered from our notoamide E feeding studies with that obtained from our genomic characterizations, we are able to propose a revised biogenesis of stephacidin A and notoamide B (Scheme 13). Starting from brevianaimde F (19), the NotF gene product catalyzes the reverse-prenylation of brevianamide F providing deoxybrevianamide E (20). Then, an as of yet unidentified oxidase converts deoxybrevianamide E into 41, which is prenylated in a normal sense by the NotC gene product to give notoamide S (42). Notoamide S is then presumed to be oxidized to the azadiene, which undergoes the IMDA reaction to provide notoamide T (45). Notoamide T would then be oxidized by another currently unidentified oxidase that mediates fashioning of the pyran ring to give stephacidin A. We have very recently applied the same methodology used to prepare 43, 44 and stephacidin A, to prepare notoamide T.[31] We are currently conscripting this synthesis to prepare double 13C-labeled notoamide T to validate it’s intermediacy as the penultimate precursor to stephacidin A.
Scheme 13.
The revised biosynthesis of stephacidin A.
Further oxidation of 4 and subsequent Pinacol rearrangement would then provide notoamide B (6); a sequence that we have recently confirmed with synthetic, double 13C-labeled stephacidin A.[32]
The identification and characterization of NotF and NotC have allowed us to determine the timing of the two prenylation steps in the biogenesis of stephacidin A and to propose a revised biogenetic pathway. However, the enzymes involved in a number of other steps are still undetermined, and until these are identified, the exact biosynthesis of 4 remains speculative.
2.6. Proposed Biosynthesis of Versicolamide B
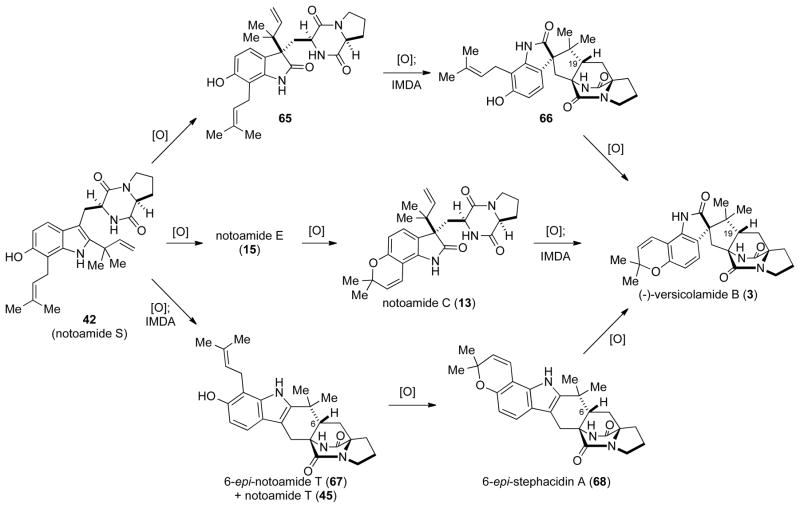
Based on the available experimental results, we are tempted to suggest that there is a branch point in the biosynthetic pathway of stephacidin A that also accommodates the construction of veriscolamide B that we currently suspect to be at the stage of notoamide S. As mention previously, versicolamide B is unique among the stephacidin and notoamide natural products due to the anti-configuration of the bridgehead hydrogen (C19) found in its bicyclo[2.2.2]diazaoctane core. To account for the stereochemical differences between 3 and 4, three possible biosynthetic pathways to veriscolamide B have been proposed as outlined in Scheme 14.
Scheme 14.
Some possible biogeneses of versicolamide B.
In the first possible route to 3, notoamide S could undergo two sequential oxidations to give notoamide C (13) via 15. Notoamide C could then undergo oxidation to the azadiene, possibly through the intermediacy of notoamide M (16), and subsequent IMDA cyclization would give (−)-veriscolamide B. In order for this route to be viable, the IMDA reaction has to occur to provide the required anti stereochemistry at C19. Fortunately, both theoretical calculations[33] and experimental results[18] have shown that the IMDA reaction of an oxindole should proceed to give the anti- product exclusively, lending support to this biogenetic pathway. The presence of notoamide M (16) among the naturally occur metabolites of Aspergillus sp.[5d] lends support to this pathway, as 16 could be the direct oxidation product of notoamide C, and the loss of water from 16 could lead directly to the requisite azadiene. For the biosynthesis of the antipodal (+)-versicolamide B, 3-epi-notoamide C would presumably be the corresponding branch point as the absolute stereochemistry at the spiro-oxindole stereogenic center would correlate to that in (+)-versicolamide B.
A similar pathway can be envisioned were the IMDA occurs prior to the formation pyran ring. In this route 42 is first converted to the oxindole 65, which can then undergo the IMDA reaction to give 66. Subsequent cyclization to form the pyran ring would then provide veriscolamide B. This pathway is analogous to the stephacidin A pathway in that the IMDA reaction occurs prior to the formation of the pyran ring. However, to date there has been no evidence suggesting the presence of 65 and/or 66 in the fungal broth, and the previously mention route seems more likely.
A third pathway involving the formation of 6-epi-stephacidin A (68) is also possible. Thus, if the IMDA reaction of the azadiene derived from 42 is not completely diastereoselective, a mixture of notoamide T (45) and 6-epi-notoamide T (67) would be produced. 6-epi-Notoamide T could then be converted to 6-epi-stephacidin A, which could be readily oxidized into 3.
However, since 6-epi-stephacidin A, or any other related 6-epi-metabolites, have not been detected as natural metabolites of any Aspergillus species to date, the likelihood of this pathway being operable is greatly diminished. Although there is currently a high level of uncertainty in the final steps of the veriscolamide B biosynthesis, future work with incorporation studies and characterization of the other Not enzymes should allow for a complete, high-resolution elucidation of the entire biosynthetic pathway.
3. Outlook
The identification and characterization of the NotF and NotC enzymes from a marine-derived Aspergillus sp. have allowed us to determine the timing of the two prenylation steps in the biogenesis of stephacidin A, and to propose a revised biogenetic pathway. However, the enzymes involved in a number of other steps are still undetermined, and until these are identified, the exact pathway remains uncertain. To this end, efforts are ongoing to identify the remaining genes involved in the pathway and parallel efforts in the genomic mining of Aspergillus versicolor, which produces the antipodal natural products, stephacidin A, notoamide B and versicolamide B, is being actively investigated. We have also undertaken the syntheses of 13C-labeled notoamides S and T, to aid in determining if 45 is truly the final precursor to both (+)- and (−)-stephacidin A.
This program has relied heavily on a robust technical platform we have built over the past two decades, to achieve the chemical synthesis of virtually every conceivable substrate and product for every stage of the biosynthetic pathways to these prenylated indole alkaloids. The discovery that Nature produces some of these metabolites in antipodal forms (and optically pure), has greatly added to the intrigue associated with how Nature fashions such beautiful structures. The technical advances in genome sequencing technology, proteomics and genomics, have provided us with the powerful tools necessary to fully understand the fascinating biogenesis of these natural substances. We are at a very exciting stage of this work and look forward to the discoveries that lie ahead.
Figure 2.
The notoamide (not) biosynthetic gene cluster
Scheme 10.
The Synthesis of substrate 44.
Scheme 12.
The prenylation of 6-hydroxydeoxybrevianamide E by NotC.
Acknowledgments
This work was supported by NIH Grant CA070375 (R.M.W.).
Biographies

Robert M. Williams recieved his B.A. degree in Chemistry in 1975 from Syracuse University. He obtained the Ph.D. degree in 1979 at MIT (W.H. Rastetter) and was a post-doctoral fellow at Harvard (1979–1980; R.B. Woodward/Yoshito Kishi). He joined Colorado State University in 1980 and was named a University Distinguished Professor in 2002. His interdisciplinary research program at the chemistry-biology interface is focused on the total synthesis of biomedically significant natural products, biosynthesis of secondary metabolites, studies on antitumor drug-DNA interactions, HDAC inhibitors, amino acids and peptides.

James D. Sunderhaus graduated from The Florida State University in 2002 with a B.S. in Chemistry and Biochemistry. He received his Ph.D. from the University of Texas - Austin in 2009 under the supervision of Prof. Stephen F. Martin. He is currently a postdoctoral fellow at Colorado State University with Prof. Robert M. Williams where he works on the synthesis of intermediates along the stephacidin and notoamide biosynthetic pathway.
Footnotes
Dedicated to Stephen F. Martin on the occasion of his 65th birthday.
References
- 1.Keller NP, Turner G, Bennett JW. Nat Rev Microbiol. 2005;3:937. doi: 10.1038/nrmicro1286. [DOI] [PubMed] [Google Scholar]
- 2.a) Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135. [Google Scholar]; b) Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J Antibiot. 1990;43:1375. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; c) Liesch JM, Wichmann CF. J Antibiot. 1990;43:1380. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; d) Blanchflower SE, Banks RM, Everett JR, Manger BR, Reading C. J Antibiot. 1991;44:492. doi: 10.7164/antibiotics.44.492. [DOI] [PubMed] [Google Scholar]; e) Blanchflower SE, Banks RM, Everett JR, Reading C. J Antibiot. 1993;46:1355. doi: 10.7164/antibiotics.46.1355. [DOI] [PubMed] [Google Scholar]
- 3.a) Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; b) Birch AJ, Russell RA. Tetrahedron. 1972;28:2999. [Google Scholar]
- 4.a) Cutrone JQ, Krampitz KD, Shu YZ, Chang LP, Lowe SE. Bristol-Myers Squibb Company; USA: 6,291,461. US Patent. 2001; b) Qian-Cutrone J, Huang S, Shu YZ, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J Am Chem Soc. 2002;124:14556. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]
- 5.a) Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew Chem, Int Ed. 2007;46:2254. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]; b) Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J Am Chem Soc. 2009;131:3834. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J Nat Prod. 2008;71:2064. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]; d) Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Org Lett. 2009;11:1297. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. J Nat Prod. 73:1438. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 6.Whyte AC, Gloer JB, Wicklow DT, Dowd PF. J Nat Prod. 1996;59:1093. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
- 7.a) Martinez-Luis S, Rodriguez R, Acevedo L, Gonzalez MC, Lira-Rocha A, Mata R. Tetrahedron. 2006;62:1817. [Google Scholar]; b) Miller KA, Figueroa M, Valente MWN, Greshock TJ, Mata R, Williams RM. Bioorg Med Chem Lett. 2008;18:6479. doi: 10.1016/j.bmcl.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KA, Williams RM. Chem Soc Rev. 2009;38:3160. doi: 10.1039/b816705m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter AEA, Sammes PG. Journal of the Chemical Society D: Chemical Communications. 1970:1103. [Google Scholar]
- 10.Baldas J, Birch AJ, Russell RA. J Chem Soc, Perkin Trans. 1974;1:50. [Google Scholar]
- 11.Williams RM, Stocking EM, Sanz-Cervera JF. Top Curr Chem. 2000;209:97. [Google Scholar]
- 12.Williams RM. Chem Pharm Bull. 2002;50:711. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]
- 13.Stocking EM, Williams RM. Angew Chem, Int Ed. 2003;42:3078. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]
- 14.a) Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2262. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]; b) Greshock TJ, Williams RM. Org Lett. 2007;9:4255. doi: 10.1021/ol701845t. [DOI] [PubMed] [Google Scholar]
- 15.a) Williams RM, Sanz-Cervera JF, Sancenon F, Marco JA, Halligan K. J Am Chem Soc. 1998;120:1090. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; b) Williams RM, Sanz-Cervera JF, Sancenon F, Marco JA, Halligan KM. Bioorg Med Chem. 1998;6:1233. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; c) Sanz-Cervera JF, Williams RM, Alberto Marco J, Maria Lopez-Sanchez J, Gonzalez F, Eugenia Martinez M, Sancenon F. Tetrahedron. 2000;56:6345. [Google Scholar]
- 16.a) Stocking EM, Sanz-Cervera JF, Williams RM. J Am Chem Soc. 2000;122:1675. doi: 10.1021/ja005655e. [DOI] [PubMed] [Google Scholar]; b) Sanz-Cervera JF, Williams RM. J Am Chem Soc. 2002;124:2556. doi: 10.1021/ja017425l. [DOI] [PubMed] [Google Scholar]
- 17.Miller KA, Welch TR, Greshock TJ, Ding Y, Sherman DH, Williams RM. J Org Chem. 2008;73:3116. doi: 10.1021/jo800116y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller KA, Tsukamoto S, Williams RM. Nat Chem. 2009;1:63. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artman GD, III, Grubbs AW, Williams RM. J Am Chem Soc. 2007;129:6336. doi: 10.1021/ja070259i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew Chem, Int Ed. 2008;47:3573. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grubbs AW, Artman GD, III, Tsukamoto S, Williams RM. Angew Chem, Int Ed. 2007;46:2257. doi: 10.1002/anie.200604377. [DOI] [PubMed] [Google Scholar]
- 22.Schkeryantz JM, Woo JCG, Siliphaivanh P, Depew KM, Danishefsky SJ. J Am Chem Soc. 1999;121:11964. [Google Scholar]
- 23.a) Somei M, Karasawa Y, Kaneko C. Heterocycles. 1981;16:941. [Google Scholar]; b) Kametani T, Kanaya N, Ihara M. J Chem Soc, Perkin Trans. 1981;1:959. [Google Scholar]
- 24.Finefield JM, Williams RM. J Org Chem. 2010;75:2785. doi: 10.1021/jo100332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAfoos TJ, Li S, Tsukamoto S, Sherman DH, Williams RM. Heterocycles. 2010;82:461. doi: 10.3987/COM-10-S(E)19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams RM, Glinka T, Kwast E, Coffman H, Stille JK. J Am Chem Soc. 1990;112:808. [Google Scholar]
- 27.Ding Y, Greshock TJ, Miller KA, Sherman DH, Williams RM. Org Lett. 2008;10:4863. doi: 10.1021/ol8019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.For other approaches to 43 see: Jin S, Wessig P, Liebscher J. J Org Chem. 2001;66:3984. doi: 10.1021/jo0100897.Baran PS, Hafensteiner BD, Ambhaikar NB, Guerrero CA, Gallagher JD. J Am Chem Soc. 2006;128:8678. doi: 10.1021/ja061660s.
- 29.Ding Y, de Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. J Am Chem Soc. 2010;132:12733. doi: 10.1021/ja1049302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundmann A, Kuznetsova T, Afiyatullov SS, Li SM. Chem Bio Chem. 2008;9:2059. doi: 10.1002/cbic.200800240. [DOI] [PubMed] [Google Scholar]
- 31.Sunderhaus J, Sherman DH, Tsukamoto S, Williams RM. (unpublished results) [Google Scholar]
- 32.Finefield JM, Greshock TJ, Sherman DH, Tsukamoto T, Williams RM. (unpublished results) [Google Scholar]
- 33.a) Domingo LR, Sanz-Cervera JF, Williams RM, Picher MT, Marco JA. J Org Chem. 1997;62:1662. [Google Scholar]; b) Domingo LR, Zaragoza RJ, Williams RM. J Org Chem. 2003;68:2895. doi: 10.1021/jo020564g. [DOI] [PubMed] [Google Scholar]