Abstract
Purpose
Distant metastasis and recurrence are major prognostic factors associated with breast cancer. Both lymphovascular invasion (LVI) and blood vessel invasion (BVI) are important routes for metastasis to regional lymph nodes and for systemic metastasis. Despite the importance of vascular invasion as a prognostic factor, application of vascular invasion as a histopathological criterion is controversial. The aim of this study was to distinguish LVI from BVI in prognosis and recurrence of breast cancer using an endothelial subtype specific immunohistochemical stain (podoplanin, D2-40, and CD31).
Methods
Sections from 80 paraffin-embedded archival specimens of invasive breast cancer were stained for podoplanin, D2-40, or CD31 expression. Immunohistochemical staining results were correlated with clinicopathological features, such as tumor size, status of lymph node metastases, estrogen receptor status, progesterone receptor status, human epidermal growth factor receptor-2 expression, and recurrence. Patients with ductal carcinoma in situ and stage IV breast cancer were excluded.
Results
A significant correlation was found between D2-40 LVI positivity and lymph node metastasis (p=0.022). We found a significant correlation between D2-40 LVI positivity and recurrence of breast cancer (p=0.014). However, no significant correlation was found between BVI and recurrence. A poorer disease free survival was shown for D2-40 positive LVI (p=0.003). In a multivariate analysis, the presence of D2-40 LVI positivity revealed a significant association with decreased disease-free survival.
Conclusion
D2-40 LVI positivity was a more prognostic predictor of breast cancer than BVI.
Keywords: Breast cancer, CD31 antigen, Monoclonal antibody D2-40, Prognosis, Survival
INTRODUCTION
Breast cancer mortality rates are declining due to early detection and systemic adjuvant therapy. However, recurrence and distant metastasis, rather than a primary tumor, are the leading causes of death [1]. Although adjuvant treatment is individualized according to the stage of cancer, nodal status, and hormonal status, not all patients show the same response to treatment. Knowledge of prognostic factors is important for predicting recurrence and metastasis and for choosing target therapies. Tumor size, axillary lymph node status, and histological grade have been established as markers for the clinical setting, and axillary lymph node status is the most influential predictor of recurrence and metastasis [2].
Known prognostic markers predict outcomes in 30% of patients; among the remaining 70%, 30% of patients will experience recurrence and metastasis. Accordingly, new markers are necessary for predicting the prognosis and for determining individualized treatment [1,2].
Lymphatic and blood vessel invasion to loco-regional lymph nodes or distant sites occur early in tumor metastasis. The 2005 St. Gallen consensus guidelines suggested lymphovascular invasion (LVI) as a predictor of postoperative prognosis [3]. This was confirmed in 2007, with some limitations [4]. However, LVI was defined using hematoxylin and eosin stained material (H&E stain), which cannot distinguish lymphatic invasion from blood vessel invasion [5]. Although there have been methodological problems distinguishing blood from lymphatic vessels, several specific markers have been discovered. Markers used to detect blood vessel invasion include elastic van Gieson stain, factor VIII-related antigen (vWF), CD34, CD31, and vascular endothelial growth factor receptor-3; and markers for lymphatic invasion include podoplanin/D2-40, lymphatic vessel endothelial hyaluronan receptor-1, laminin, type IV collagen, and homeobox prospero-like protein [6]. Among vascular markers, CD31 is a 130-kDa transmembrane glycoprotein platelet endothelial cell adhesion molecule-1 in the immunoglobulin superfamily [6]. It is expressed on monocytes, platelets, selected T-cell subsets, and endothelial cells and is found more commonly on blood vascular endothelial cells than lymphatic endothelial cells [7]. In contrast, podoplanin is a 38-kDa integral transmembrane glycoprotein originally detected on the surface of renal glomerular podocytes in mice [8]. D2-40 is an IgG2a monoclonal antibody to the oncofetal M2A antigen, which is usually expressed in germ cell tumors and fetal testis [9]. Both D2-40 and podoplanin are widely used to distinguish lymphatic from blood vessels, as they are expressed in lymphatic endothelium [8,10], and D2-40 and podoplanin are now recognized as one of the most specific and sensitive markers to determine tumor cells in lymphatic vessels, which is known to be an important determinant of outcome [11]. However, despite the importance of blood vessel invasion as a prognostic factor, controversy still exists regarding the application of blood vessel invasion (BVI) as a histopathological criterion.
Therefore, the aim of this study was to distinguish between the role of LVI and BVI in the prognosis and recurrence of invasive ductal breast cancer using an endothelial subtype specific immunohistochemical stain (podoplanin, D2-40, and CD31).
METHODS
Patients
From January 2005 to February 2007, data from charts and pathological reports as well as tissues from consecutive patients with invasive ductal carcinoma of the breast at Korea University Anam Hospital were reviewed retrospectively. Patients without surgical treatment, those diagnosed with ductal carcinoma in situ, metastasis, and those with insufficient follow up data, pathology slides, and tissue blocks were excluded. All patients gave written informed consent for the tumor evaluation. However, Institutional Review Board approval was not required at the time of the study, as the evaluation of the tumor was by immunohistochemistry.
Of 80 patients, 41 underwent breast conserving surgery, and 39 underwent a mastectomy; 43 patients underwent sentinel lymph node biopsy, and axillary lymph node dissection was performed according to the results. The remaining patients underwent conventional axillary lymph node dissection. Patients received treatment with radiation, hormones, or chemotherapy according to their pathological reports. The mean follow-up period was 35±11.026 months.
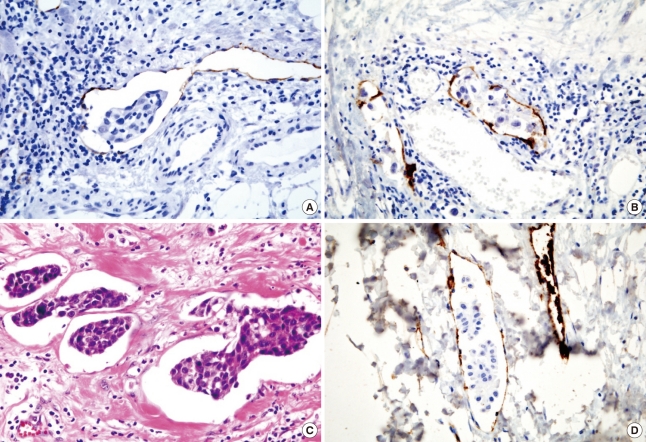
The baseline characteristics of the patients are summarized in Table 1. Tumor size and nodal status were categorized according to the TNM system criteria from the American Joint Committee on Cancer classification, and histological grade was evaluated using the Nottingham modification of the Bloom and Richardson histological grading criteria [12].
Table 1.
Patient characteristics
LVI=lymphovascular invasion; BVI=blood vascular invasion; H&E=haematoxylin and eosin stain.
Immunohistochemistry
Four paraffin-embedded tissue samples were cut into 4-µm thick sections. One section was routinely stained with H&E, whereas the others were stained with D2-40 (1:50; DakoCytomation, Glostrup, Denmark), podoplanin (1:50; DakoCytomation), or CD31 (1:40; DakoCytomation). Sections were deparaffinized in xylene and rehydrated in a graded series of ethanol to water. Peroxidase activity was inhibited by precipitation in methanol with 3% H2O2 for 5 minutes. Sections were cooked with citrate buffer (pH 6.0) in a pressure cooker at 12℃ for 5 minutes for antigen retrieval and incubated with the respective primary antibody at room temperature for 30 minutes. After washing, the sections were incubated with a secondary antibody (ChemMate Dako Envision; Dako) at room temperature for 30 minutes. Subsequently, the sections were subjected to DAB (substrate buffer+DAB chromogen [×50]) for 5 minutes. The slides were counterstained with hematoxylin and then mounted.
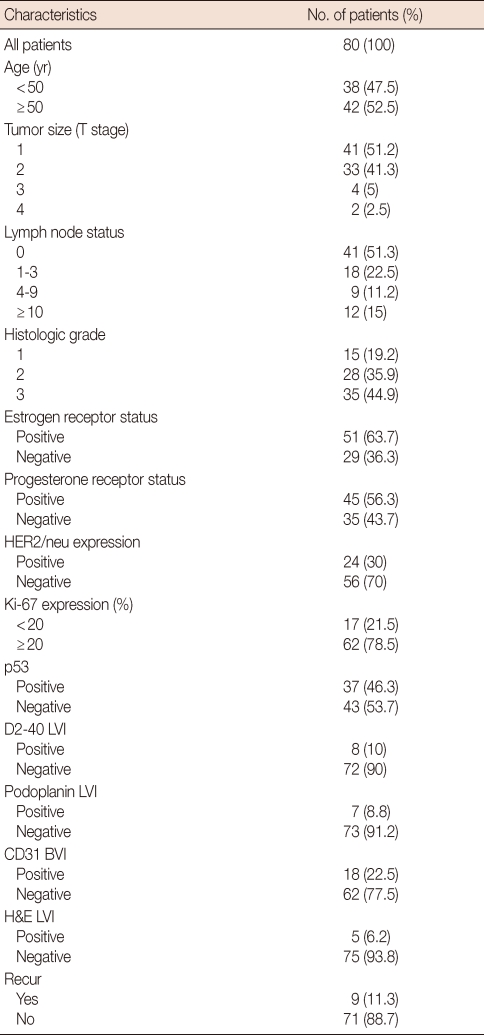
LVI was considered positive when tumor emboli were detected in podoplanin, D2-40, and H&E positive vessels, and BVI was reported positive when tumor emboli were detected in CD31 positively stained vessels (Figure 1). Tumor emboli detected in the clear space without stained vessels was not considered positive for LVI and BVI in this study.
Figure 1.
Tumor emboli in lymphatic vessel stained immunohistochemically with (A) D2-40, (B) podoplanin, (C) hematoxylin and eosin (H&E) stain, and tumor emboli in blood vessel stained with (D) CD31 (×400).
Both estrogen (ER) and progesterone receptor (PR) status were assessed by standard immunohistochemical methods and were considered positive if the nuclear staining value was greater than 10%. Evaluations were performed by a single pathologist using a light microscope.
Results were correlated with clinicopathological features, such as tumor size, status of lymph node metastasis, hormonal receptor status, human epidermal growth factor receptor2 (HER2)/ neu expression, and recurrence. The relationship between LVI, BVI, and disease-free survival (DFS) was also analyzed.
Statistical analysis
The statistical analysis was conducted using SPSS version 12.0 software (SPSS Inc., Chicago, USA). Correlations of clinicopathological factors and recurrence with D2-40, podoplanin, CD31, and H&E staining were assessed using chi-square tests. To predict recurrence, logistic regression with a forward procedure was used for statistically significant factors in a multivariate analysis. Kaplan-Meier survival curves by the log-rank test were used for the univariate, and Cox-regression for multivariate analysis was performed to determine DFS. A p-value <0.05 was regarded as statistically significant.
RESULTS
All patients were female, and ranged in age from 28 to 76 years (mean, 49.4±10.727). LVI was detected by D2-40, podoplanin and H&E stain in eight (10%), seven (8.8%), and five (5.2%) tumors, respectively. BVI was detected by CD31 stain in 18 tumors (22.5%). Among LVI detected cases, all podoplanin and H&E stain cases were included in D2-40 stained cases. Seven tumors were positive for both D2-40 LVI and CD31 BVI.
Only one patient developed locoregional recurrence in the breast, and eight patients developed distant metastasis after surgery.
Correlation between D2-40, podoplanin, H&E stain LVI positivity, and clinicopathological factors
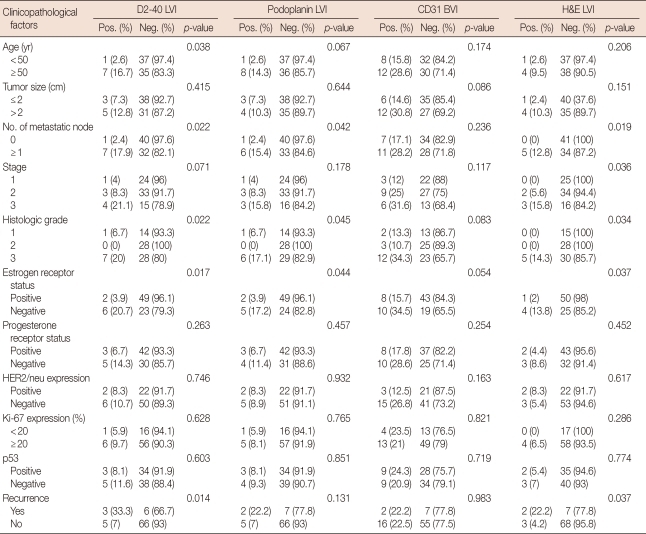
The relationship between D2-40, podoplanin LVI, and the clinicopathological factors is shown in Table 2. Recurrence was associated with positive LVI detected by D2-40 and H&E stain (p=0.014, p=0.037, respectively), but not by podoplanin stain (p=0.131). Tumors with D2-40 and podoplanin LVI were associated with a positive axillary lymph node (p=0.022, p=0.042, respectively), higher grade (p=0.022, p=0.045, respectively), and negative ER (p=0.017, p=0.044, respectively). Variables significantly associated with H&E stain LVI positivity included lymph node status (p=0.019), TNM stage (p=0.036), and ER status (p=0.037).
Table 2.
Correlation between D2-40, podoplanin, CD31, H&E stain, and clinicopathologic factors
LVI=lymphovascular invasion; BVI=blood vascular invasion; H&E=haematoxylin and eosin stain; Pos.=no. of positive cases; Neg.=no. of negative cases.
Correlation between CD31 BVI positivity and clinicopathological factors
No correlation was found between CD31 BVI positivity and other clinicopathological factors such as age, tumor size, lymph node status, histological grade, hormonal status, or recurrence (Table 2).
Correlation between prognostic factors, LVI, BVI, and DFS
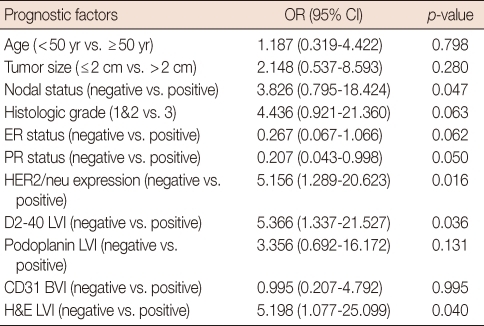
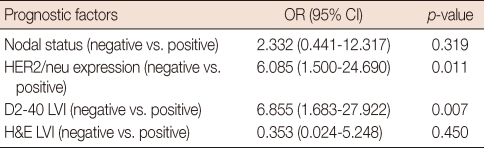
Univariate analysis using the Cox regression model demonstrated the status of lymph node metastasis, HER2/neu expression, LVI status stained by D2-40 and H&E to be prognostic factors for DFS (Table 3). Among these factors, only negative HER2/neu expression (odd ratio [OR], 6.085; p=0.011) and positive D2-40 LVI positivity (OR, 6.855; p=0.007) was significantly related to poorer outcome on multivariate analysis (Table 4).
Table 3.
Univariate analysis of prognostic factors associated with disease-free survival in breast cancer patients
OR=odds ratio; CI=confidence interval; ER=estrogen receptor; PR=progesterone receptor; LVI=lymphovascular invasion; BVI=blood vascular invasion; H&E=haematoxylin and eosin stain.
Table 4.
Multivariate analysis of prognostic factors associated with disease-free survival in breast cancer patients
OR=odds ratio; CI=confidence interval; ER=estrogen receptor; PR=progesterone receptor; LVI=lymphovascular invasion; H&E=haematoxylin and eosin stain.
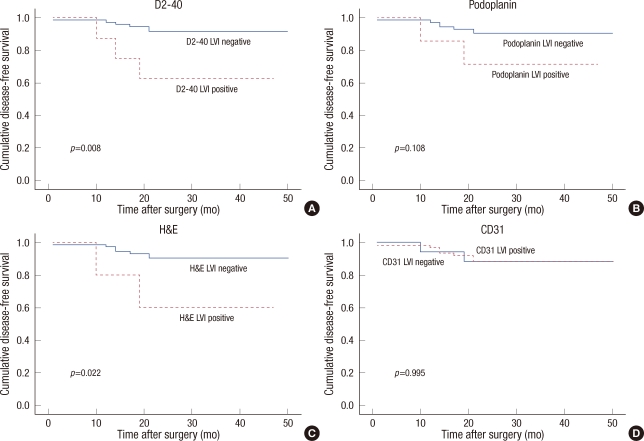
Patients with negative LVI D2-40 had better DFS than those with positive tumors (p=0.003), and similar results were shown for podoplanin and the LVI H&E stain (p=0.048, p=0.011, respectively) on Kaplan-Meier survival curve (Figure 2). However, no significance was found between positivity of BVI CD31 and DFS (p=0.951).
Figure 2.
Comparison of disease-free survival between positive and negative lymphovascular invasion (LVI) stained by (A) D2-40, (B) podoplanin, (C) hematoxylin and eosin (H&E) stain, and blood vessel invasion stained by (D) CD31.
DISCUSSION
Because one of the main causes of cancer death is distant metastasis, understanding the mechanism of tumor metastasis is important. Metastasis is often is explained by the metastatic cascade, in which the tumor cells first go through a process known as tumor cell dissociation in which they are separated from the primary tumor. They then invade the extracellular matrix, and enter the lymphatic or vascular system for transport to distant organs in a process known as intravasation. Extravasation occurs if tumor cells reach other organs where they can proliferate [1,13,14]. Based on this knowledge, if the existence of tumor cells in the lymphatic or vascular system can be predicted, the possibility of metastasis could also be predicted. As mentioned earlier, LVI was detected in the past using H&E stain in cases in which BVI could not be distinguished. However, new markers have been discovered along with advances in immunohistochemical technique. Using D2-40 as a marker, several studies have concluded that LVI is a prognostic factor, not only in breast cancer [15,16] but also in malignant melanoma, pulmonary adenocarcinoma, colorectal cancer, and gastric cancer [17-21]. Rabban and Chen [22] questioned the specificity of D2-40 to detect lymphatic vessels and its use as a marker to detect LVI without additional staining, as D2-40 is also expressed in myoepithelial cells which is a misinterpretation of true LVI from ductal carcinoma in situ (DCIS). However, their study showed that only few ducts with DCIS were D2-40 positive, and the entire circumference of the duct did not express D2-40. p63, or smooth muscle myosin immunostaining, which confirms the presence of myoepithelium, was only necessary for those patients with solid DCIS or in difficult cases that were morphologically confusing. Moreover, Arnaout-Alkarain et al. [23] proposed that D2-40 positivity in myoepithelial cells does not create a problem, as it is not difficult to distinguish from lymphatic channels. Usually ducts are much larger than vessels, and myoepithelium in smaller ducts is discontinuous, whereas endothelial lining is continuous in lymph vessels.
We used D2-40 and podoplanin as markers for LVI to determine its relationship with other clinicopathological factors and the prognosis. The LVI detection rate with D2-40 and podoplanin was 10% and 8.8%, respectively, which was quite low compared with other reports, which were between 8.8% and 86% [11,24]. In this study, we demonstrated that D2-40 stain could detect LVI the most and H&E stain the least. Moreover, despite the low detection rate, there was significant relationship between LVI and prognosis which also shows that LVI detection by D2-40 stain could be a significant marker for prognosis. Despite a short follow-up period, LVI-positive tumors stained with D2-40 and H&E indicated decreased DFS (p=0.008, 0.022, respectively), supporting results from other studies [15,17].
Tumor size is one of the strongest predictive factors for local recurrence, and tumors greater than 2 cm lead to decreased DFS [25]. In our study, there appeared to be more cases with tumor sizes greater than 2 cm in the D2-40 or podoplanin LVI positive group; however, no statistical significance was observed, which may be a result of the small study population. Although not as strong as tumor size and axillary lymph node status, tumor grade is associated with prognosis, and higher grade tumors show aggressive behaviors [2]. In our results, most LVI positive tumors were histological grade 3, which is consistent with other reports [17,26], and this can be explained by the speculation that aggressive tumors are more capable of invading lymphatic vessels. A significant relationship between LVI and ER status, with p-values of 0.017, 0.044, and 0.037 for D2-40, podoplanin, and H&E stain, respectively, was also observed. Controversy exists regarding the prognostic value of hormone receptors, because overall survival and DFS are influenced by hormonal therapy for ER-positive tumors; however, ER-positive tumors tend to have more favorable characteristics, such as low-grade histology and a low proliferative index [2]. We suggest that LVI negativity in ER-positive tumors provides prognostic value.
Axillary lymph node status is an important prognostic factor [2], and the metastatic route is through the lymphatic system. Braun et al. [26] reported on the correlation between LVI and axillary lymph node metastasis, particularly in small (T1) tumors, in 247 cases using D2-40 as a marker. Similarly, Wong et al. [27] investigated the significance of LVI in node-negative tumors for predicting the necessity for radiation therapy or an axillary dissection. They concluded that LVI negative patients with small tumors have a low risk for lymph node metastasis and recommended that LVI positive patients undergo full radiation therapy and/or axillary dissection. However, they did not mention the staining method used to detect LVI positivity. In our current study, LVI positivity stained with all three methods was correlated with lymph node status, supporting results from recent studies. Moreover, D2-40 LVI positivity and axillary lymph node metastasis were associated with recurrence, although in the multivariate analysis, D2-40 LVI positivity was the only significant factor.
BVI is an important factor associated with the hematogenous spread of tumor cells [1,13]. Unlike LVI studies, fewer studies have been conducted and less controversy exists regarding the prognostic significance of BVI [5,28,29]. Elastic van Gieson stain or vWF could be used to detect BVI; however, CD31 or CD34 has been preferred in some studies due to their specificity [6]. BVI positivity ranged from 4.2% to 33% [29,30] in previous studies, and positivity was 22.5% when we used CD31 as a marker for BVI. We found no significant relationship with other prognostic markers, and BVI was not associated with recurrence or DFS. Kato et al. [30], who investigated BVI in Japanese patients using elastic van Gieson stain and the VIII-related antigen, found a significant association between tumor size, lymph node status, histological grade, and survival. Lauria et al. [29] evaluated a large population consisting of 1,408 patients and reported that BVI was associated with a worse prognosis and with lymph node metastasis; however, BVI was not significant in the multivariate analysis. But, LVI and BVI were distinguished with H&E stain, defining lymphatic vessels with a clear endothelial lining, blood vessels with a fibrin clot, and erythrocytes in the endothelial-lined space. Differences in reports regarding BVI could be due to different staining methods.
The purpose of this study was to determine whether or not podoplanin, D2-40, or CD31 were significant prognostic factors. Not only are prognostic factors for breast cancer important for predicting patient prognosis, but they might also be important with regard to treatment. Some patients could be over-treated with chemotherapy and suffer side effects, which could also lead to other serious conditions. If a patient's risk for developing metastasis or recurrence could be accurately predicted, treatment could be individualized according to own his risk. Efforts should be made to determine the prognostic significance of LVI and BVI and to discover other prognostic factors.
In summary, positive LVI detected by D2-40 was a predictor of prognosis and BVI did not have any significance. However further prospective studies with a larger population are necessary to commonly use D2-40 stains as specific markers in clinical settings.
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 2.Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin. 1997;47:28–51. doi: 10.3322/canjclin.47.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 5.van den Eynden GG, van der Auwera I, van Laere SJ, Colpaert CG, van Dam P, Dirix LY, et al. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer. 2006;94:1643–1649. doi: 10.1038/sj.bjc.6603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammed RA, Ellis IO, Lee AH, Martin SG. Vascular invasion in breast cancer: an overview of recent prognostic developments and molecular pathophysiological mechanisms. Histopathology. 2009;55:1–9. doi: 10.1111/j.1365-2559.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 7.Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–16074. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks A, Sutherland DR, Bailey D, Iglesias J, Law J, Lei M, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer. 1999;80:569–578. doi: 10.1038/sj.bjc.6690393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukunaga M. Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology. 2005;46:396–402. doi: 10.1111/j.1365-2559.2005.02098.x. [DOI] [PubMed] [Google Scholar]
- 11.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 12.Robbins P, Pinder S, de Klerk N, Dawkins H, Harvey J, Sterrett G, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995;26:873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 13.Engers R, Gabbert HE. Mechanisms of tumor metastasis: cell biological aspects and clinical implications. J Cancer Res Clin Oncol. 2000;126:682–692. doi: 10.1007/s004320000148. [DOI] [PubMed] [Google Scholar]
- 14.Böhle AS, Kalthoff H. Molecular mechanisms of tumor metastasis and angiogenesis. Langenbecks Arch Surg. 1999;384:133–140. doi: 10.1007/s004230050183. [DOI] [PubMed] [Google Scholar]
- 15.Ito M, Moriya T, Ishida T, Usami S, Kasajima A, Sasano H, et al. Significance of pathological evaluation for lymphatic vessel invasion in invasive breast cancer. Breast Cancer. 2007;14:381–387. doi: 10.2325/jbcs.14.381. [DOI] [PubMed] [Google Scholar]
- 16.Cunnick GH, Jiang WG, Douglas-Jones T, Watkins G, Gomez KF, Morgan MJ, et al. Lymphangiogenesis and lymph node metastasis in breast cancer. Mol Cancer. 2008;7:23. doi: 10.1186/1476-4598-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valencak J, Heere-Ress E, Kopp T, Schoppmann SF, Kittler H, Pehamberger H. Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer. 2004;40:358–364. doi: 10.1016/j.ejca.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Adachi Y, Nakamura H, Kitamura Y, Taniguchi Y, Araki K, Shomori K, et al. Lymphatic vessel density in pulmonary adenocarcinoma immunohistochemically evaluated with anti-podoplanin or anti-D2-40 antibody is correlated with lymphatic invasion or lymph node metastases. Pathol Int. 2007;57:171–177. doi: 10.1111/j.1440-1827.2007.02077.x. [DOI] [PubMed] [Google Scholar]
- 19.Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol. 2006;19:1317–1323. doi: 10.1038/modpathol.3800651. [DOI] [PubMed] [Google Scholar]
- 20.Youn SI, Kim BG, Cha SJ, Park SJ, Chang IT, Park SI, et al. Angiogenic and lymphangiogenic microvessel density in terms of the expression of CD31 and D2-40 in colon cancer. J Korean Surg Soc. 2007;73:315–320. [Google Scholar]
- 21.Kim TH, Kim YS, Choi YC, Kim BK, Lee TJ, Park YG. The significance of the lymphatic micro vessel density and vascular endothelial growth factor- C expression for colorectal cancer. J Korean Surg Soc. 2007;73:406–411. [Google Scholar]
- 22.Rabban JT, Chen YY. D2-40 expression by breast myoepithelium: potential pitfalls in distinguishing intralymphatic carcinoma from in situ carcinoma. Hum Pathol. 2008;39:175–183. doi: 10.1016/j.humpath.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183–191. doi: 10.1038/modpathol.3800728. [DOI] [PubMed] [Google Scholar]
- 24.Nime FA, Rosen PP, Thaler HT, Ashikari R, Urban JA. Prognostic significance of tumor emboli in intramammary lymphatics in patients with mammary carcinoma. Am J Surg Pathol. 1977;1:25–30. doi: 10.1097/00000478-197701010-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kuru B, Camlibel M, Gulcelik MA, Alagol H. Prognostic factors affecting survival and disease-free survival in lymph node-negative breast carcinomas. J Surg Oncol. 2003;83:167–172. doi: 10.1002/jso.10264. [DOI] [PubMed] [Google Scholar]
- 26.Braun M, Flucke U, Debald M, Walgenbach-Bruenagel G, Walgenbach KJ, Höller T, et al. Detection of lymphovascular invasion in early breast cancer by D2-40 (podoplanin): a clinically useful predictor for axillary lymph node metastases. Breast Cancer Res Treat. 2008;112:503–511. doi: 10.1007/s10549-007-9875-2. [DOI] [PubMed] [Google Scholar]
- 27.Wong JS, O'Neill A, Recht A, Schnitt SJ, Connolly JL, Silver B, et al. The relationship between lymphatic vessell invasion, tumor size, and pathologic nodal status: can we predict who can avoid a third field in the absence of axillary dissection? Int J Radiat Oncol Biol Phys. 2000;48:133–137. doi: 10.1016/s0360-3016(00)00605-2. [DOI] [PubMed] [Google Scholar]
- 28.Kato T, Kameoka S, Kimura T, Nishikawa T, Kobayashi M. The combination of angiogenesis and blood vessel invasion as a prognostic indicator in primary breast cancer. Br J Cancer. 2003;88:1900–1908. doi: 10.1038/sj.bjc.6600921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauria R, Perrone F, Carlomagno C, De Laurentiis M, Morabito A, Gallo C, et al. The prognostic value of lymphatic and blood vessel invasion in operable breast cancer. Cancer. 1995;76:1772–1778. doi: 10.1002/1097-0142(19951115)76:10<1772::aid-cncr2820761014>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Kato T, Kameoka S, Kimura T, Nishikawa T, Kobayashi M. Blood vessel invasion as a predictor of long-term survival for Japanese patients with breast cancer. Breast Cancer Res Treat. 2002;73:1–12. doi: 10.1023/a:1015224703057. [DOI] [PubMed] [Google Scholar]