Abstract
Purpose
The role of first-line trastuzumab-based therapy has been firmly established in patients with human epidermal growth factor receptor-2 (HER2) positive metastatic breast cancer. In this trial, we evaluated the efficacy and safety of a vinorelbine and trastuzumab combination chemotherapy in patients who were pretreated with anthracyclines and taxanes.
Methods
Thirty-three patients with HER2 overexpressing metastatic breast cancer, all of whom had previously been treated with anthracyclines and taxanes, were included in this study. The patients were treated with 25 mg/m2 of vinorelbine (over a 15-minute infusion) on days 1 and 8 every 3 weeks. Additionally, trastuzumab was administered at an initial dose of 4 mg/kg over 90 minutes, and was subsequently administered at weekly doses of 2 mg/kg (over 30 minutes).
Results
The median age of the patients was 53 years (range, 39-72 years). The overall response rate was 30.3% (10 patients; 95% confidence interval [CI], 23-57%). The median time to progression was 6.8 months (95% CI, 5.3-8.2 months). The median overall survival was 12.4 months (95% CI, 10.3-14.6 months). In the 194 cycles of treatment, the incidence rates of grade ≥3 neutropenia and anemia were 7.2% and 1.0%, respectively. Neutropenic fever was detected in three cycles (1.5%). The non-hematological toxicities were not severe: grade 1 or 2 nausea or vomiting was detected in 15.2%, and grade 2 neuropathy was noted in 6.1% of patients. None of the patients experienced any serious cardiac toxicity, and no treatment-related deaths occurred.
Conclusion
These results show that a combination chemotherapy consisting of vinorelbine and trastuzumab is useful in patients with HER2-overexpressing metastatic breast cancer who were pretreated with anthracyclines and taxanes, with a favorable toxicity profile.
Keywords: Breast neoplasms, Metastasis, Trastuzumab, Vinorelbine
INTRODUCTION
Human epidermal growth factor receptor-2 (HER2) is a key contributor to normal cell growth and differentiation [1]. However, HER2 is associated with neoplastic transformation of cells when overexpressed. Approximately 15-20% of breast cancers overexpress HER2 (3+ by immunohistochemistry [IHC]) and/or amplification of the HER2 gene. HER2 overexpression is generally associated with a more aggressive tumor phenotype, and women with HER2 positive disease tend to have poor overall prognoses and faster relapse times at all stages of cancer development [2]. The advent of trastuzumab, a humanized monoclonal antibody against the extracellular domain of HER2, represents a major breakthrough in the treatment of women suffering from HER2 positive metastatic breast cancer (MBC).
As a first-line, single-agent therapy, trastuzumab has an overall response rate of 26% [3]. Among patients treated previously with chemotherapy for MBC, trastuzumab monotherapy resulted in an overall response rate of 15% [4]. Trastuzumab exerts synergistic effects with vinorelbine, cisplatin, docetaxel, thiotepa, cyclophosphamide, and etoposide; exerts additive effects with doxorubicin, paclitaxel, methotrexate, and vinblastine; and exerts antagonistic effects with 5-fluorouracil [5]. In two randomized trials, the addition of trastzumab to either paclitaxel [6] or docetaxel [7] yielded a significant survival benefit in patients with previously untreated MBC. The results of these two trials have firmly established the combination of trastuzumab with a taxane as standard first-line treatment for patients with HER2-overexpressing MBC. However, when first-line chemotherapy fails, non-crossresistant treatments with minimal toxic effects that could be readily administered on an outpatient basis are warranted.
Vinorelbine is a semisynthetic vinca alkaloid, with higher liposolubility and a higher tissue concentration than its analogues [8]. When employed as a single agent against MBC, vinorelbine exhibits response rates ranging from 32% to 50% as a first-line treatment, and from 15% to 36% as a second-line treatment [9]. In the preclinical setting, a synergism has been detected between trastuzumab and vinorelbine. The mean combination index value for trastuzumab and vinorelbine ranged from 0.24 (p<0.001) to 0.78 (p=0.034), in which a statistically significant value of <1.0 indicated synergy [5].
The use of trastuzumab and vinorelbine to treat MBC results in objective response rates for the combination in the range of 44-86% (51-86% as first-line treatment), and a median duration of response of 10-17.5 months [10]. However, no previous reports have assessed the efficacy of a therapy consisting of vinorelbine plus trastruzumab in cases of heavily treated MBC. The treatment for intensively pretreated MBC is basically palliative, and the objective is to provide antitumor activity and prolong survival, but without any significant deterioration in quality of life.
Therefore, we conducted a phase II study to evaluate the efficacy and toxicity of vinorelbine plus trastuzumab in patients with HER2-positive MBC who had been treated previously with anthracyclines and taxanes.
METHODS
Eligibility
To be eligible for this study, patients were required to have a histologically confirmed diagnosis of MBC, in addition to at least one measurable lesion. All patients were ≥18 years and were required to have previously treated with anthracycline and taxane-based chemotherapy. Only patients with an Eastern Cooperative Oncology Group (ECOG) performance status of grade 0-2 were enrolled in this study. Adequate bone marrow function (an absolute neutrophil count ≥1,500×103/µL and a platelet count ≥100,000×103/µL), adequate renal function (a serum creatinine level ≤1.5 mg/dL), and adequate hepatic function (aspartate aminotransferase and alanine aminotransferase levels ≤3×upper limit of normal and a total bilirubin level ≤1.5×upper limit of normal) were required for the patients enrolled in this study. Patients were also required to have a left ventricular ejection fraction (LVEF) of ≥50%. Patients were allowed to have received prior adjuvant chemotherapy (including taxane- or anthracycline-containing regimens), as long as chemotherapy was received ≥6 weeks prior to entry into the study. Patients were also allowed to have received adjuvant trastuzumab if the final dose was received ≥12 months prior to study entry.
Patients were ineligible if they had local relapse only, tumor in the contralateral breast only, symptoms of brain metastases or leptomeningeal involvement, peripheral neuropathy of at least grade 2 (according to National Cancer Institute Common Toxicity Criteria, NCI-CTC) or serious conditions including cardiac disease, symptomatic dyspnea, uncontrolled hypercalcemia, or clinically relevant active infection. Patients were also excluded if they were pregnant or breast-feeding, had participated in another clinical trial within 30 days prior to inclusion in this study, had a history of another malignancy, except for adequately treated basal cell carcinoma of the skin or in situ cervical carcinoma, or previously exposed to trastuzumab or lapatinib. All patients provided informed consent, and this study was approved by the Institutional Review Board of Dong-A University Medical Center (No.11-32).
HER2 status
A primary antibody directed against HER2 (Neo Markers, Fremont, USA) was used. Patients overexpressing HER2 at the 3+ level of IHC staining were immediately eligible for inclusion. HER2 expression at the 2+ level required confirmation by HER2 gene amplification using fluorescent in situ hybridization (FISH) with PathVision® HER2 DNA probe kit (Vysis Inc., Downers Grove, USA). HER2 testing was conducted on a primary tumor sample or a biopsy from the metastatic site.
Treatment protocol
The patients were treated with 25 mg/m2 of vinorelbine (over a 15-minute infusion period) on days 1 and 8, at 3-week intervals. The vinorelbine dose was adjusted each week on the day of therapy, based on hematological and/or non-hematological toxicity. Adjustments were made in the dose of vinorelbine (25% dose reduction) in patients who experienced grade ≥3 hematological toxicity, grade 4 febrile neutropenia, grade 4 neutropenia persisting for 7 days, or grade 4 thrombocytopenia. The granulocyte colony stimulating factor was used for cases in which treatment was delayed for more than 2 weeks because of neutropenia or febrile neutropenia. One-week delays in vinorelbine dosing were applied in cases of grade 2 or higher neutropenia and thrombocytopenia. The vinorelbine dosage was reduced by 25% in cases of grade 2 neurologic toxicity, until it resolved to grade 1 or lower. In cases of non-hematologic grade 3, vinorelbine administration was delayed until the side effects were resolved to grade 1 or lower. If toxicity persisted for more than 3 weeks, patients were dismissed from the study, as were patients with grade 4 non-hematologic toxicity.
Trastuzumab was administered at an initial dose of 4 mg/kg over 90 minutes, and was subsequently administered at weekly doses of 2 mg/kg (over a 30-minute period). If patients experienced an infusion syndrome characterized by rigor, fever, or other symptoms of hypersensitivity to trastuzumab, treatment was halted, and patients were assessed and treated with supportive measures (acetaminophen, diphenhydramine, H2 antagonists, dexamethasone, or meperidine) as needed. Treatment was reinstituted when vital signs stabilized. The study protocol did not include dose adaptations or delays for trastuzumab. All patients received electrocardiograms and LVEF measurements upon enrollment, and every 9 weeks thereafter or more frequently when dictated by clinical circumstances. Trastuzumab application was paused when LVEF was reduced by >20% from baseline or was reduced to >10% below the lower limit of normal. If a reevaluation conducted after 1 month reflected stabilized cardiac function, trastuzumab treatment was resumed, albeit accompanied by short-term controls. Otherwise, trastuzumab treatment was halted. Trastuzumab was immediately halted in New York Heart Association (NYHA) III or IV cardiac dysfunction. Vinorelbine and trastuzumab were administered until disease progression, unacceptable toxicity, or patient refusal.
Assessment of response
A physical examination, complete blood counts, blood chemistry, and chest X-rays were acquired after each cycle. Computed tomography scans were repeated every three cycles or earlier for cases in which clinical deterioration was detected. The primary endpoint of this study was the tumor response rate, and the secondary endpoints included the time to progression (TTP), overall survival (OS), and toxicities. Patients who had been treated with at least two cycles of therapy were assessable for response; all patients who received treatment were assessable for toxicity.
Tumor responses were evaluated using the Response Evaluation Criteria in Solid Tumors criteria. A complete response (CR) was defined as the disappearance of all measurable lesions for a minimum duration of 8 weeks. Partial response (PR) was defined as a reduction of 30% or more in the sum of products of the greatest diameters of measurable lesions, no increase in lesion size, and no new lesions. Stable disease (SD) was defined as a reduction of less than 30% and an increase of less than 20% without any appearance of new lesions. Progressive disease (PD) was defined as an increase of greater than 20% in tumor size or the appearance of new lesions. Toxicity was evaluated using the NCI-CTC criteria (version 3.0), and was recorded per patient as the worst episode that appeared during a treatment cycle.
Statistical methods
This trial was designed to detect a response rate of 35%, as compared to a minimal clinically meaningful response rate of 20%. The two-stage optimal design devised by Simon was adopted, with a statistical power of 80% to accept the hypothesis and 5% significance to reject it. Allowing for a follow-up loss rate of up to 10%, the total required sample size was 31 patients with measurable disease. The TTP and the OS were calculated from treatment initiation to the initial observation of disease progression or death, respectively. The Kaplan-Meier method was applied to estimate overall and progression-free survival outcomes. No multivariate analyses were conducted, due to the limited sample size. All data were analyzed with SPSS software version 14.0 (SPSS Inc., Chicago, USA).
RESULTS
Patient characteristics
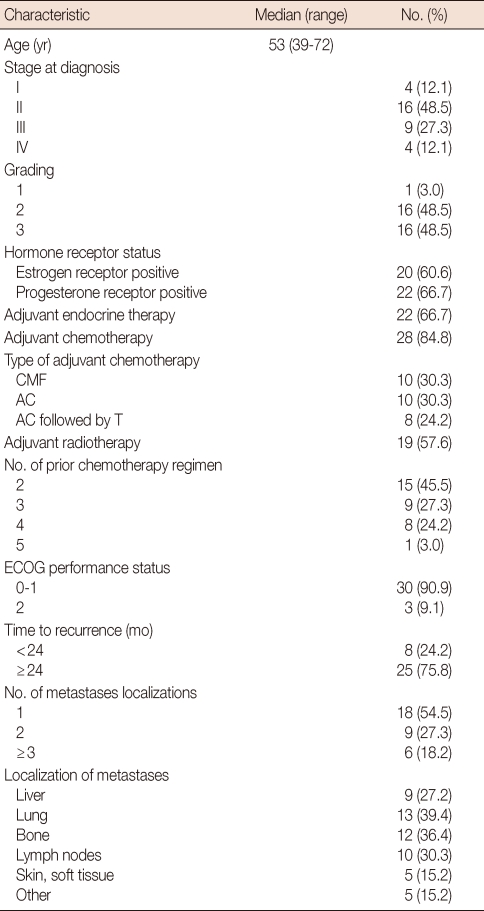
Between August 2004 and August 2008, 33 patients with MBC, who were pretreated with anthracycline and taxane-based therapy, were scheduled for treatment at the Dong-A University Medical Center, Busan, South Korea. The characteristics of the 33 patients enrolled in this study are described in Table 1. The median patient age was 53 years (range, 39-72 years). The performance status (ECOG) was grade 0-1 in the majority of the patients; three patients (9.1%) were grade 2.
Table 1.
Patient characteristics (n=33)
CMF=cyclophosphamide, methotrexate, 5-fluorouracil; AC=adriamycin, cyclophosphamide; T=taxol; ECOG=Eastern Cooperative Oncology Group.
Estrogen receptor (ER) expression was positive in 20 patients (60.6%) and progesterone receptor (PR) expression was positive in 22 patients (66.7%). All patients with hormone receptor-positive tumors had received adjuvant hormonal therapy, but only six of the 22 patients (27.3%) had previously received palliative hormonal therapy. Twenty-nine of the patients (88%) were +3 overexpression of HER-2 IHC, four possessed tumors with +2 overexpression and were FISH-positive.
One organ was involved in 18 patients (54.5%), two organs were involved in nine (27.3%) and three or more organs were involved in six (18.2%). The lung was the most common metastatic site (13 patients, 39.4%), and bone (12 patients, 36.4%), lymph nodes (ten patients, 30.3%), liver (nine patients, 27.2%) and skin and soft tissue (five patients, 15.2%) were involved in a combined fashion. Fifteen patients (45.5%) received vinorelbine plus trastuzumab treatment as a third-line chemotherapy, nine patients (27.3%) as a fourth-line, and nine patients (27.3%) as a fifth (or more)-line therapy. Enrolled patients were previously treated with doxorubicin and cyclophosphamide, paclitaxel or docetaxel, capecitabine, and gemcitabine. None of the patients received prior adjuvant or metaststic setting.
Tumor response and survival
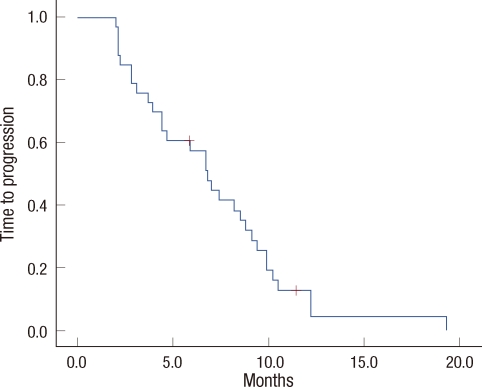
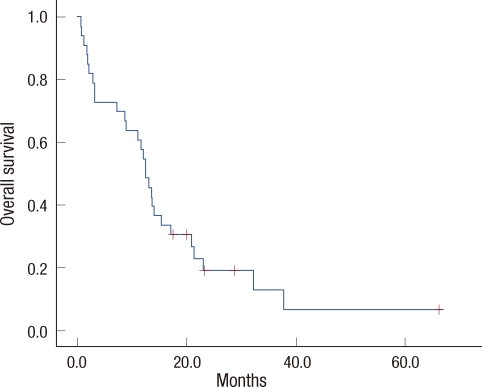
Of the 33 patients who were assessed for responses, PR was noted in ten patients (30.3%; 95% confidence interval [CI], 23-37%), SD was detected in 12 patients (36.4%), and PD was observed in 11 patients (33.3%). The response rate of third line treatment was not better than that of later line therapy (46.7% vs. 16.7%, p=0.062). The median follow-up duration was 12.9 months. The median TTP was 6.8 months (95% CI, 5.3-8.2 months) (Figure 1). The median OS was 12.4 months (95% CI, 10.3-14.6 months) (Figure 2). The OS of third line therapy tended to be longer than that of later line therapy, but the difference was not significant (13.5 vs. 8.9 months, p=0.192). At the time of study analysis, four patients remained in treatment, and 29 patients had discontinued the study. Twenty-seven patients had experienced tumor progression. Of those patients with PD, four (12.1%) were discovered to have new central nervous system metastases as sites of disease progression. An additional two patients withdrew their consent for physician or patient preference.
Figure 1.
Time to progression curve.
Figure 2.
Overall survival curve.
Dose administration and toxicity
A total of 194 treatment cycles were administered to 33 patients, with a median number of three cycles (range, 2-12 cycles). The number of dose-reduced cycles was 74 (38.1%) among the 194 cycles. The reasons for dose reduction were neutropenia and/or febrile neutropenia. The relative dose intensities were 15 mg/m2/wk (90.6%) for vinorelbine and 1.9 mg/kg/wk (95.0%) for trastuzumab.
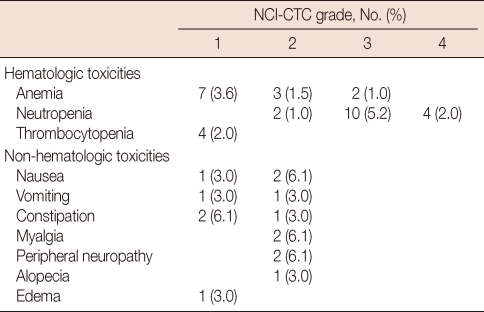
The incidence and severity of acute toxicity associated with combined vinorelbine and trastuzumab therapy was quite low. All treatment-related toxicities noted in this study are shown in Table 2. Grade 3 or 4 neutropenia was observed in 14 cycles of treatment (7.2%), but only three episodes of non-fatal neutropenic fever were detected. Grade 1 or 2 vomiting was noted in five patients (15.2%), and grade 1 or 2 peripheral sensory neuropathy was detected in two patients (6.1%). Many of the women with neuropathy had pre-existing symptoms from previous taxane-based treatments. Fewer than one-third of patients showed any alopecia, and the observed gastrointestinal symptoms were also mild, with less than 10% of patients exhibiting grade 2 constipation and no patients exhibiting grade 3 or 4 gastrointestinal toxicities. Notably, no case of congestive heart failure or asymptomatic reductions in LVEF ≥20% occurred in the study population. Additionally, no treatment-related deaths occurred during this study.
Table 2.
Hematologic (per cycle) and non-hematologic (per patient) toxicities
NCI-CTC=National Cancer Institute-Common Toxicity Criteria.
DISCUSSION
After the pioneering research of Slamon et al. [6], as well as another report by Marty et al. [7], the combination of trastuzumab and taxane is currently the standard first-line treatment for patients with HER2-positive MBC. However, such combinations are not reasonable for patients who have already received taxanes and trastuzumab as an adjuvant treatment, a group whose numbers will undoubtedly increase.
Trastuzumab has also proven clinically beneficial in combination with other chemotherapeutic agents, including vinorelbine, capecitabine, gemcitabine, liposomal doxorubicin, and platinum salts [11]. Among them, vinorelbine rarely induces total alopecia or severe gastrointestinal events or symptomatic cardiac events. The most frequent dose-limiting hematological toxicity associated with vinorelbine is neutropenia. Common non-hematological side-effects include grade 1/2 peripheral neuropathy, constipation, and phlebitis [9]. Several phase II trials of trastuzumab in combination with vinorelbine have been conducted [12-16]. The overall response rates (ORRs) were generally highest in patients who received the trastuzumab-vinorelbine combination as a first-line treatment for MBC (range, 51-86%). Encouraging ORRs were also observed after prior chemotherapy for MBC, and the use of adjuvant chemotherapy does not appear to influence clinical efficacy [13,14]. Trastuzumab-vinorelbine has yielded progression-free survival (PFS) and OS results comparable to other trastuzumab-chemotherapy combination [12-16].
A phase III, multicenter, randomized trial was conducted to assess the efficacy of trastuzumab in combination with either vinorelbine or a taxane for treating of HER2-positive MBC: this was called the trastuzumab and vinorelbine or taxane (TRAVIOTA) study [17]. The available results reflect at least comparable activity between the treatment arms: the ORR was 51% for trastuzumab-vinorelbine and 40% for trastuzumab-taxane, and the median TTPs were 8.5 and 6.0 months, respectively. Notably, that study did not possess power sufficient to detect statistically significant treatment differences, due to its early closure. In another retrospective comparison, the combination of docetaxel and trastuzumab was associated with higher ORR (77% vs. 57%, p=0.01), and longer OS relative to vinorelbine and trastuzumab (35 vs. 23 months, p=0.04), with no difference in TTP (12 vs. 10 months, p=0.53) [18]. Obviously, because of the retrospective nature of our study, several biases must be considered when interpreting our findings.
The selection of chemotherapeutic regimens is a challenging issue for MBC whose disease has failed to respond to anthracyclines and/or taxanes. In particular, treating patients who are experiencing severe myelosuppression with these cytotoxic therapies has been particularly problematic. In a previous report, the efficacies of gemcitabine and trastuzumab after previous exposure to anthracyclines, docetaxel, and/or vinorelbine and trastuzumab were assessed [19]. The RR was 19.2%, the TTP was 3 months, and the OS was 17 months. Other results of the phase II trial demonstrated that capecitabine administered in combination with trastuzumab is highly effective (RR 45%, PFS 6.7 months, OS 28 months) in patients with anthracycline- and taxane-pretreated HER2 overexpressing MBC [20].
In this study, the RR was 30.3%, the TTP was 6.8 months, and the OS was 12.4 months, which were lower than in many previous reports; this might be attributable to the fact that all enrolled patients had received both anthracylines and taxanes, that >50% of the patients enrolled received vinorelbine and trastuzumab as fourth-line and fifth-line chemotherapy. Four patients (12.1%) developed cerebral metastases as initial progression sites while on therapy, a problem frequently encountered in HER2-positive disease [21]. This phenomenon is probably attributable to the prolonged survival due to trastuzumab treatment, in connection with the observation that antibodies are unable to pass the blood-brain-barrier.
The toxicity observed when using trastuzumab in combination with vinorelbine did not differ appreciably from that anticipated from previous studies [12-16]. Neutropenia was the grade 3/4 adverse event most frequently associated with combination therapy. Grade 3/4 neutropenia was noted in 7.2% of treatment courses; however, the duration of neutropenia was both brief and noncumulative. Few non-hematological grade 3/4 adverse effects were observed in this study. Although patients should be monitored for cardiac toxicity while on trastuzumab therapy, vinorelbine does not appear to enhance trastuzumab-induced cardiac toxicity. It is worth noting that no cardiac dysfunction was observed in conjunction with heart failure symptoms in this study, which constitutes an improvement even upon the excellent cardiac tolerance observed in other phase II studies using similar combinations. Finally, none of the patients in this study died of any treatment-related complications.
The number of cases with a reduced dose of vinorebine was 74 (38.1%) among the total of 194 cycles, which was due to neutropenia and/or febrile neutropenia. Therefore, the dose and schedule of vinorelbine employed in this phase II trial appears feasible for use in heavily pretreated populations.
Although this study enrolled only a small number of patients and its duration was relatively short, the combination treatment consisting of trastuzumab and vinorelbine generated a response rate of 30.3% as a more than third-line therapy with a median OS of 12.4 months. It is difficult to compare this current phase II study of trastuzumab and vinorelbine directly with other various phase II/III studies due to different patient selection criteria, particularly differences in previous therapies and determinations of HER2 overexpression. Although it would clearly be inappropriate to consider this combination as a standard treatment for patients with HER2-overexpressing MBC before more controlled trials are conducted, this schedule may be regarded as an acceptable option for MBC who have previously undergone heavy treatment.
We conclude that the combination of vinorelbine plus trastuzumab appears to be a feasible and safe treatment option for HER2 positive MBC who have previously undergone heavy treatment.
Footnotes
This paper was supported by Dong-A University Research Fund.
Conflict of interest relevant to this article was not reported.
References
- 1.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Ménard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a prognostic factor in breast cancer. Oncology. 2001;61(Suppl 2):67–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 3.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 5.Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 8.Krikorian A, Rahmani R, Bromet M, Bore P, Cano JP. Pharmacokinetics and metabolism of Navelbine. Semin Oncol. 1989;16(2 suppl 4):21–25. [PubMed] [Google Scholar]
- 9.Bonneterre J, Penel N. Vinorelbine in breast cancer. Expert Opin Pharmacother. 2008;9:2901–2910. doi: 10.1517/14656566.9.16.2901. [DOI] [PubMed] [Google Scholar]
- 10.Chan A. A review of the use of trastuzumab (Herceptin) plus vinorelbine in metastatic breast cancer. Ann Oncol. 2007;18:1152–1158. doi: 10.1093/annonc/mdl476. [DOI] [PubMed] [Google Scholar]
- 11.Bell R, Verma S, Untch M, Cameron D, Smith I. Maximizing clinical benefit with trastuzumab. Semin Oncol. 2004;31(5 suppl 10):35–44. doi: 10.1053/j.seminoncol.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Chan A, Martin M, Untch M, Gil MG, Guillem-Porta V, Wojtukiewicz M, et al. Vinorelbine plus trastuzumab combination as first-line therapy for HER 2-positive metastatic breast cancer patients: an international phase II trial. Br J Cancer. 2006;95:788–793. doi: 10.1038/sj.bjc.6603351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein HJ, Harris LN, Marcom PK, Lambert-Falls R, Havlin K, Overmoyer B, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol. 2003;21:2889–2895. doi: 10.1200/JCO.2003.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Papaldo P, Fabi A, Ferretti G, Mottolese M, Cianciulli AM, Di Cocco B, et al. A phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: changing the natural history of HER2-positive disease. Ann Oncol. 2006;17:630–636. doi: 10.1093/annonc/mdj110. [DOI] [PubMed] [Google Scholar]
- 15.Schilling G, Bruweleit M, Harbeck N, Thomssen C, Becker K, Hoffmann R, et al. Phase II trial of vinorelbine and trastuzumab in patients with HER2-positive metastatic breast cancer. A prospective, open label, non-controlled, multicenter phase II trial (to investigate efficacy and safety of this combination chemotherapy) Invest New Drugs. 2009;27:166–172. doi: 10.1007/s10637-008-9166-8. [DOI] [PubMed] [Google Scholar]
- 16.De Maio E, Pacilio C, Gravina A, Morabito A, Di Rella F, Labonia V, et al. Vinorelbine plus 3-weekly trastuzumab in metastatic breast cancer: a single-centre phase 2 trial. BMC Cancer. 2007;7:50. doi: 10.1186/1471-2407-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 18.Redana S, Donadio M, Nolè F, Jacomuzzi ME, Beano A, Martinello R, et al. Trastuzumab with either docetaxel or vinorelbine as first-line treatment for patients with HER2-positive advanced breast cancer: a retrospective comparison. BMC Cancer. 2010;10:28. doi: 10.1186/1471-2407-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartsch R, Wenzel C, Gampenrieder SP, Pluschnig U, Altorjai G, Rudas M, et al. Trastuzumab and gemcitabine as salvage therapy in heavily pre-treated patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62:903–910. doi: 10.1007/s00280-008-0682-1. [DOI] [PubMed] [Google Scholar]
- 20.Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007;25:3246–3250. doi: 10.1200/JCO.2006.09.6826. [DOI] [PubMed] [Google Scholar]
- 21.Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]