Abstract
Pandemic 2009 influenza A (H1N1) virus (H1N1pdm) is different from contemporary seasonal human viruses in that it can cause infection deep in the lungs of critical care patients. Here we establish a mammalian animal model and assessed the efficacy of the neuraminidase (NA) inhibitor oseltamivir treatment against H1N1pdm virus infection. Oseltamivir (25 mg/kg/day twice daily for 5 days) was orally administered to groups of ferrets, starting either 2 or 24 h after inoculation with 106 PFU of A/California/04/2009 (H1N1) influenza virus. We determined that virus replication was restricted to 1 or 2 of 4 lung lobes in oseltamivir-treated animals, while virus was consistently isolated from 4 of 4 lung lobes in control animals (1.5–3.8 log10PFU/g). Analysis of arterial blood oxygenation revealed less pronounced changes in partial oxygen and carbon dioxide pressure in oseltamivir-treated ferrets, and histologic examination confirmed reduced pneumonia. Treated animals had significantly decreased inflammatory responses in the upper respiratory tract (P<0.05), less fever and weight loss, and less reduction of activity. Virus titers in the nasal washes of treated and control ferrets did not differ significantly. NA sequencing and fluorescence-based phenotypic assays identified no oseltamivir-resistant variants. Overall, oseltamivir treatment decreases the signs of infection and reduced the spread of H1N1pdm influenza virus in the lungs of ferrets and therefore impeded the development of viral pneumonia.
Keywords: pandemic 2009 H1N1 virus, neuraminidase inhibitor, oseltamivir, ferret model
1. Introduction
Pandemic influenza A (H1N1) virus (H1N1pdm) emerged in April 2009 in Mexico and rapidly spread to more than 200 countries. Genetic analyses showed that the virus was generated by multiple reassortment events and that each of its precursor gene segments had circulated in swine for more than 10 years (Garten et al., 2009; Smith et al., 2009). The M and NA genes, which are the targets of specific anti-influenza drugs (M2 ion channel blockers and NA inhibitors), belong to the Eurasian swine lineage of influenza viruses and are wholly derived from avian influenza viruses (Smith et al., 2009). Most of the circulating H1N1pdm viruses carry the N31S mutation in the M2 protein and are resistant to M2 ion channel blockers (WHO, 2009; Gubareva et al., 2010). However, few of the viruses have been found to be resistant to the NA inhibitor oseltamivir (CDC, 2009; Kidd et al., 2009); resistance has been detected predominantly in either hospitalized patients (most of whom were immunosuppressed and received prolonged oseltamivir therapy) (Kidd et al., 2009; Gaur et al., 2010) or those in whom post-exposure oseltamivir prophylaxis failed (WHO, 2009).
Human infection with H1N1pdm viruses is characterized by a broad spectrum of clinical symptoms, ranging from afebrile upper respiratory illness to diffuse viral pneumonia (Cao et al., 2009; Writing Committee, 2010). Systemic (e.g., gastrointestinal) symptoms are frequent. The pathogenesis of H1N1pdm influenza viruses has been investigated in several animal models. Studies in mice, ferrets, pigs, and non-human primates found that H1N1pdm viruses replicated in the upper respiratory tract (URT), thus showing receptor-binding properties similar to those of seasonal H1N1 and H3N2 viruses. However, H1N1pdm viruses also possess the receptor specificity necessary for replication in lung tissue (Itoh et al., 2009; Maines et al., 2009; Munster et al., 2009). Previously the ability to replicate in lung tissue was predominantly a characteristic feature of highly pathogenic H5N1 influenza viruses; in contrast, the seasonal human influenza viruses replicate only to a limited extent in lung tissue (Shinya et al., 2006; Gambotto et al., 2008).
Given the fact that H1N1pdm viruses possess the ability to replicate deep in the human lungs, evaluation of the benefits of approved anti-influenza drugs is of high priority. Here we studied oseltamivir effectiveness against H1N1pdm virus in a ferret animal model, in particular the capacity to inhibit virus replication in lung tissue. We applied a novel method to evaluate the extent of pulmonary infection based on alterations of lung functions as determined by arterial blood oxygenation. Emergence of oseltamivir-resistant variants was not determined by NA sequencing and fluorescence-based phenotypic assays of nasal wash, nasal turbinate, and lung isolates.
2. Materials and methods
2.1. Viruses and cells
Wild-type A/California/04/2009 (A/CA/04/09) (H1N1) influenza virus was provided by Dr. Y. Kawaoka (University of Wisconsin-Madison) The virus was isolated from the nasal swab of a patient by culturing in Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, VA), followed by two passages in MDCK cells that were maintained in minimal essential medium supplemented with 5% newborn calf serum, 2 mM L-glutamine, 0.2% sodium bicarbonate, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate in a humidified atmosphere of 5% CO2.
2.2. Compound
Oseltamivir carboxylate ([3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]-1-cyclohexene-1-carboxylic acid) and the prodrug oseltamivir phosphate (oseltamivir) [ethyl(3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate] were provided by F. Hoffmann-La Roche Ltd.
2.3. Plaque assay
Plaque assays were performed in MDCK cells to determine virus yield and plaque diameter. Briefly, confluent MDCK cells were incubated for 1 h at 37°C with 10-fold serial dilutions of virus in 1 ml infection medium. The cells were then washed and overlaid with freshly prepared MEM containing 0.3% BSA, 0.9% SeaPlaque agarose (Lonza, Rockland, ME), and 1 μg/ml TPCK trypsin (Worthington Biochemical Corp.). The plaques were assessed after incubation at 37°C for 3 days.
2.4. Virus susceptibility to NA inhibitor in vitro
A modified fluorometric assay using the fluorogenic substrate 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) (Sigma-Aldrich) was used to determine viral NA activity (Potier et al., 1979). The fluorescence of the released 4-methylumbelliferone was measured in a Synergy 2 multi-mode microplate reader (BioTek) using excitation and emission wavelengths of 360 and 460 nm, respectively. The drug concentration required to inhibit 50% of the NA enzymatic activity (IC50) was determined by plotting the percent inhibition of NA activity as a function of compound concentration calculated in the GraphPad Prism 4 software from the inhibitor-response curve.
2.5. Assessment of drug efficacy in ferrets
All animal experiments were approved by the Animal Care and Use Committee of St. Jude Children’s Research Hospital and complied with National Institutes of Health policies and the Animal Welfare Act. Young adult female ferrets (Marshall’s Farms, North Rose, NY) aged 3 to 5 months were screened by HI test to ensure seronegativity to currently circulating human H1N1 and H3N2 influenza A viruses, influenza B viruses, and H1N1pdm influenza A viruses. Ferrets were anesthetized by IM administration of ketamine 5 mg/kg body weight, xylazine 0.5 mg/kg body weight, and atropine 0.04 mg/kg body weight, and by inhalation of isoflurane. Three groups, each of 7 ferrets were inoculated intranasally with 106 plaque-forming units (PFU) of A/CA/04/09 (H1N1) influenza virus in 0.5 ml PBS. Five animals were observed for clinical signs of infection and 2 were euthanized on day 4 post-inoculation (p.i.) to determine virus titers in the internal organs. Control (uninfected, untreated) animals received 0.5 ml of sterile PBS delivered intranasally.
Oseltamivir (25 mg/kg/day given as 2 daily doses of 12.5 mg/kg for 5 days) was mixed 1:1 with sterile sugar syrup and was given orally beginning either 2 or 24 h after virus inoculation. Control inoculated ferrets received sterile PBS mixed 1:1 with sterile sugar syrup (placebo) on the same schedule. Clinical signs of illness, relative inactivity index (RII) (Reuman et al., 1989), weight, and temperature were recorded daily. Body temperature was measured once daily by subcutaneous implantable temperature transponders (Bio Medic Data Systems Inc, Seaford, DE). A rise in body temperature greater than three S.D. above baseline was considered a significant elevation.
2.6. Inflammatory responses in the upper respiratory tract
Inflammatory cell counts were determined in nasal washes obtained on days 2, 4, 6, 8, and 10 p.i. (Govorkova et al., 2007). Briefly, the nasal washes were centrifuged at 1000 × g for 10 min. The cell pellet was resuspended in PBS, and the cells were counted in an automated cell counter (Invitrogen Corp., Carlsbad, CA). The inflammatory cell count was calculated on the basis of the initial volume of nasal wash. The protein concentration in the cell-free nasal wash supernatant was determined by using a standard protein assay (Bio-Rad, Hercules, CA).
2.7. Measurement of arterial blood oxygenation
Three animals per experimental group were anesthetized by IM administration of ketamine 5 mg/kg body weight on days 0, 4, and 8 p.i., and arterial blood was collected from the tail arteries. Partial pressure of oxygen (pO2, used as a measure of blood oxygenation) was measured by using a portable i-STAT handheld analyzer (Abott Lab., Abott Park, IL).
2.8. Virus load in the upper and lower respiratory tracts
On days 2, 4, 6, 8, and 10 p.i. ferrets were anesthetized by IM injection of ketamine (25 mg/kg), and 0.5 ml sterile PBS containing antibiotics was instilled into each nostril and collected. Virus in the nasal wash specimens was titrated in MDCK cells and expressed as log10PFU/ml. Two animals in each treatment and control group were euthanized on day 4 p.i. (~12 h after administration of oseltamivir or placebo) by intracardiac injection of Euthanasia V solution. Tissue samples (~0.5 g) were collected from the nasal turbinate, trachea, and each of the 4 lung lobes. Samples were homogenized in 1 ml sterile PBS with antibiotics and the virus titer (log10PFU/g) was determined in MDCK cells.
2.9. Histologic analysis
Tissues (nasal turbinate, trachea and lung) collected on day 4 p.i. were fixed in 10% neutral-buffered formalin and embedded in paraffin. Five-micron sections were stained with hematoxylin and eosin and examined by light microscopy.
2.10. Emergence of resistance-associated mutations
Viral RNA was isolated directly from nasal washes collected on days 6 and 8 p.i. and from lung, nasal turbinate, and trachea homogenates collected on day 4 p.i. by using the RNeasy Mini kit (Qiagen). For clonal analysis of the virus population, we analyzed viral RNA extracted from individual plaques obtained in MDCK cells after inoculation with nasal wash samples or from the various tissue homogenates. The HA (HA1 region) and NA genes were sequenced by RT-PCR as described elsewhere (Hoffmann et al., 2001) by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude to identify mutations associated with NA inhibitor resistance. DNA sequences were completed and edited by using the Lasergene sequence analysis software package (DNASTAR).
2.11. Serological tests
Serum samples were collected from ferrets 21 days p.i., treated with receptor-destroying enzyme, heat-inactivated at 56 °C for 30 min, and tested by HI assay with 0.5% packed chicken red blood cells.
2.12. Statistical analysis
Virus titers in ferret organs and nasal wash samples were compared by unpaired two-tailed t-test. A probability (P) value of 0.05 was prospectively chosen to indicate that the result was not attributable to chance.
3. Results
3.1. Effect of oseltamivir treatment on clinical signs
To evaluate the effect of oseltamivir treatment on the reduction of clinical signs we inoculated ferrets with 106 PFU of A/CA/04/09 (H1N1) influenza virus and treated them with the drug at a dose of 25 mg/kg/d starting either 2 h or 24 h p.i. Control (infected, untreated) ferrets showed mild signs of inactivity (RII=1.0), a 1–2 °C increase in body temperature on days 2–4 p.i., and continuous weight loss during days 1–6 p.i.; weight loss was maximal (8.5%) on day 6 p.i. (Fig. 1A, B). Common respiratory signs, including nasal discharge, coughing, and sneezing, were observed in 2/5 control ferrets. The oseltamivir-treated ferrets showed no signs of reduced activity (RII~0), developed less pronounced fever that did not peak on day 4 p.i. as in controls (Fig. 1A,B), showed no respiratory signs, and had markedly less weight loss than the control group. Cell counts in the nasal washes of oseltamivir-treated animals did not change and did not differ appreciably, whereas a 5-fold elevation of cell counts was observed in control untreated animals on day 6 p.i. (P= 0.0001) (Fig. 1C). The protein concentrations of the nasal washes showed a similar pattern (Fig. 1D). All ferrets showed a strong serum antibody response on day 21 p.i. (HI reciprocal geometric mean titers of 1280–2560) that was comparable across regimens and groups (data not shown). Thus, although the oseltamivir treatment regimens studied did not protect against infection, they reduced morbidity and hastened recovery.
Fig. 1.
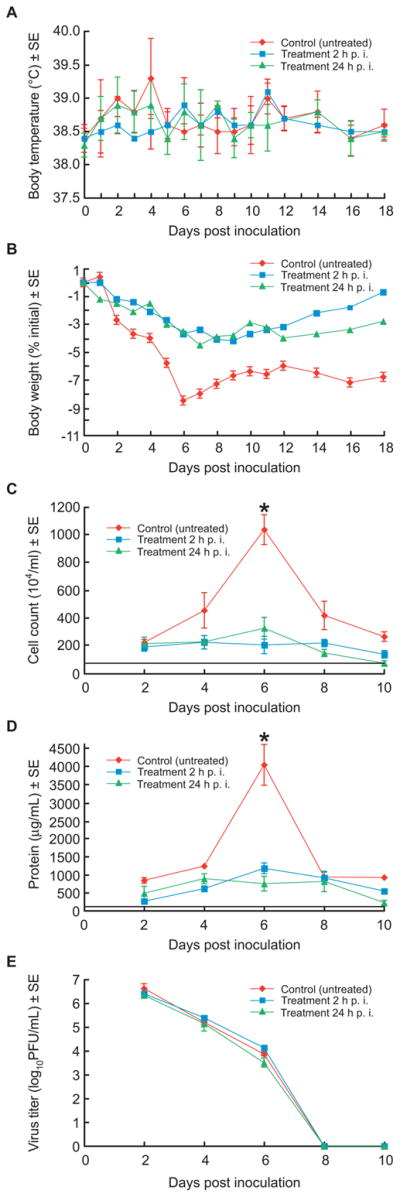
Pathogenicity of A/CA/04/09 (H1N1) influenza virus in the upper respiratory tract of oseltamivir-treated and control ferrets. Oseltamivir (25 mg/kg/day twice daily for 5 days) was administered orally to groups of 5 ferrets starting either 2 h or 24 h after inoculation with 106 PFU of A/CA/04/09 (H1N1) influenza virus. Virus-inoculated control animals received sterile PBS on the same schedule. Body temperature (A), percent weight change (B), inflammatory cell counts (C), protein concentration (D), and virus titers (E) were determined on the indicated days p.i. Horizontal lines indicate the mean inflammatory cell counts (C) and protein concentrations (D) in the uninfected ferrets. All values are the mean ± S.E. * P<0.05 as compared to control value (unpaired two-tailed t-test).
3.2. Effect of oseltamivir treatment on virus replication in upper and lower respiratory tracts
To determine the effect of oseltamivir treatment on virus replication in the nasal cavities and lung tissue we examined virus load in the nasal washes of ferrets on days 2, 4, 6, 8 and 10 p.i. and in each 4 lung lobes. Virus-inoculated ferrets shed high titers of virus from the upper respiratory tract on day 2 p.i. (mean peak titer, 5.9–6.9 log10 PFU/ml) and continued shedding through day 6 p.i. No virus shedding was detected on days 8 and 10 p.i. in the control or treatment groups (Fig. 1E). These virus titers did not differ significantly in control vs. treated ferrets (P>0.05) at any time point.
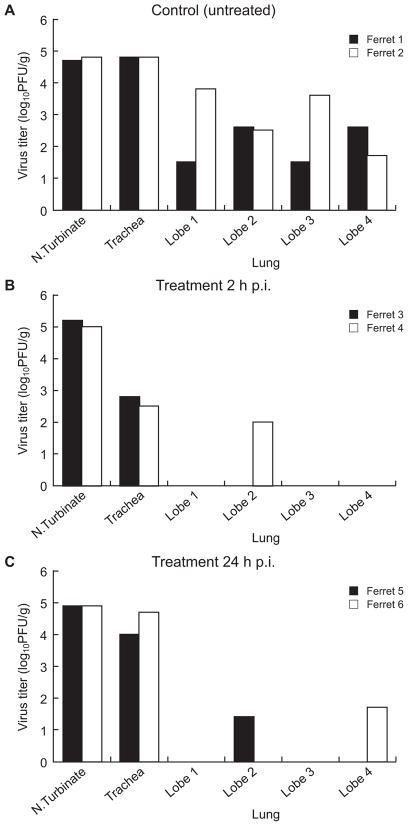
There was clear evidence of A/CA/04/09 (H1N1) virus replication in the lung tissue. Virus was isolated from all 4 lung lobes in both of the untreated control animals tested on day 4 p.i.; titers were 1.5–3.8 log10PFU/g (Fig. 2A). Virus titers were even higher in the nasal turbinates and tracheas of the two control animals (4.8 log10PFU/g). When treatment with oseltamivir was initiated 2 h p.i. (Fig. 2B), virus was isolated from only a single lung lobe in only one of the 2 animals tested (titer, 2.0 log10PFU/g); tracheal titers were ~2.0 log10PFU/g lower than those in control ferrets, although titers in the nasal turbinates were comparable. When oseltamivir treatment was delayed until 24 h p.i. (Fig. 2C) virus replication was detected in one lung lobe in each treated ferret but nasal turbinate and tracheal titers did not differ substantially from those in controls.
Fig. 2.
Effect of oseltamivir treatment on virus titers in the nasal turbinates, tracheas, and lungs of ferrets inoculated with A/CA/04/09 (H1N1) influenza virus. Control animals (A) inoculated with the same virus dose received sterile PBS on the same schedule. Oseltamivir (25 mg/kg/day twice daily for 5 days) was administered orally beginning either 2 h (B) or 24 h (C) after inoculation with 106 PFU of A/CA/04/09 (H1N1) influenza virus. Virus titers (log10PFU/gram tissue) were determined in each of the 4 pulmonary lobes, nasal turbinates, and tracheas of two ferrets per group on day 4 p.i. Each point represents the results from a single ferret.
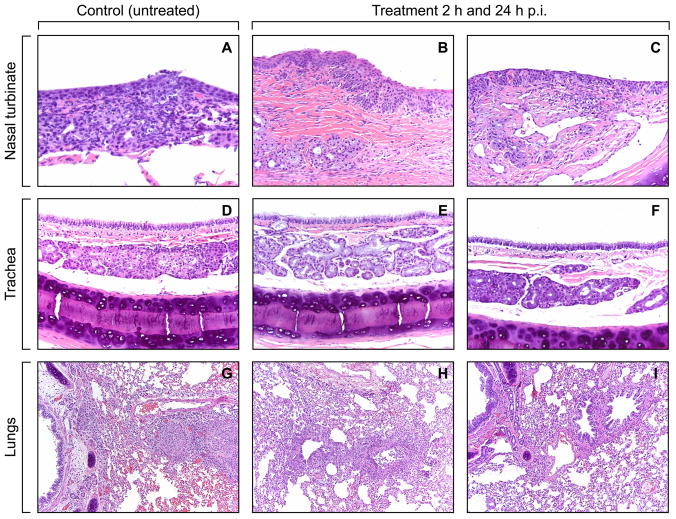
Histopathological analysis revealed mild to moderate rhinitis in control animals, characterized by multifocal necrosis of submucosal mucus glands and inflammatory cell infiltrates in the nasal turbinates (Fig. 3A). In the trachea, 50%-75% of the submucosal mucus glands were necrotic and filled with cellular debris and inflammatory cells (Fig. 3D). Histopathologic changes in the nasal turbinates and trachea of oseltamivir-treated ferrets were minor, and aggregates of necrotic cellular debris and mononuclear inflammatory cells were detected in <5% of the tissues (Fig. 3B, C, E, F). Moderate bronchiolitis characterized by epithelial necrosis and sloughing and acute inflammation affected 50% of two lung lobes and more than 90% of two lung lobes in both control animals (Fig. 3G). In some areas the inflammation extended into the alveoli adjacent to the bronchioles. In the oseltamivir-treated ferrets, histopathologic changes in the tissues studied were quite variable among lung samples, and the extent of lung pathology differed markedly from that in controls. In each treatment group (2 h and 24 h p.i.), one ferret showed bronchiolitis with acute inflammation affecting ~30% of the 4 lobes (Fig. 3H) and the other ferret showed acute inflammation of a single bronchiole in one lobe (~1% tissues), with little involvement (< 1% tissues) of other lung tissue (Fig. 3I).
Fig. 3.
Histologic changes in the nasal turbinates, tracheas, and lungs of oseltamivir-treated and control ferrets inoculated with A/CA/04/09 (H1N1) influenza virus. Photomicrographs shown are hematoxylin-and-eosin-stained sections of nasal turbinate (A, B, C), trachea (D, E, F) and lung tissue (G, H, I). Ferrets were inoculated with 106 PFU of A/CA/04/09 (H1N1) influenza virus; oseltamivir (25 mg/kg/day twice daily for 5 days) or sterile PBS was administered orally either 2 h or 24 h after inoculation. Tissue samples were obtained on day 4 p.i. Sections were stained with hematoxylin and eosin. Lesions and inflammation are apparent in nasal turbinate, trachea, and lung sections from control (untreated) ferrets, with extensive epithelial necrosis and inflammatory cell infiltration of the bronchial submucosal mucus glands and bronchioles (A, D, G). The inflammatory cell infiltrates extend into the alveoli surrounding the bronchioles. Lesions and inflammation were minimal to mild (B, E, H) or unremarkable to minimal (C, F, I) in nasal turbinate, trachea, and lung sections from oseltamivir-treated ferrets. Magnification, ×20.
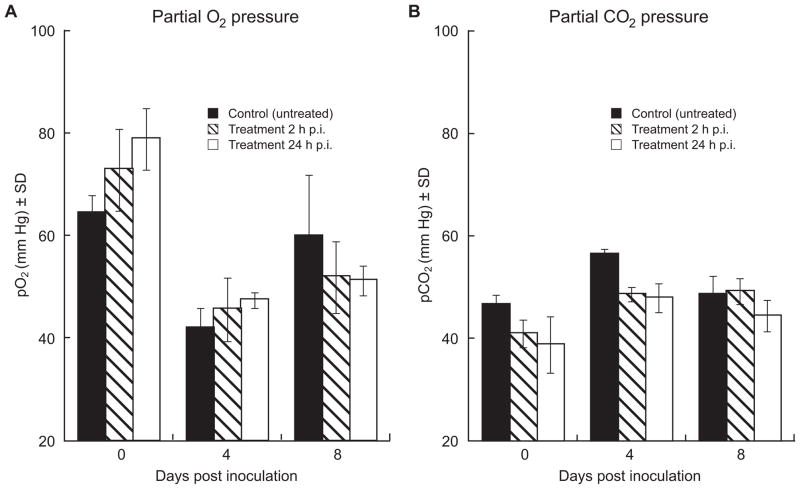
We evaluated the effect of oseltamivir treatment on lung function by measuring partial oxygen pressure (pO2) and partial carbon dioxide pressure (pCO2) in the arterial blood of ferrets on days 0, 4, and 8 p.i. (Fig. 4A, B). In the control group, the pO2 declined from ~65 to ~42 mmHg on day 4 p.i. and returned to near-baseline levels on day 8 p.i.; the changes in pCO2 were consistent with the observed changes in pO2. In the oseltamivir-treated groups, changes in pO2 and pCO2 were less pronounced but were not found to differ significantly from control values.
Fig. 4.
Arterial blood oxygenation in oseltamivir-treated and control ferrets inoculated with A/CA/04/09 (H1N1) influenza virus. Partial oxygen (pO2) (A) and carbon dioxide (pCO2) (B) pressure in arterial blood was measured on days 0, 4, and 8 p.i. in oseltamivir-treated and control ferrets inoculated with 106 PFU of A/CA/04/09 (H1N1) influenza virus. Ferrets were given sterile PBS or 25 mg/kg/day of oseltamivir twice daily for 5 days, beginning either 2 h or 24 h p.i.. Data points are the mean (mm Hg) ± S.D. from 3 ferrets.
3.3. Emergence of oseltamivir resistance
To determine whether oseltamivir-resistant variants emerged during treatment, we sequenced the NA and HA (HA1 region) genes of viruses isolated from nasal wash (days 6 and 8 p.i.), nasal turbinate, trachea, and lung (day 4 p.i.) samples. Direct sequencing of the NA genes from nasal wash samples and analysis of virus clones obtained from individual plaques in MDCK cells revealed no sequence change in amino acid residues H275Y (Table 1), I223R and N295S (results not shown), associated with oseltamivir resistance in the N1 NA subtype. Moreover, all isolates remained susceptible to oseltamivir carboxylate on day 6 p.i. (mean IC50, 2.6 –5.3 nM) in NA enzyme inhibition assays. In an analysis of 12 clones, we identified polymorphism of three amino acid residues (G155E, S183P, and L191I) in the HA1 region of the stock A/CA/04/09 (H1N1) virus (Table 1). Clones with different amino acids at these positions were identified in samples from inoculated control animals. Two HA mutations (155E and 191I) consistently appeared together in the control ferrets. In the oseltamivir-treatment groups, clones with the S183P and L191I polymorphisms were identified, but they showed only glycine at residue 155. Although these HA mutations can affect virus properties, they did not alter susceptibility to oseltamivir carboxylate. No resistant variants were detected in this study.
Table 1.
Assessment of NA and HA sequence changes during oseltamivir treatment of ferrets inoculated with A/CA/04/09 (H1N1) influenza virus
| Virus, oseltamivir regimen | Origin of sample | Amino acid residue at position no. (no. of clones/total no. sequenced) a:
|
|||
|---|---|---|---|---|---|
| NA H275Y |
HA (HA1)
|
||||
| G155E | S183P | L191I | |||
| A/CA/04/09, wild type | MDCK cells | H275 (12/12) | 155E (10/12) | 183P (3/12) | 191I (9/12) |
| 0 mg/kg/day | Nasal wash | – | – | 183P (2/6) | 191I (1/6) |
| Lungs | – | 155E (4/6) | 183P (1/6) | 191I (4/6) | |
| Nasal turbinate | – | 155E (3/6) | 183P (1/6) | 191I (2/6) | |
| 25 mg/kg/day, 2 h delay | Nasal wash | – | – | – | 191I (1/6) |
| Nasal turbinate | – | – | 183P (2/6) | 191I (1/6) | |
| 25 mg/kg/day, 24 h delay | Nasal wash | – | – | 183P (2/6) | 191I (3/6) |
| Nasal turbinate | – | – | 183P (1/6) | – | |
NA and HA (HA1) amino acid mutations were identified by sequence analysis of 6–12 virus clones obtained from individual plaques grown in MDCK cells from nasal wash, nasal turbinate, or lung specimens. Amino acid numbering is based on N1 NA and on H1 HA numbering.
–, no mutations detected.
4. Discussion
The main purpose of our study was to establish mammalian animal model for determination whether oseltamivir can offer a clinical benefit to humans infected with H1N1pdm virus, which, unlike contemporary seasonal influenza viruses, is reported to replicate deep in the lungs and cause viral pneumonia. In a ferret model we found that orally administered oseltamivir effectively reduced the spread of A/CA/04/09 (H1N1) virus in the lungs, thus reducing the risk of development of viral pneumonia. This finding is consistent with a clinical observation that oseltamivir appears to reduce the risk of radiographically confirmed pneumonia and that treatment within 2 days of symptom onset can reduce the duration of fever and virus shedding (Yu et al., 2010). The effect of oseltamivir on virus load and on the duration of virus shedding is not often addressed in clinical trials. We consider that the extent to which treatment with oseltamivir might control viral replication in the URT and lung tissues is an important determinant which can affect virus transmission and development of complications.
Preclinical data suggest that the effectiveness of oseltamivir treatment depends on the virus load and that higher doses and more prolonged treatment are required for more virulent H5N1 influenza virus infection (Yen et al., 2005; Govorkova et al., 2007). This study used a high inoculation dose of H1N1pdm virus (106 PFU/ferret), which may explain why virus replication in the URT was not inhibited by oseltamivir. This inoculation dose was selected based on preliminary experiments on the pathogenicity of H1N1pdm influenza virus in ferrets, and resulted in detectable clinical signs of infection which can be monitored under antiviral drug treatment (results not shown). Most human infections are likely to involve a smaller virus inoculum and therefore may respond more favorably to oseltamivir. Two small observational studies of 2009 H1N1pdm influenza also suggested that early oseltamivir treatment reduces the duration of virus shedding (Ling et al., 2010), virus load, and duration of fever (Li et al., 2010). The pharmacokinetic parameters of oseltamivir carboxylate are well established in humans; its oral bioavailability is reported to be ~80%, peak plasma concentration is achieved 3–4 hours after administration, and plasma half-life is 6–10 h (McClellan and Perry, 2001). However, limited information is available about the pharmacokinetics and tissue distribution of the drug in a ferret model. It is possible that insufficient oseltamivir was available in the upper respiratory tract of ferrets to promote virus clearance.
Improved methods are needed to monitor the respiratory condition of experimental animals infected with H1N1pdm influenza virus, which is known to replicate in the lungs (Itoh et al., 2009; Maines et al., 2009; Munster et al., 2009). This study is the first, to our knowledge, to assess the effect of oseltamivir treatment on lung function in a ferret model, although arterial blood gas parameters (pO2, pCO2, pH) have been used in mice to characterize pulmonary function during infection with H1N1 (Thomas et al., 2009) and H5N1 (Xu et al., 2006) viruses. We observed a tendency to less impaired lung functions from day 0 to day 4 p.i. in the oseltamivir-treated ferrets as compared to those in control. Inoculation of ferrets with A/CA/04/09 (H1N1) virus in this study caused mild disease, and it is possible that more severe respiratory impairment would have revealed significant differences. The use of arterial blood gas measurements was also limited by the necessity to collect blood samples on multiple days and potential adverse side effects of anesthesia on the levels of blood gases. Anesthesia can have marked effects not only on cardiopulmonary function and tissue oxygenation but also on vascular regulation (Wilson et al., 2006) and therefore limits this approach to evaluate lung functions in an animal model. The suitability of non-invasive methods such as pulse-oximetry for use in ferrets should be assessed and it could be a valuable parameter for evaluation of drug efficacy in pre-clinical experiments.
The development and dissemination of resistance can significantly compromise the clinical benefits of NA inhibitors. A single H275Y NA mutation is the most common cause of resistance in the N1 NA subtype and has been observed in oseltamivir-resistant H1N1pdm viruses (Deyde et al., 2010). A novel I223R NA mutation identified in H1N1pdm viruses caused relatively modest resistance to both oseltamivir and zanamivir; in combination with H275Y, this mutation significantly increased resistance to oseltamivir and peramivir but only marginally increased resistance to zanamivir (Nguyen et al., 2010; van der Vries et al., 2010). Here we examined virus samples from nasal washes and tissues (nasal turbinate, trachea and lungs) for known molecular markers of NA inhibitor resistance by sequence analysis of dominant virus populations and individual clones. We did not detect H275Y, I223R and N295S NA mutations in multiple samples tested. Phenotypic assay confirmed that the viruses isolated from treated ferrets retained high susceptibility to oseltamivir, consistent with the low incidence of oseltamivir resistance in samples isolated from patients, including those in high-risk groups (WHO, 2009; Gubareva et al., 2010).
We did not detect D222G substitutions in HA which was associated with more severe disease and altered receptor specificity and cell tropism of H1N1pdm viruses (Chen et al., 2010; Liu et al., 2010). The A/CA/04/09 (H1N1) virus used in our study possessed a mixture of amino acids at HA positions 155, 183 and 191. The G155E HA mutation has been shown to increase growth rate in MDCK cells and eggs due to the negatively charged glutamic acid (Chen et al., 2010). The L191I mutation in the glycan binding region is conserved in 2009 H1N1pdm and seasonal H1N1 viruses. Alignment of 100 HA sequences of H1N1pdm available in GenBank revealed polymorphism of these three residues (results not shown), and thus potential differences in replication efficiency and tissue tropism.
Recent reports suggest that NA inhibitors offer a benefit against 2009 H1N1pdm influenza viruses (Kidd et al., 2009; Dominguez-Cherit et al., 2009; Jain et al., 2009; Yu et al., 2010). They were shown to slow the transmission of H1N1pdm viruses when administered as prophylaxis to contacts within households, schools, and workplaces (Anderson et al., 2009), and oseltamivir provided effective ring prophylaxis when given after exposure in military camps (Lee et al., 2010). However, despite numerous epidemiological and clinical reports on H1N1pdm, few have evaluated how oseltamivir treatment affects virus load and duration of virus shedding (Charlier et al., 2009; Li et al., 2010). Therefore, our findings in a ferret model further elucidate the antiviral activity of oseltamivir against 2009 H1N1pdm virus infection and possible role in prevention of viral pneumonia. It leads to the main conclusion of the study that specific anti-influenza therapy should be considered as an important part in the management of severe cases of the disease if administered early enough in the clinical course, in addition to other therapeutic interventions that can reduce clinical symptoms and provide more rapid recovery.
Acknowledgments
We gratefully acknowledge the editorial assistance of Sharon Naron, the excellent technical support of Patrick Seiler and the Center for Biotechnology, and Klo Spelshouse for help with illustrations. This work was supported by Contract HHSN266200700005C from the National Institute of Allergy and Infectious Diseases and Cancer Center Support (CORE) grant P30 CA 21765 from the National Cancer Institute, National Institutes of Health; the American Lebanese Syrian Associated Charities (ALSAC); and the F. Hoffmann La-Roche Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM. How well are we managing the influenza A/H1N1 pandemic in the UK? BMJ. 2009;339:b2897. doi: 10.1136/bmj.b2897. [DOI] [PubMed] [Google Scholar]
- Cao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, Liang ZA, Liang L, Zhang SJ, Zhang B, Gu L, Lu LH, Wang DY, Wang C National Influenza A Pandemic (H1N1) Clinical Investigation Group of China., 2009. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361:2507–2517. doi: 10.1056/NEJMoa0906612. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:433–435. [PubMed] [Google Scholar]
- Charlier C, Enouf V, Lanternier F, Grandadam M, Amazzough K, Blanche S, Lecuit M, Lortholary O, van der Werf S. Kinetics of nasopharyngeal shedding of novel H1N1 (swine-like) influenza A virus in an immunocompetent adult under oseltamivir therapy. Clin Microbiol Infect. 2009;15:1189–1191. doi: 10.1111/j.1469-0691.2009.03007.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Wen X, To KK, Wang P, Tse H, Chan JF, Tsoi HW, Fung KS, Tse CW, Lee RA, Chan KH, Yuen KY. Quasispecies of the D225G substitution in the hemagglutinin of pandemic influenza A(H1N1) 2009 virus from patients with severe disease in Hong Kong, China. J Infect Dis. 2010;201:1517–1521. doi: 10.1086/652661. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wang W, Zhou H, Suguitan AL, Jr, Shambaugh C, Kim L, Zhao J, Kemble G, Jin H. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol. 2010;84:44–51. doi: 10.1128/JVI.02106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyde VM, Sheu TG, Trujillo AA, Okomo-Adhiambo M, Garten R, Klimov AI, Gubareva LV. Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by pyrosequencing. Antimicrob Agents Chemother. 2010;54:1102–1110. doi: 10.1128/AAC.01417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart TE, Fowler RA. Critically ill patients with 2009 influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009;10:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur AH, Bagga B, Barman S, Hayden R, Lamptey A, Hoffman JM, Bhojwani D, Flynn PM, Tuomanen E, Webby R. Intravenous zanamivir for oseltamivir-resistant 2009 H1N1 influenza. N Engl J Med. 2010;7:88–89. doi: 10.1056/NEJMc0910893. [DOI] [PubMed] [Google Scholar]
- Govorkova EA, Ilyushina NA, Boltz DA, Douglas A, Yilmaz N, Webster RG. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob Agents Chemother. 2007;51:1414–1424. doi: 10.1128/AAC.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Trujillo AA, Okomo-Adhiambo M, Mishin VP, Deyde VM, Sleeman K, Nguyen HT, Sheu TG, Garten RJ, Shaw MW, Fry AM, Klimov AI. Comprehensive assessment of 2009 pandemic influenza A (H1N1) virus drug susceptibility in vitro. Antivir Ther. 2010;15(8):1151–1159. doi: 10.3851/IMP1678. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki-Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Takahashi K, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K, Kawaoka Y. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–1025. doi: 10.1038/nature08260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- Kidd IM, Down J, Nastouli E, Shulman R, Grant PR, Howell DC, Singer M. H1N1 pneumonitis treated with intravenous zanamivir. Lancet. 2009;19:1036. doi: 10.1016/S0140-6736(09)61528-2. [DOI] [PubMed] [Google Scholar]
- Lee VJ, Yap J, Cook AR, Chen MI, Tay JK, Tan BH, Loh JP, Chew SW, Koh WH, Lin R, Cui L, Lee CW, Sung WK, Wong CW, Hibberd ML, Kang WL, Seet B, Tambyah PA. Oseltamivir ring prophylaxis for containment of 2009 H1N1 influenza outbreaks. N Engl J Med. 2010;362:2166–2174. doi: 10.1056/NEJMoa0908482. [DOI] [PubMed] [Google Scholar]
- Li IW, Hung IF, To KK, Chan KH, Wong SS, Chan JF, Cheng VC, Tsang OT, Lai ST, Lau YL, Yuen KY. The natural viral load profile of patients with pandemic 2009 influenza A (H1N1) and the effect of oseltamivir treatment. Chest. 2010;137:759–768. doi: 10.1378/chest.09-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling LM, Chow AL, Lye DC, Tan AS, Krishnan P, Cui L, Win NN, Chan M, Lim PL, Lee CC, Leo YS. Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection. Clin, Infect, Dis. 2010;50:963–969. doi: 10.1086/651083. [DOI] [PubMed] [Google Scholar]
- Liu Y, Childs RA, Matrosovich T, Wharton S, Palma AS, Chai W, Daniels R, Gregory V, Uhlendorff J, Kiso M, Klenk HD, Hay A, Feizi T, Matrosovich M. Altered receptor specificity and cell tropism of D222G hemagglutinin mutants isolated from fatal cases of pandemic A(H1N1) 2009 influenza virus. J Virol. 2010;84:12069–12074. doi: 10.1128/JVI.01639-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan K, Perry CM. Oseltamivir: a review of its use in influenza. Drugs. 2001;61:263–283. doi: 10.2165/00003495-200161020-00011. [DOI] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HT, Fry AM, Loveless PA, Klimov AI, Gubareva LV. Recovery of a multidrug-resistant strain of pandemic influenza A 2009 (H1N1) virus carrying a dual H275Y/I223R mutation from a child after prolonged treatment with oseltamivir. Clin Infect Dis. 2010;51:983–984. doi: 10.1086/656439. [DOI] [PubMed] [Google Scholar]
- Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Reuman PD, Keely S, Schiff GM. Assessment of signs of influenza illness in the ferret model. J Virol Methods. 1989;24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;25:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vries E, Stelma FF, Boucher CA. Emergence of a multidrug-resistant pandemic influenza A (H1N1) virus. N Engl J Med. 2010;363:1381–1382. doi: 10.1056/NEJMc1003749. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Lee WMF, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J of Appl Physiol. 2006;101:1648–1656. doi: 10.1152/japplphysiol.00394.2006. [DOI] [PubMed] [Google Scholar]
- World Health Organization. [Accessed 14 March 2011];Pandemic (H1N1) 2009 – update 60. 2009 www.who.int/csr/don/2009_08_04/en/print.html.
- Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- Xu T, Qiao J, Zhao L, Wang G, He G, Li K, Tian Y, Gao M, Wang J, Wang H, Dong C. Acute respiratory distress syndrome induced by avian influenza A (H5N1) virus in mice. Am J Respir Crit Care Med. 2006;174:1011–1017. doi: 10.1164/rccm.200511-1751OC. [DOI] [PubMed] [Google Scholar]
- Yu H, Liao Q, Yuan Y, Zhou L, Xiang N, Huai Y, Guo X, Zheng Y, van Doorn HR, Farrar J, Gao Z, Feng Z, Wang Y, Yang W. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. doi: 10.1136/bmj.c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Monto AS, Webster RG, Govorkova EA. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192:665–672. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]