Abstract
Among the Weapons of Mass Destruction, chemical warfare (CW) is probably one of the most brutal created by mankind in comparison with biological and nuclear warfare. Chemical weapons are inexpensive and are relatively easy to produce, even by small terrorist groups, to create mass casualties with small quantities. The characteristics of various CW agents, general information relevant to current physical as well as medical protection methods, detection equipment available and decontamination techniques are discussed in this review article. A brief note on Chemical Weapons Convention is also provided.
Keywords: Blister agents, chemical warfare, decontamination, detection, mustards, nerve agents, protection
Among the Weapons of Mass Destruction (WMD), chemical warfare (CW) is probably one of the most brutal created by mankind. CW agents are extremely toxic synthetic chemicals that can be dispersed as a gas, liquid or aerosol or as agents adsorbed to particles to become a powder. These CW agents have either lethal or incapacitating effects on humans.[1] They differ from explosive chemicals in which the destructive effects are caused by shear force and are localized. Thousands of toxic substances are known, but only some of them are considered as CW agents based on their characteristics, viz. high toxicity, imperceptibility to senses and rapidity of action after dissemination and persistency, and are listed as scheduled chemicals in the Chemical Weapons Convention (CWC).[2] According to the CWC, chemical weapons are defined as toxic chemicals and their precursors, munitions and devices, and any equipment specifically designed for use directly in connection with such weapons.
The use of poisonous chemicals from plant extracts to poison individuals is widely documented throughout the Middle Ages and Renaissance, but it was not until the expansion of industrial chemistry in the 19th century that mass production and deployment of CW agents in war became a possibility. Thus, the birth of modern CW was ushered in by the German gas attack with chlorine on 22nd April 1915 at Ypres, Belgium. The use of these toxic chemicals, including phosgene, sulfur mustard and lewisites caused 100,000 deaths and 1.2 million casualties in World War I (WWI).[3] Millions of innocent civilians were killed by the Nazis with Zyklon B gas (hydrogen cyanide gas) during World War II. Agent Orange – a defoliant – was used by the USA during the Vietnam War. The only major use of CW since WWI occurred during the Iran–Iraq War in the 1980s. The largest single CW attack killing around 5,000 people followed an Iraqi nerve agent attack on the Kurdish civilian population of Halabja. This attack illustrates the one single characteristic of CW agents that allows them to be considered as WMD.[4] This incident, together with the relative simplicity of their production, makes them an attractive terrorist weapon. This has been made particularly evident by the Sarin attacks by a Japanese cult in Matsumoto city (1994) and the Tokyo subway system (1995), causing 5,500 injuries and 12 deaths. The threat of using CW agents in domestic terrorist attack was demonstrated for the first time in these cases. However, mass casualties were prevented not as a consequence of the medical response but because of the inefficiency of the delivery method.[5] Subsequent investigations revealed that the sect had also produced Tabun, Soman, Vx and Botulinum toxin.[6] Thus, even small groups of individuals can use CW agents to create massive and extensive human suffering without any warning.
Terrorists have previously used more conventional means of violence, such as bombings, assassinations and hostage taking, to promote their causes. Terrorism and criminal activities achieved a whole new quality after incidents like the repeated assaults on the World Trade Center in New York culminating in its destruction on September 11, 2001 and the subsequent dissemination of anthrax-letters.[7] On April 6, 2004, news agencies reported that the British police foiled a plot by members of the El-Qaeda to prepare and detonate a bomb containing OsO4 in London.[8] Following these, over the last decade, it has been feared that terrorists might be tempted to acquire and use such weapons against innocent civilians. The major reasons for the production and use of such weapons are manifold. First, chemical weapons are cost-effective, particularly when used against concentrated forces or populations. Second, they may be used at lower levels of concentration with an aim to cause panic and disorder among civilians. Among the CW agents, chlorine, phosgene and cyanides are widely used in the manufacturing processes of various chemical or pharmaceutical industries. Thus, the act of terrorism might also occur in the form of a toxic chemical release, e.g. when such industrial plants, stocks or transports become a target of terrorist attacks.[9] In the Tokyo subway attack, a simple plastic bag containing the CW agent Sarin was kept on an underground train, allegedly piercing it with umbrella tips before the cult's escaped.[10] Therefore, intentional release of such agents during National or International events can be easily achieved by transporting them in the form of water bottles, cold drink cans, ampoules or even pens, etc. to the site. The effect of intentional release of CW agent varies greatly, depending on several factors, including the toxicity of the compound, its volatility and concentration, the route of exposure, the duration of the exposure and the environmental conditions. The release of such agents in an enclosed place could deliver doses high enough to injure or kill a large number of people, whereas in an open area, chemical cloud would become less concentrated as it spreads, leading possibly to numerous mild casualties.
In the present time, all over the world, chemical terrorism is a serious threat to the security of mankind, whose scale essentially exceeds the impact of use of the most modem firearms.[11] Probably about 70 different chemicals or mixtures of chemicals have been used or stockpiled as agents for the purpose of CW during the 20th century.[12] Taking recent technological advances into consideration, easy access to raw materials, the ready availability of technical information on the internet, increasing crime and corruption and state-sponsored terrorism and globalization, it is not difficult for the terrorists to use CW agents to achieve their goals.[13] This review provides a comprehensive account of the CW agents, which includes current status of protective equipment available, detection and decontamination methods. The role of the CWC is also briefly mentioned.
Classification of CW Agents
The CW agents possess different characteristics and belong to various classes of compounds with pronounced physicochemical, physiological and chemical properties.[14,15] Thus, they are classified in many ways. Based on their volatility, they are classified as persistent or non-persistent agents. The more volatile an agent, the quicker it evaporates and disperses. The more volatile agents like chlorine, phosgene and hydrogen cyanide are non-persistent agents whereas the less volatile agents like sulfur mustard and Vx are persistent agents. Based on their chemical structure, they can be classified as organophosphorus (OP), organosulfur and organofluorine compounds and arsenicals. In general, classification in terms of physiological effects produced on humans by the CW agents is used for many decades. Thus, the CW agents used in warfare are classified as follows:
Nerve agents
Vesicants (blistering agents)
Bloods agents (cyanogenic agents)
Choking agents (pulmonary agents)
Riot-control agents (tear gases)
Psychomimetic agents
Toxins
Nerve agents
Nerve agents acquired their name because they affect the functioning of the nervous system. Nerve agents do not occur naturally and belong to a group of OP compounds. The first known nerve agent, Tabun (GA), was first developed by the German chemist, Gerhard Schrader, in the 1930s during his research in the development of new OP insecticides. Following this, a series of nerve agents known as the G-agents, which include Sarin (GB) and Soman (GD), were developed. Germany had stockpiles of nerve agent munitions during World War II, but did not use them.[15] A variety of nerve agents were developed till 1960 for military use. Much importance was given to increase their potency and environmental persistence. Thus, the V-agents, as more stable versions of the “G” agents, were developed. Thus VX, a sulfur-containing OP, is more potent than sarin, is more stable, less volatile and less water-soluble, acting through direct skin contact, and persisting in the environment up to several weeks after release. Nerve agents are more toxic than the other reported CW agents. They are highly toxic and can cause death within few minutes to few hours after exposure, depending on the concentration. Nerve agents were not used during World War II. The only known battlefield use of nerve agents was in the Iraq-Iran conflict during the 1980–8 war; Iraq reportedly used nerve agents against Iranian troops and later against members of its Kurdish population in northern Iraq.[16] The chemical structures and some of the properties of the more powerful nerve agents are given below [Figure 1, Table 1].[17,18]
Figure 1.

Chemical structure of nerve agents
Table 1.
Physical properties of nerve agents
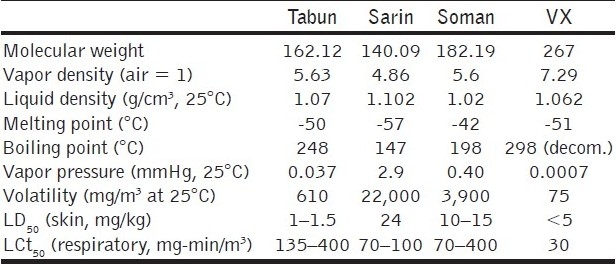
In the pure state, all nerve agents are colorless liquids. The G-agents give off a fruity odor whereas the V-agents give off an amine odor. Sarin is infinitely soluble in water, Soman is sparingly soluble in water and Tabun and Vx are of intermediate character in this respect. The reactions of nerve agents take place through P-X bond cleavage. Thus, the reaction of G-agents with alkali gives non-toxic phosphonic acid. On the other hand, the hydrolysis product of Vx leads to another extremely toxic product.
The mode of action of nerve agents is well documented in the literature.[19–22] Nerve agents exert their biological effects by irreversibly inhibiting the enzyme acetyl cholinesterase (AChE). This enzyme is responsible for hydrolyzing acetylcholine (ACh), a neurotransmitter liberated at the nerve synapse, nerve–muscle (neuromuscular) junction and nerve–gland junction. In a normal individual, a small quantity of ACh is continuously liberated and hydrolyzed by AChE. Inhibition of AChE causes accumulation of Ach, leading to overexcitation or paralysis. As soon as the nerve agents enter the system, symptoms of poisoning appear.
The effects of nerve agents are the result of the action on the muscurinic and nicotinic receptors on the receptors within the central nervous system. They include constriction of the pupil (meiosis), increased production of saliva, running nose, increased perspiration, urination, defecation, bronchosecretion, bronchoconstriction, decreased heart rate and blood pressure, muscular twitches and cramps, cardiac arrhythmias, tremors and convulsions. The most critical effects are paralysis of the respiratory muscles and inhibition of the respiratory center. Ultimately, death results due to respiratory paralysis. If the concentration of the nerve agent is high, death is immediate.
The treatment of nerve agent poisoning requires constant attention by the medical personnel.[23–26] Three drugs, atropine, pralidoxime chloride and diazepam, are used to treat nerve agent exposure. Atropine competes with ACh for the muscuranic ACh receptors and thus helps to protect accumulation of excess ACh during nerve agent poisoning. Atropine is active against all nerve agents. Thus, atropine should be administered immediately and should be repeated, starting with an initial dose of 2 mg intramuscularly or intravenously. The administration of atropine should be continued till it is adequate, as indicated by dryness of mucosa of nose and mouth, and an increase in heart rate. The dosage of atropine should not hinder the performance of a non-intoxicated individual. Side-effects of 2 mg atropine in a normal individual are increased heart rate, drying of secretions, mydriasis (dilatation of pupil) and paralysis of accommodation. Most of the effects are reversible. 2-pyridine aldoxime chloride (2-PAM or pralidoxime) is used for the nicotinic effects of nerve agents, which include muscle fascilation followed by depolarization paralysis.[24] Sometimes, 2-PAM helps to regenerate AChE, thereby restoring muscle repolariztion. Other than pralidoxime, obidoxime, also known as toxogonin, can also be used. But, these oximes are not effective for Soman poisoning. For this, H-series oximes are preferred (HI-6). The oximes should be administered in combination with atropine. The dose of pralidoxime chloride is 15–25 mg/kg by slow intravenous injection. The usual dose of toxogonin is 300 mg. Because these oximes are quickly excreted, further doses may be needed. The convulsions induced by nerve agent poisoning may cause brain damage. Diazepam is used as an adjunct to reduce the convulsions. The usual dose of diazepam is 5–10 mg, intramuscularly. It is important that the antidotes should be administered very quickly in the field itself in the form of first aid.
This is done by the use of autoinjectors, which does not require the help of medical personnel to inject the drug. Atropine and PAM chloride autoinjectors are available for immediate use. There are no accepted prophylactic antidotes for nerve agent poisoning, i.e. drugs administered before exposure to the agent. Pyridostigmine bromide has been introduced as a prophylactic drug. The dose is 30 mg, three-times a day. Although it may give some protection against nerve agent poisoning, it has side-effects.
Blistering agents
Blistering agents[27–38] or vesicants are toxic compounds that produce skin injuries resembling those caused by burns. These agents on inhalation affect the upper respiratory tract as well as the lungs, producing pulmonary edema. These agents can also cause severe eye injuries. There are two forms of vesicants: mustards and arsenicals. The most important substance in this class of CW agents is sulfur mustard and is called as king of CW agents. Other members include nitrogen mustards (HN1, HN2 and HN3) and lewisites (L1, L2 and L3) [Figure 2].
Figure 2.

Chemical structures of blister agents
Pure sulfur mustard is a colorless and odorless liquid; the impure product has a characteristic smell similar to mustard or garlic. It has low volatility. It is soluble in organic solvents readily but is barely soluble in water (0.8 g/L). Nitrogen mustards are also colorless liquids in the pure state. They are less volatile, less soluble and more resistant to oxidizing agents than sulfur mustard, but are less stable in storage. Pure lewisites are also colorless liquids with metallic odor and solubility in water is similar to sulfur mustard, these are but relatively unstable. Thus, nitrogen mustards and lewisites lack the basic requirement, i.e. storage stability of a CW agent. Some of the characteristics of blister agents are given in Table 2.
Table 2.
Physical properties of blister agents
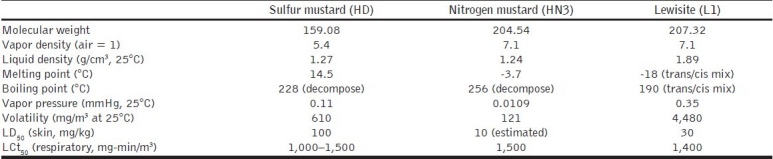
Sulfur mustard is the vesicant with the highest military significance since its use in WWI. The nitrogen mustards were synthesized in the 1930s but were not produced in large amounts for warfare. Mechlorethamine (HN2, Mustargen) has found more peaceful applications as a cancer chemotherapeutic agent and has remained the standard compound for this purpose for many years. Lewisite (L) was synthesized in 1918 for military purpose due to its non-flammable property and toxicity similar to mustard, but has probably not been used on a battlefield.
The mustards are radiomimetic and are extremely toxic to dividing cells. Mustards are lipophilic and readily penetrate the skin, most textiles and rubber. After passing through the cellular membrane, sulfur mustard is converted to highly reactive sulphonium ion. It irreversibly alkylates DNA, RNA and protein, causing cell death; the most important target is DNA. Mustard alkylates the purine bases of DNA and damages them.[33] Lewisite is absorbed by the skin ten-times faster, and it causes immediate pain and irritation in the affected organ and produces more systematic symptoms. It directly binds to the sulfhydryl groups and inactivates them.[19]
The clinical hallmark of mustard exposure is the relative lack of symptoms following exposure.[34] The length of this latent period and rapidity and intensity of symptom development depend on the mode of exposure, concentration of the agent and environmental conditions. In the form of gas or aerosol, mustard attacks the skin, eyes and the respiratory tract. Chemical damage begins in 1–2 min after contact, but onset of pain is delayed for 4–6 h. Eye damage ranges from conjunctivitis to corneal opacification, ulceration and rupture. Skin injury ranges from sun burn-like erythema to vesicles, which coalesce into blisters. Liquid exposure can cause coagulation necrosis of the dermis.[35] The effect of mustard gas on the respiratory tract also depends on the degree of exposure. If the exposure is mild, swelling and erythema will be present in the nose, larynx and trachea. The laryngeal edema and necrosis may lead to respiratory obstruction. There is a danger of bacterial infection of the lungs, which may result in bronchopneumonia. The latter may be responsible for death following mustard exposure. Severe exposure to mustard may induce bone marrow damage, leading to leucopenia. Ingestion of food or water contaminated by liquid mustard produces nausea, vomiting, pain and diarrhea. Even exposure to the skin alone can cause malaise, vomiting and cardiac abnormalities.
There is no specific antidote for mustard toxicity and the treatment is similar to that of burn injuries.[34,36,38] The eyes should be washed with uncontaminated water. If the eyelids are sticky, then sterile petroleum jelly can be applied. Blepharospasm can be relieved by instilling one drop of atropine solution (1%) three to four-times a day. Secondary infection, if any, can be treated by instilling ciprofloxacillin eye drops. The skin area should be immediately decontaminated using Fuller's Earth. Povidone–iodine ointment or framycetin ointment should be applied on the mustard blisters. Systemic analgesics and antihistamines can be used to relieve itching and pain. Pharyngitis due to inhalation exposure can be relieved by taking alkaline gargle. For persistent cough, codeine can be taken.
Unlike mustard, specific treatment for lewisite poisoning is available. 2,3-dithiocaptopropanol, commonly known as British Anti Lewisite (BAL), is a specific chelating agent for arsenicals.[19] Lewisite is a potent inhibitor of anaerobic glycolysis, an effect reversible with BAL. If the eye or skin is contaminated, BAL ointment should be applied immediately. The other treatment measures are similar to that of mustard. If the contamination is very severe, BAL should be administered systemically. BAL in oil (10% solution) should be given by deep intramuscular injection at a dose of 5 mg/kg and repeated after 4, 8 and 12 h. Due to the toxicity and side-effects of BAL, less-toxic and more-specific new arsenic antidotes such as meso-dimercaptosuccinic acid (DMSA), 2,3-dimercapto-1-propanesulphonic acid (DMPS) and 2,3-dithioerythritol (DTE) are being studied.[39,40]
Blood agents
Blood agents[41–47] are cyanide group of chemicals that affect bodily functions by preventing the normal utilization of oxygen by body tissues. The term “blood agent” is a misnomer because these agents do not typically affect the blood, although they may interrupt the production of blood components. Rather, they exert their toxic effect at the cellular level by interrupting the electron transport chain in the inner membranes of the mitochondria. These agents are also known as systemic agents as they inhibit certain specific enzymes. Hydrogen cyanide (HCN) and cyanogen chloride (CNCl) are the main CW agents in this class. The properties of these agents are given in Table 3.
Table 3.
Physical properties of blood agents
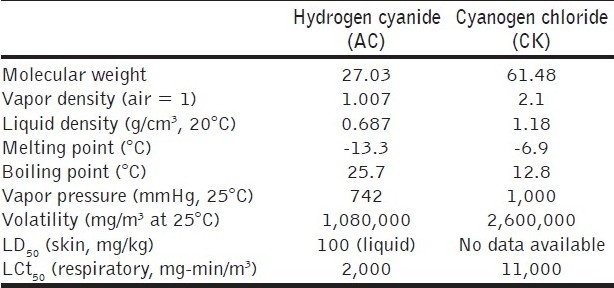
Hydrogen cyanide, first discovered by a Swedish chemist in 1872, was used as an industrial chemical long before the realization of its potential as a CW agent during the first World War. The French were the first to consider it for this purpose and used shells made from this material in the battle of Somme in 1916. Cyanogen chloride was also available in plenty as a commercial product having applications as an industrial intermediate during the first World War. It was introduced to overcome the disadvantages encountered in the use of HCN, i.e. a heavier gas with cumulative effects in low concentrations. Cyanogen chloride has powerful lachrymatory and choking effects also. The agent penetrates the filter elements of a gas mask more readily than any other agent. The worldwide industrial development has become a major threat so far as cyanide is concerned. This was used by Iraq in her conflict with Iran during the 1983–88 war. The very high volatility of the blood agents makes them less useful as CW agents, but their use as a terrorist weapon rests on their efficacy if deployed in enclosed spaces.
Cyanide has a very high affinity for iron in the ferric (Fe+3) state. On entering the biological system, it readily reacts with trivalent iron of cytochrome oxidase (an end-chain enzyme of cellular respiration) to form a complex, thereby impairing the utilization of oxygen in the tissues. Eventually, death follows as a result of respiratory failure.[45] The onset and intensity of symptoms depend on the concentration of inhaled toxic vapor and duration of the exposure. Symptoms on exposure to low doses of HCN are weakness, giddiness, headache, confusion and, sometimes, nausea and vomiting. Clinical signs appear only at high levels of exposure, which include fast and painful respiration, lack of coordination of movement, cardiac irregularities, hypoxic convulsions, coma and respiratory failure culminating in death. Diagnosis may be aided by characteristic odor of cyanide (bitter almond) or a faint pale-red hue of the skin.
Artificial respiration and oxygen are given as first aid to victims of cyanide poisoning. The patient should be removed from the contaminated environment. The aim of the treatment is to dissociate the cyanide ion from the cytochrome oxidase–cyanide complex. This can be accomplished by binders like amyl nitrite, sodium nitrite and 4-dimethylaminophenol (DMAP), which oxidize hemoglobin to methemoglobin, which subsequently sequesters the cyanide ion to form cyanmethemoglobin. Amyl nitrite is usually administered by inhalation while sodium nitrite (10 ml of 3%) is given intravenously (i.v.). Sodium thiosufhate (50 ml of 25% solution, i.v.), a sulfur donor to enzyme rhodanese present in the liver, augments the elimination of cyanide as thiocyanate. Other chelating compounds like hydroxocobalamin (vitamin B12a, 20 mg, i.v.) and kelocyanor (cobalt-EDTA) are also effective antidotes. Recent studies have also shown that disodium 2-ketoglutarate, either alone or in combination with sodium thiosulfate, is very effective in antagonizing lethal cyanide poisoning in experimental animals. Hyperbaric oxygen also potentiates the efficacy of known antidotes. Other supportive therapy include diazepam (3 mg × 10 mg, i.v.), sodium bicarbonate and methylene blue (30 ml of 1%, i.v.). Treatment of cyanide poisoning must be rapid to be effective.[47]
Choking agents
Choking agents injure an individual mainly in the respiratory tract, i.e. in the nose, throat, and particularly, the lungs. In extreme cases, membranes swell, the lungs become filled with liquid and death results from lack of oxygen; thus, these agents “choke” the unprotected individuals. Fatalities of this type are referred to as “dry-land drownings.”[48] Chlorine and phosgene are the best known among this class, although diphosgenes, nitric oxide and perfluoroisobutylene (PFIB) also belong to this group. Choking agents were among the first CW agents produced in large quantities and were used extensively during WWI. They are generally heavier than air. Both chlorine and phosgene are used in many chemical industrial processes, making the control of these compounds difficult, and these can be used as a devastating low-tech weapon in the hands of terrorists.[49]
At room temperature, chlorine is a pungent, green-yellow gas. It can be liquefied under moderate pressure. Even at toxic levels, phosgene gas has little distinguishing odor and usually kills its victims only after a considerable delay (up to 24 h). Phosgene was alone responsible for about 80% death by chemicals in WWI.[50] Being liquid at room temperature, diphosgene, another WWI agent, is generally easier to handle than phosgene, and it is more persistent than either chlorine or phosgene. Diphosgene is basically phosgene with chloroform grafted onto it, and it has the capability to penetrate through canisters. PFIB is an industrial gas generated as a byproduct from overheating and during the production of polytetrafluoroethylene (Teflon). Like phosgene, PFIB has a latency period between exposure and symptoms. Because of its high toxicity (about 10-times as toxic as phosgene), its protective filters breakthrough property and wide availability, PFIB is listed in the Scheduled 2 toxic substance in the CWC. The properties of the choking agents are given in Table 4.
Table 4.
Physical properties of choking agents

There is no uniform view regarding the mechanism of action of phosgene intoxication. Phosgene is highly reactive and combines with the –SH, –NH2 and –OH groups of biological macromolecules, including enzymes, and this may account for its toxic effects. Poisoning is mainly attributed to acylation of certain tissue elements of the lungs and increased permeability of the alveolar mucous membrane resulting in pulmonary edema with consequent anoxia and death. Inhalation of low concentrations of phosgene produces rapid and shallow breathing, reduced respiratory volume, bradycardia and hypotension. Many cholinergic symptoms like increased salivation, nausea, micturition and defecation are also observed. Exposure to higher concentration may produce more specific effects on the vital functions of the lungs and, eventually, development of pulmonary edema culminating in death. The immediate cause of death is usually paralysis of the respiratory center due to anoxia.[51–53]
Treatment of phosgene poisoning is essentially palliative. The main objective of the treatment is to prevent the development of pulmonary edema and other secondary effects arising out of anoxia. Treatment is extended in three steps. Under first aid, the victim should be allowed fresh air and should be kept warm. The treatment is phased in a manner to provide basic therapy within 30 min of exposure followed by selected additional therapy. Immediate medical aid involves artificial respiration along with the administration of cortisone (hexamethasone or beclamethasone) and sodium bicarbonate assisted by positive pressure breathing. Coughing worsens the prognosis and can be suppressed with codeine. Sedatives are not recommended. Antibiotic therapy is recommended when bronchitis or pneumonitis develops. The selected additional therapy includes supplementary oxygen and i.v. injection of sodium bicarbonate. Relief from airway obstruction may be achieved by theophylline and prostaglandin E1 (PGE1) followed by surfactant supplementation with dipamitoyl phosphatidylcholine or cholesterol palmitate aerosols. Intensive care and supervision is required for more than 24 h.[54–56]
Riot control agents
Riot control agents are compounds that cause temporary incapacitation by irritation of the eyes (tearing and blepharospasm), causing them to close, and irritation of the upper respiratory tract. They are often called irritants, lachrymators and harassing agents. The general public usually calls them tear gases. Many tear-stimulating substances were tested as CW agents.[57] On exposure, they cause pain in the eyes, flow of tears and skin irritation. Sensations like skin irritation and lachrymation caused by the tear gases are so strong that victims cannot behave rationally, which will hinder and incapacitate the performance of coordinated activities of the exposed people. Three types of riot control agents are recognized: lachrymators, which primarily cause lachrymation and eye irritation; sternutators, which mainly cause sneezing and irritation of the upper respiratory tract; and vomiting agents, which additionally cause vomiting. Among a long series of chemical substances used as riot control agents, only three chemical agents, viz. CN, CS and CR are of significant importance.[58] The properties of these are given in Table 5. [Figure 3].
Table 5.
Physical properties of riot control agents

Figure 3.
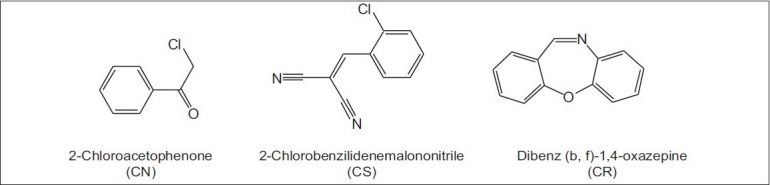
Chemical structures of riot control agents
CN, CS and CR are solid at room temperature and are dispersed as aerosols. They are relatively insoluble in water but soluble in most of the organic solvents. They are characterized by rapid onset of effects, short duration of action and a high safety ratio. The eye is the most sensitive organ, with pain arising rapidly, accompanied by conjunctivitis, blepharospasm and lacrymation.[59,60] The symptoms are felt within 10–30 s. For each of the acute symptoms of pain, the probable mode of action is a direct chemical action on sensory receptors in skin and mucosa that involves a nicotinamide adenine dinucleotide hydrogenase (NADH)-dependent enzymatic process. The peripheral nature of their site of action distinguishes these agents pharmacologically from other incapacitating agents that affect the central nervous system, such as psychochemicals.
The treatment for lachrymator exposure requires immediate decontamination of the clothing and eyes. The victim should be brought to fresh air and the eyes and the skin should be decontaminated with water. Inflammation of skin may be treated with calamine lotion.[61]
Psychomimetic agents
Chemical agents that consistently produce changes in thought, perception and mood, without causing any major disturbances in the autonomic nervous system or other serious disability, are classified as psychomimetic agents.[62–64] Therefore, this group of agents usually includes substances which, when administered in low doses (<10 mg), cause conditions similar to psychotic disorders or other symptoms emanating from the central nervous system, such as loss of feeling, paralysis, hallucinations, etc.
The civil use of this type of agents dates back to antiquity and includes the use of plants such as thorn apple (Datura stramonium)that contain various anticholinergic alkaloids. Psychomimetic agents were used for the first time as an incapacitating agent during war time in 600 BC, when Solon's soldiers threw hellebore roots into streams supplying water to enemy troops, who then developed diarrhea. Hannibal's army in 184 BC used belladonna plants to induce disorientation in the enemy. During World War II, the US military investigated a wide range of possible non-lethal, psychobehavioral chemical incapacitating agents containing indole moiety such as lysergic acid diethylamide (LSD) and marijuana derivatives as well as several glycolate anticholinergics. One of the anticholinergic compounds, 3-quinuclidinyl benzilate, was developed and weaponized in the 1960s as a new chemical agent for battlefield use as a psychochemical[64] and assigned the NATO code BZ [Figure 4].
Figure 4.
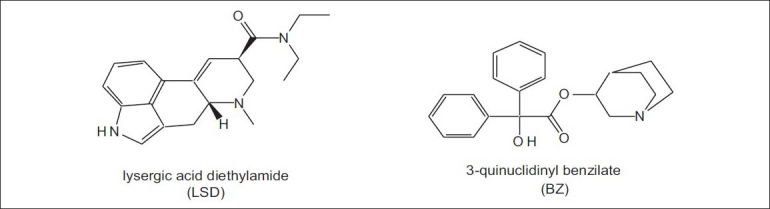
Chemical structures of psychomimetic agents
The psychedelics group includes a large variety of compounds. Among them, LSDis the most well known member. LSD remained as a drug of great interest from the 1950s. Systematic testing of LSD as a possible incapacitating agent was carried out mostly from 1959 to 1965. LSD was proved to be a highly potent incapacitating agent that could produce complete incapacitation, i.e. producing unpredictable behavior by giving an oral dose of approximately 2.5 μg/kg. Affected individuals usually can neither follow a series of instructions given to them nor concentrate on any task. Studies conducted in simulated military exercises demonstrated conclusively that even well-trained units become totally disorganized by giving oral doses of <200 μg. Psilocybin, ibogaine and harmine are also examples of psychedelics, but none of these share the potency of LSD. The aerosolized dose of LSD that capacitates 50% of the exposed individuals (ID50) is estimated to be 6 μg/kg.[65]
BZ is a potent anticholinergic drug that is 25-times more potent than atropine. The ID50 for BZ is 6 μg/kg. Less than 1 mg of BZ can produce acute brain syndrome, characterized by delirium lasting for 2–3 days, which can be reversed by physostigmine and other anticholinesterases.[41]
The common signs and symptoms produced by the psychomimetic agents are:
-
a)
restlessness, dizziness or giddiness; failure to obey orders, confusion, erratic behavior; stumbling or staggering; vomiting
-
b)
dryness of mouth, tachycardia at rest, elevated temperature, flushing of face; blurred vision, pupillary dilation; slurred or non-sensical speech; hallucinatory behavior; disrobing; mumbling and picking behavior; stupor and coma
-
c)
inappropriate smiling or laughter, irrational fear, distractibility, difficulty expressing self, perceptual distortions and phobias
The mechanism by which LSD causes such profound effects on the human perception still has not been established.[66,67] LSD stimulates centers of the sympathetic nervous system in the midbrain, which leads to pupillary dilation, increase in body temperature and rise in the blood sugar level. LSD also has a serotonin-blocking effect. Serotonin is a hormone-like substance occurring naturally in various organs of warm-blooded animals. Concentrated in the midbrain, it plays an important role in the propagation of impulses in certain nerves and, therefore, in the biochemistry of psychic functions. LSD also influences neurophysiologic functions that are connected with dopamine, which is another naturally occurring hormone-like substance. Most of the brain centers receptive to dopamine become activated by LSD, while the others are depressed. The structure of LSD is very similar to other hallucinogenic drugs such as mescaline and psilocybin, all of which contain a substituted indole ring (or a related structure).
Clinical effects from ingestion or inhalation of psychedelics appear after an asymptomatic or latent period that may be as little as 30 min or as long as 20 h; the usual range is 0.5–4 h. However, there is no effect even after 36 h on skin exposure to BZ and other psychedelics. General supportive management of the patient includes decontamination of skin, clothing, weapons and other related items from the patient. The greatest risks to the patient's life are injuries from his or her own erratic behavior and hyperthermia, especially in patients who are in hot or humid environments or are dehydrated from overexertion or insufficient water intake. Management of heat stress assumes a high priority in a severely exposed patient.
Toxins
Poisonous chemical compounds synthesized in nature by living organisms such as bacteria, fungi, terrestrial or marine animals are called toxins.[68,69] They are classified on the basis of chemical nature, molecular weight, source, preferred targets in the body and mechanism of action. Thus, they are two groups: protein toxins consisting of long, folded chains of amino acids and non-protein toxins, which generally are small molecules with a complex chemical nature.[70] Because of the hybrid nature of toxins, they have sometimes been considered CW agents and sometimes BW agents. Similarly, based on their mechanism of action, they are grouped as cardiotoxins, dermatotoxins, hepatotoxins, neurotoxins, etc. The most potent toxins are neurotoxins such as botulinum toxin and tetanus toxin, but there also are others such as staphylococcal enterotoxin. Different bacteria produce each of these biotoxins. Botulinum toxin is the most toxic agent known ever to man[71] and is a very potent neurotoxin, which blocks the release of AChE from the cholinergic nerves of the human nervous system. The lethal dose for humans of such toxins is in the sub-microgram range, which is many times lower (more toxic) than the dosage for nerve agents. Some of the toxins, their origin and mode of action are given in the Table 6.
Table 6.
Characteristics of some potent toxins
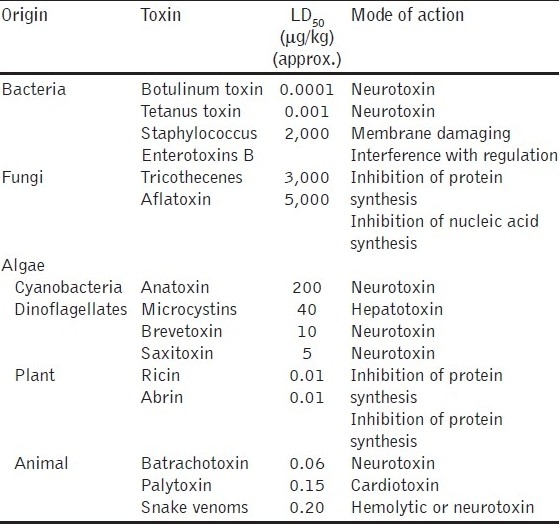
Botulinum toxin is also known as agent X.[71] Arnon et al. estimated that if 1 g of this toxin is aerosolized, it would kill more than one million people.[72] The lethal dose for a 70-kg human is estimated to be approximately 0.7 μg if inhaled or 70 μg if ingested.[73]
Ricin is a very potent toxin of plant origin, isolated from the seeds of caster oil, Ricinus communis. It inhibits ribosome proteins, and the toxic dose for humans is about 0.1–1.0 μg/kg, depending on the mode of administration. It was used in the famous “umbrella tip” assassination of the Bulgarian journalist. Iraq produced and weaponized several highly potent toxins such as botulinum toxin and aflatoxin.[74]
Considerations such as production, weaponization, delivery, environmental stability and host factors place practical limits on their use; toxins as warfare agents are restricted to assassinations or strictly localized terrorist attacks.[69] The two most important toxin threats are botulinum toxin due to its toxicity and Staphylococcus Enterotoxins B, an incapacitating toxin, which cannot be destroyed by boiling. Cases of food poisoning, especially during summer, are due to this bacterial toxin. Ricin and the trichothecene mycotoxins, including T-2 mycotoxin, are of lesser concern, but are still potential threats. The treatment for toxins is passive immunization with antitoxins, with a variety of antitoxins currently being investigated.[75]
Protection During Chemical Emergencies
In case of a suspected or proven release of unknown chemicals or CW agents, the important elements of emergency response are: (i) detection and identification of the agent, (ii) physical and medical protection against the agent and (iii) decontamination.
Detection
The rapid identification and qualitative and quantitative determination[76–82] of the unknown agent is necessary for the selection of adequate protective measures (protective masks and clothing as well as medical treatment), the mapping of contamination area and decontamination procedures. For onsite verification, especially involving CW agents, several handheld detection devices are available, including Three Color Detector (TCD) paper, Residual Vapor Detection (RVD) Kit, Water Poison Detection Kit (WPDK) and chemical agent monitors. These devices have several limitations, such as low specificity and inability to detect all CW agents. Definitive identification of an agent can be carried out onsite in a mobile analytical laboratory or in an off-site laboratory, and this will generally take many hours.
Clinical symptoms and signs in exposed individuals may be the most useful indicators of the likely agent. This is followed by an on-the-spot analysis with detection paper (TCD). The paper, impregnated with reagents, reacts with the liquid CW agents to produce three distinct colors. The paper sticks to any surface and the color change occurs within 30 s, mustard agent turns it red, G-nerve agent turns it yellow and V-nerve agent turns it green. The disadvantage is that it may give false-positive results with many other substances.
The RVD kit is a portable, disposable CW agent detector kit that can detect CW vapors in air with a high specificity. The detection tubes are made of glass with break-off tips and are filled with impregnated chemicals on silica. A definite volume of air is sucked by pump strokes. Color change of the silica indicates the presence of a particular agent. Detection tubes are available for individual agents. Blister and nerve agents, phosgene, cyanogens chloride and hydrogen cyanide in the atmosphere can be detected by this kit. Similarly, poisonous substances present in water sources can be detected with the help of WPDK. This kit contains different reagents marked with numbers, test procedures for known CW agents and results based on simple color change for detection.
Many instruments are available for point detection of CW agents. These are mainly based on three principles: ion mobility spectrometers (IMS), gas chromatograph (GCs) and surface acoustic wave sensors (SAWS). Hand-held chemical agent monitors, based on the principle of ion-mobility spectrometry (IMS), are the most common devices used for the detection of CW agents, particularly blister and nerve agents.[79,80] The GID-3 (Smiths) is a British IMS for onsite detection of common CW agents. The GID-2A is another version for fixed locations designed for unattended operation and continuous monitoring. The hand-held improved chemical agent monitor (ICAM) is a portable IMS device. The M8A1 is another IMS-based automatic CW alarm detector used during the 1991 Gulf war and it is replaced with the Automatic Chemical Agent Alarm (ACADA), which is a high-resolution IMS. ACADA can detect agents in a few seconds and can clean itself for fresh detection within 1 min. The other variants of IMS are RAID (Bruker's) and M-90 (Environics).
Handheld CW agent detectors, viz. AP2C (France) or CHASE (Israel) based on the flame photometry principle, is also frequently used. It can detect chemiluminescent reactions of sulfur or phosphorus-containing organic compounds in a hydrogen/air flame.
Surface Acoustic Wave Sensors (SAWS) work in the same manner as do Quartz Crystal Microbalances (QCMs), but at higher frequencies and greater sensitivities. The Joint Chemical Agent Detector (JCAD) employs an SAW-based technology. The JCAD is a handheld, lightweight CW detector and it combines with an electrochemical cell.
Portable gas chromatograph is used for automatic real-time continuous agent monitoring. In this, air is used as carrier gas and air sample is drawn through a pre-concentrator loop filled with an adsorbent material. A photoionization detector (PID) is used to detect the agents. The entire cycle from sample collection to detection takes about 5–10 min.
Gas chromatograph coupled with mass detector (GCMS) fitted on a vehicle can be used for unambiguous detection of most of the organic compounds, including CW agents, at very low concentrations.[81] However, this hyphenated technique is complex, requires a skilled operator, is difficult to maintain and does not provide much advantage in comparison with off-site analysis, especially when chemical incident occurs at remote site when the mobile lab cannot approach easily. The commercially available portable GCMS-based instruments include HAPSITE (Inficon's), EM series (Bruker's), MM1 or MM2. No suitable onsite detection equipment or methods for the detection of psychomimetic agents are available except GCMS.
In addition to the above electrochemical-based detectors for nerve agents (NAD), standoff detection instruments (M21, RAPID) are also available. The M21 remote sensing chemical agent alarm (RSCAAL) is based on a passive infrared detector and is capable of detecting nerve and blister agents in the vapor phase from a distance of up to 5,000 m.[78] Instruments based on other techniques, such as molecularly imprinted polymer (MIP) sensors, biosensors, surface plasmon resonance (SPR), conductive polymer sensors, etc. are under development stage.
All the detection methods have susceptibility to interferences or false-positive results. The best way is to use two types of detectors working on different principles to obtain accurate data.[82]
Protection
The main twin aspects of physical protection[83–87] are the creation of an artificial barrier between the toxic agent and the individuals and the provision of “breathable air.” The barrier has to provide protection against liquids, aerosols or gases. The breathable air is accomplished by two ways: directly connected to a source (oxygen or pure air cylinder) or detoxification of contaminated air by passing through a canister. However, the use of a respirator can cause physiological discomforts such as breathing resistance, heat stress and vision and communication problems, resulting in the loss of efficiency.
The canister contains a “high-efficiency particulate aerosol (HEPA) filter media” for the aerosol retention and the gaseous contaminants from air are removed by the impregnated carbon through adsorption, catalytic decomposition and chemisorption processes. Thus, full-face protective masks provide good protection for both the respiratory tract and the eyes. The face mask should have a good field of vision and speech transmission preferably with a microphone. These respiratory protective masks in different sizes are required to suit different face sizes.
In case of body protection, liquid-impermeable full protective gears should be used for highly contaminated areas whereas toxic vapor-impermeable/air-permeable protective suits can be used. The impermeable suit is made up of nylon fabric coated outside with neoprene and another side with butyl rubber. The permeable protective clothing consists of three layers: the upper layer is oil, water and fire retardant nylon fabric, the middle layer is a carbon-coated non-woven fabric and the third layer is a simple cotton fabric layer. The protection gloves consist of an outer butyl rubber glove for chemical protection and an inner cotton glove for the absorption of perspiration water. Similar to gloves, overboots made up of butyl rubber are worn over the already used shoes to protect the footwear against contamination. The term “Personal Protective Equipment” (PPE) is used to denote complete protective gears i.e. face mask, suits, gloves and boots.
Collective protection to a group of people in vehicles or shelters is provided by providing purified air by filtration of contaminated air through large filters. The advantage of this is that individuals inside the protected area are not required to wear masks separately.
There are four levels of personal protection for dealing with hazardous substances. These are described as levels A, B, C and D for chemical protective clothing in combination with different types of respiratory protection.[86] Level A protection should be worn when the highest level of respiratory, skin, eye and mucous membrane protection is needed. This protection consists of a fully encapsulated, vapor-tight, chemical-resistant suit, boots and gloves together with a self-contained breathing apparatus (SCBA). Level B protection should be selected when the highest level of respiratory protection is needed, but a lesser degree of skin and eye protection is required. This equipment consists of a chemical-resistant suit, chemical-resistant boots and gloves and SCBA. Level C protection should be selected when the types of airborne substance are known, concentration is measured, criteria for using air-purifying respirators are met and skin or eye exposures are unlikely. This PPE consists of a chemical-resistant suit, full-face mask with air-purifying canister-equipped respirator and chemical-resistant boots and gloves. Level D protection provides no respiratory protection and minimal skin protection and should not be worn on any site when respiratory or skin hazards exist.
A recent review brings out NBC protective clothing, their types, various clothing materials used, their evaluation procedures, washing methodologies and decontamination methods as well as the futuristic trends, such as detection and decontamination-integrated NBC protective clothing based on electrospun nanofiber technology and molecularly imprinted polymer technology.[87]
Decontamination
Decontamination[88–102] is the conversion of toxic chemicals into harmless products either by destruction or by detoxification. Decontamination is a complex process involving processes involving highly toxic chemicals with diverse properties, adverse conditions and a wide variety of subjects to be treated, e.g. human skin and eyes, equipment with metal/wooden/plastic surface, field, etc. In terms of CW agents, decontamination can be defined as reduction or removal of chemical agents. This may be accomplished by physically removing the agents or by chemically neutralizing them. Decontamination is most effective when conducted within 1 min of exposure, but this is practically rarely possible.[89]
Physical decontamination is easy and acts universally, but is not as effective as chemical decontamination. It involves mainly adsorption of the toxic chemical on adsorbents such as Fuller's earth, kaolin, talc, activated carbon, etc., or removal of agents by spraying simple water or soap water under pressure. In emergency situations, the adsorbent may even be flour, saw dust or soil. There are many dry formulations containing Fuller's earth, such as PDK-1, PDK-2, M-291 kit that are available for decontamination of skin and equipment. The main disadvantage of the physical method is that the adsorbent or water used for decontamination should be detoxified later and disposed off carefully. Water spray should not be used in case of head or skin contaminated with mustards as it will spread to other body surfaces.
Chemical decontamination converts the toxic CW agents into innocuous products that can be handled safely. The chemical reactions that are generally applied in chemical decontamination methods are either nucleophilic reactions or oxidations.[97] A commonly used skin decontaminant is the 0.5% hypochlorite solution or household bleach. Alkali (sodium or potassium hydroxide) dissolved in methanol or ethanol detoxifies most of the CW agents, but should not be used in decontaminating skin other than in extreme emergencies. Chloramines are effective against mustards and V-agents but are ineffective against G-agents. Sodium carbonate solution renders G-type nerve agents harmless, but with V-agents, it produces a toxic product.
Many ready-to-use effective chemical formulations have been developed and are commercially available. For example, DS2 is a universal decontamination solution for equipment and field use. This is investigated in depth and is available under different names. It is a composition of sodium hydroxide (2%), ethylene glycol monomethylether (28%) and diethylenetriamine (70%). The active ingredient in this formulation is ethylene glycol monomethylether, which acts as a nucleophile and hydrolyses the CW agents into non-toxic products. It is a clear, amber-colored solution and is used in the portable decontamination apparatus. DS2 reacts with GB and HD to effectively reduce their hazards within 5 min. Within 30 min contact time, DS2 neutralizes all known toxic chemical agents. It can damage paints as well as plastics, rubber and leather materials.
The American decontamination kit called M258A1 and M280 contain the active ingredients ethanol (72%), phenol (10%), NaOH (5%), ammonia (0.2%) and water (12%). Similarly, the German emulsion C8 is composed of tetrachloroethylene (15%), water (76%), anionic surfactant (1%) and calcium hypochlorite (8%). It is non-corrosive and can penetrate into paint to dissolve and react with the imbedded agent without damaging the paint.
The microemulsion-based (multi-purpose chemical, biological decontaminant) MCBD system contains water (60%), tetrachloroethylene (7%), n-cetyl trimethyl ammonium chloride (28%) and traces of cosurfactant, (n-Bu)4 NOH. To this microemulsion, Fichlor (4%), sodium 2-nitro-4-iodoxybenzoate (0.1%) and sodium borate are added for reactions with the agents. The MCBD system was designed to be superior to the C8 system because it is a more stable emulsion at a lower pH of 10, contains less tetrachloroethylene and is partially catalytic.
The variables that determine the effectiveness of decontamination are contamination time, temperature, contamination density and decontamination medium, nature of agent and nature of decontaminants. The requirements of an ideal decontaminant or decontamination formulation are rapid in action, efficiency, harmless to humans, non-corrosive, stability in long-term storage and washable with water. There is a great deal of recent discussion and re-evaluation of decontamination agents and strategies and, therefore, careful consideration of the most current recommendation is important.[103] Decontamination must be performed on all victims and responders before they cross into the clean area.
CWC
On April 29, 1997, the world's first multilateral disarmament Treaty “Chemical Weapons Convention” entered into force.[104] The CWC text has a preamble, 24 articles and three annexes, namely an annex on chemicals, an annex on verification and a confidentiality annex. The main objective of the CWC is to prohibit development, production, acquisition, retention, stockpiling transfer and use of chemical weapons. It mandated the Organisation for the Prohibition of Chemical Weapons (OPCW), with its headquarters at The Hague, the Netherlands, to eliminate the scourge of chemical weapons forever. It requires every country signatory to this Convention to destroy existing CW stockpiles and chemical weapons production facilities under its jurisdiction. The most unique feature of the Treaty is its comprehensiveness and a strict inspection and verification regime. In addition, each State Party undertakes to provide protection and assistance through the Organisation if chemical weapons have been used against a State Party or if a State Party is threatened by such weapons. The CWC also provides for international cooperation among States Parties in the pursuit of chemistry for peaceful purposes. As on date, 188 countries are signatories to the convention. OPCW inspectors also verify the consistency of industrial chemical declarations and, together with State Parties, monitor the non-diversion of chemicals for activities prohibited under this convention. It has been estimated that more than 100,000 compounds are controlled under the CWC, although, in practical terms, the actual number of CW agents, precursors and degradation products that are contained in the OPCW database is in thousands. The OPCW Central Analytical Database is a compilation maintained by the OPCW laboratory, and it contains analytical data of the chemicals that fall under the CWC.
Indian Scenario
Preparing the nation to address the dangers from the chemical disaster is a major challenge. The National Disaster Management Authority, Government of India, has formulated guidelines for mitigation and response to various types of chemical disasters.[103,105,106] The Defence Research and Development Organisation (DRDO) has made useful contributions by developing several products using indigenous technologies for defence against the CW threat. Some of the products are listed below:
-
i)
TCD paper to detect nerve/blister agents
-
ii)
RVD kit to detect all CW agents
-
iii)
WPDK kit
-
iv)
portable gas chromatograph (PGC) to detect CW agents
-
v)
NAD
-
vi)
FPD-based handheld CW agent sensor
-
vii)
mobile reconnaissance laboratory
-
viii)
NBC reconnaissance vehicle
-
ix)
reusable autoinjectors (for nerve agents poisoning)
-
x)
first aid kit for CW (Types A and B)
-
xi)
NBC canister (for physical protection against CBW)
-
xii)
NBC filters for collective protection (type: FAT-100M, FAT-200M, FAS-200M, FAS RV-200M, FAS-400M and FAS-850 M)
-
xiii)
integrated field shelter
-
xiv)
personal decontamination kits (PDK-1, 2 and 3)
-
xv)
decontamination solution (DS2)
-
xvi)
portable decontamination apparatus (for spraying DS2)
-
xvii)
α-KG: an oral antidote to cyanide poisoning
-
xviii)
DRDE-07: the first and only antidote for sulfur mustard
In addition to the R & D work, DRDO has also been engaged in conducting various NBC defences training programmes to the Army, Navy, Air force, Paramilitary Forces (CISF, ITBP, CRPF, and BSF), forensic scientists and civil authorities.
Conclusion
Individual training and regular mock drill exercises in planning, detection, protection and decontamination are necessary to face any chemical attack situation. Area monitoring should be carried out to check the presence of any CW agents before any event starts. The nearest hospitals should be identified and necessary arrangements (decontamination facility, antidotes stock, isolated wards, protective gears, NBC-trained staff) have to be made to deal with any emergency.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Aas P. The threat of mid-spectrum chemical warfare agents. Prehosp Disaster Med. 2003;18:306–12. doi: 10.1017/s1049023x00001254. [DOI] [PubMed] [Google Scholar]
- 2.Convention on the Prohibition of the Development, Production, Stockpiling and use of Chemical Weapons and Destruction, Technical Secretariat of Organization for Prohibition of Chemical Weapons, The Hague, accessible through internet. 1997. Available from: http://www.opcw.org .
- 3.Smart JK. History of Chemical and Biological Warfare: An American Perspective. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare. Washington, DC: Office of the Surgeon General; 1997. p. 15. [Google Scholar]
- 4.Riley B. The toxicology and treatment of injuries from chemical warfare agents. Curr Anaesth Crit Care. 2003;14:149. [Google Scholar]
- 5.Okumura T, Suzuki K, Fukuda A. The Tokyo subwy sarin attack: Disaster management, Part 2: Hospital response. Acad Emerg Med. 1998;5:618–24. doi: 10.1111/j.1553-2712.1998.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 6.Stock T, Haug M, Radler P. In: Stockholm. International Peace Research Institute Yearbook 1996: Armaments,disarmament and International security. London: Oxford University Press; 1996. Chemical and biological weapon developments and arms control; p. 661. [Google Scholar]
- 7.Schwenk M, Kluge S, Jaroni H. Toxicological aspects of preparedness and aftercare for chemical-incidents. Toxicology. 2005;214:232–48. doi: 10.1016/j.tox.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Makarovsky I, Markel G, Hoffman A, Schein O, Finkelstien A, Nissimov TB, et al. Osmium Tetroxide: A New Kind of Weapon. Isr Med Assoc J. 2007;9:750–2. [PubMed] [Google Scholar]
- 9.Small L. Toxic industrial chemicals: A future weapons of mass destruction threat. US Government Reports Announcements and Index. 2002 [Google Scholar]
- 10.Tu AT. Overview of sarin terrorist attacks on Japan. Am Chem Soc Symp Ser. 2000;745:304. [Google Scholar]
- 11.Patocka J, Fusek J. Chemical agents and chemical terrorism. Cent Eur J Public Health. 2004;12:S75–7. [PubMed] [Google Scholar]
- 12.Volans GN, Karalliedde L. Long term effects of chemical weapons. Lancet. 2002;360:S35–6. doi: 10.1016/s0140-6736(02)11813-7. [DOI] [PubMed] [Google Scholar]
- 13.Heymann WR. Threats of biological and chemical warfare on civilian populations. J Am Acad Dermatol. 2004;51:452–3. doi: 10.1016/j.jaad.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Prentiss AM. Chemicals in warfare. New York: McGraw-Hill Book Company; 1937. p. 579. [Google Scholar]
- 15.López-Muñoz F, Alamo C, Guerra JA, García-García P. The development of neurotoxic agents as chemical weapons during the National Socialist period in Germany. Rev Neurol. 2008;47:99–106. [PubMed] [Google Scholar]
- 16.Stuart JA, Ursano RJ, Fullerton CS, Norwood AE, Murray K. Belief in exposure to terrorist agents: Reported exposure to nerve or mustard gas by Gulf War veterans. J Nerv Ment Dis. 2003;191:431–6. doi: 10.1097/01.NMD.0000081634.28356.6B. [DOI] [PubMed] [Google Scholar]
- 17.Hoenig SL. Compendium of chemical warfare agents. New York, USA: Springer Science; 2007. [Google Scholar]
- 18.Sidell FR, Borak J. Chemical warfare agents: II. Nerve agents. Ann Emerg Med. 1992;21:865–71. doi: 10.1016/s0196-0644(05)81036-4. [DOI] [PubMed] [Google Scholar]
- 19.Somani SM. Chemical Warfare Agents. USA: Academic Press Inc; 1992. p. 67. [Google Scholar]
- 20.Bajgar J, Fusek J, Kassa J, Kuca K, Jun D. Chemical aspects of pharmacological prophylaxis against nerve agent poisoning. Curr Med Chem. 2009;16:2977–86. doi: 10.2174/092986709788803088. [DOI] [PubMed] [Google Scholar]
- 21.Bajgar J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 22.Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica (Hradec Kralove) 2005;48:3–21. [PubMed] [Google Scholar]
- 23.Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology. 2007;233:145–54. doi: 10.1016/j.tox.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 24.Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25:297–323. doi: 10.2165/00139709-200625040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Jokanović M. Medical treatment of acute poisoning with organophosphorus and carbamate pesticides. Toxicol Lett. 2009;190:107. doi: 10.1016/j.toxlet.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Niven AS, Roop SA. Inhalational exposure to nerve agents Respir. Care Clin N Am. 2004;10:59–74. doi: 10.1016/S1078-5337(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra RC, Gansean K, Sugendran K, Swamy RV. Chemistry and toxicology of bis(2-chloroethyl) sulphide (sulfur mustard) - a review. Def Sci J. 1999;49:97. [Google Scholar]
- 28.Medema J. Mustard gas: The science of H. NBC Defense Technol Int. 1986:66. [Google Scholar]
- 29.Borak J, Sidell FR. Agents of chemical warfare: Sulfur mustard. Ann Emerg Med. 1992;21:303–8. doi: 10.1016/s0196-0644(05)80892-3. [DOI] [PubMed] [Google Scholar]
- 30.Geraci MJ. Mustard gas: Imminent danger or eminent threat? AnnPharmacother. 2008;42:237–46. doi: 10.1345/aph.1K445. [DOI] [PubMed] [Google Scholar]
- 31.Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacol Rev. 1996;48:289–326. [PubMed] [Google Scholar]
- 32.Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Davis KG, Aspera G. Exposure to liquid sulfur mustard. Ann Emerg Med. 2001;37:653–6. doi: 10.1067/mem.2001.114322. [DOI] [PubMed] [Google Scholar]
- 36.Smith WJ. Therapeutic options to treat sulfur mustard poisoning--the road ahead. Toxicology. 2009;263:70–3. doi: 10.1016/j.tox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Kehe K, Thiermann H, Balszuweit F, Eyer F, Steinritz D, Zilker T. Acute effects of sulfur mustard injury–Munich experiences. Toxicology. 2009;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 38.Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical defense against mustard gas: Toxic mechanisms and pharmacological implications. Boca Raton, Fla: CRC Press; 1991. [Google Scholar]
- 39.Aposhian HV, Mershon MM, Brinkley FB, Hsu CA, Hackley BE. Anti-lewisite activity of meso-dimercaptosuccinic acid, 2,3-dimercapto-1-propanesulphonic acid. Life Sci. 1982;31:2149. doi: 10.1016/0024-3205(82)90108-4. [DOI] [PubMed] [Google Scholar]
- 40.Boyd VL, Harbell JW, O’Connor RJ, McGown EL. 2,3-Dithioerythritol, a possible new arsenic antidote. Chem ResToxicol. 1989;2:301–6. doi: 10.1021/tx00011a006. [DOI] [PubMed] [Google Scholar]
- 41.Sidell FR, Takafuji ET, David RF, editors. (Textbook of Military Medicine Series, Part 1. Warfare, Weaponry, and the Casualty) Washington, DC: Office of the Surgeon General, Department of the Army; 1997. Medical Aspects of Chemical and Biological Warfare. [Google Scholar]
- 42.Bartlett JG, Sifton DW, Kelly GL, editors. PDR guide to biological and chemical warfare response. 1st ed. Montvale, NJ: Thompson Healthcare Publications; 2002. pp. 1–404. [Google Scholar]
- 43.Marrs TC, Robert LM, Frederick RS. Chemical warfare agents: Toxicology and treatment. Chichester, England: John Wiley and Sons; 1997. [Google Scholar]
- 44.Bhattacharya R. Antidotes to cyanide poisoning: Present status. Indian J Pharmacol. 2000;32:94. [Google Scholar]
- 45.Raza SK, Jaiswal DK. Mechanism of cyanide toxicity and efficacy of its antidotes. Def Sci J. 1994;44:331. [Google Scholar]
- 46.Borowitz JL, Isom GE, Baskin SI. Chemical warfare agents: Toxicity at low levels. Boca Raton: CRC Press; 2001. p. 305. [Google Scholar]
- 47.Cummings TF. The treatment of cyanide poisoning Occupational Med. 2004;54:82–5. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 48.James AF. Compton, Military chemical and biological agents. Cadwell, New Jersey: The Telford press; 1987. p. 111. [Google Scholar]
- 49.Croddy E, Armendariz CP, Hart J. Chemical and biological warfare. New York: Springer-Verlag; 2002. p. 93. [Google Scholar]
- 50.Lohs K. Synthetic poisons. 2nd ed. East Berlin: Deutscher Militarverlag; 1963. p. 51. [Google Scholar]
- 51.Buckling S. Medical manual of chemical warfare. Brooklyn, New York: Chemical Publishing Co; 1943. [Google Scholar]
- 52.Cucinell SA. Review of the toxicity of long-term phosgene exposure. Arch Environ Health. 1974;28:272–5. doi: 10.1080/00039896.1974.10666485. [DOI] [PubMed] [Google Scholar]
- 53.Glass WI, Harries WE, Witlock RM. Phosgene poisoning: A case report. N Z Med J. 1971;74:386–9. [PubMed] [Google Scholar]
- 54.Regan RA. Review of clinical experience in handling phosgene exposure cases. Toxicol Ind Health. 1985;1:69–72. doi: 10.1177/074823378500100207. [DOI] [PubMed] [Google Scholar]
- 55.Diller WF. Therapeutic strategy in phosgene poisoning. Toxicol Ind Health. 1985;1:93–9. doi: 10.1177/074823378500100210. [DOI] [PubMed] [Google Scholar]
- 56.Diller WF, Zante R. A literature review: Therapy for phosgene poisoning. Toxicol Ind Health. 1985;1:117–28. doi: 10.1177/074823378500100212. [DOI] [PubMed] [Google Scholar]
- 57.Beswick FW. Chemical agents used in riot control and warfare. Hum Toxicol. 1983;2:247–56. doi: 10.1177/096032718300200213. [DOI] [PubMed] [Google Scholar]
- 58.Ballantyne B. In: Riot control agents (Biomedical and health aspects of the use of chemicals in civil disturbances) Scott RB, Frazer J, editors. Bristol: Wright and Sons; 1977. p. 7. [Google Scholar]
- 59.Sanford JP. Medical aspects of riot control (harassing) agents. Annu Rev Med. 1976;27:412–9. doi: 10.1146/annurev.me.27.020176.002225. [DOI] [PubMed] [Google Scholar]
- 60.Rengstorff RH. Tear gas and riot control agents: A review of eye effects. Optometr Week. 1969;60:25. [Google Scholar]
- 61.Olajos EJ, Salem H. Riot Control Agents: Pharmacology, toxicology, biochemistry and chemistry. J Appl Toxicol. 2001;21:355–91. doi: 10.1002/jat.767. [DOI] [PubMed] [Google Scholar]
- 62.Gordon M. In Psychopharmacological Agents Medicinal Chemistry. New York: Academic Press; 1964. p. 1. [Google Scholar]
- 63.Hoffer A, Osmond H. The Hallucinogens. New York: Academic Press; 1967. [Google Scholar]
- 64.Shulgin AT, Ivsersen LL, Snyder SH, editors. Handbook of Psychopharmacology. Vol. 11. New York: Plenum Press; 1978. pp. 243–333. [Google Scholar]
- 65.Kinston W, Rosser R. Disaster: Effects on mental and physical state. J Psychosom Res. 1974;18:437–56. doi: 10.1016/0022-3999(74)90035-x. [DOI] [PubMed] [Google Scholar]
- 66.Hofmann A, Tscherter H. Isolation of lysergic acid alkaloids from the Mexican drug ololiuqui (Rivea corymbosa (L.) Hall.f.) Experientia. 1960;16:414. doi: 10.1007/BF02178840. [DOI] [PubMed] [Google Scholar]
- 67.Hofmann A. Teonanácatl and Ololiuqui, two ancient magic drugs of Mexico. Bull. Narcotics. 1971;23:3–14. [Google Scholar]
- 68.Madsen JM. Toxins as weapons of mass destruction.A comparison and contrast with biological-warfare and chemical-warfare agents. Clin Lab Med. 2001;21:593–605. [PubMed] [Google Scholar]
- 69.Bigalke H, Rummel A. Medical aspects of toxin weapons. Toxicol. 2005;214:210–20. doi: 10.1016/j.tox.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 70.Tucker J. Dilemmas of a dual-use technology: Toxins in medicine and warfare. Politics and Life Sci. 1994:51. [Google Scholar]
- 71.Gill DM. Bacterial toxins: A table of lethal amounts. Microbiol Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. Botulinum toxin as a biological weapon: Medical and public health management. J Amer Med Assoc. 2001;285:1059–70. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 73.Albin RL. Basal ganglia neurotoxins. Neurol Clin. 2000;18:665–80. doi: 10.1016/s0733-8619(05)70217-6. [DOI] [PubMed] [Google Scholar]
- 74.Pearson G. The UNSCOM saga: Chemical and biological weapons non-proliferation. London: MacMillan Press Ltd; 1999. [Google Scholar]
- 75.Martin CO, Adams HP., Jr Neurological aspects of biological and chemical terrorism: A review for neurologists. Arxh Neurol. 2003;60:21–5. doi: 10.1001/archneur.60.1.21. [DOI] [PubMed] [Google Scholar]
- 76.Murray GM, Southard GE. Sensors for chemical weapons detection IEEE Instrumentaion and Measurement magazine 2002.p. 2002:12–21. [Google Scholar]
- 77.Gripstad B. FOA briefing book on Chemical Warfare Agents. Stockholm, Sweden: Swedish National Defence Research Establishment; 1992. [Google Scholar]
- 78.Murray GM, Lawrence DS. In: Chemical Weapon Convention Chemical Analysis. Mesikaakso M, editor. Chichester: John Wiley and sons Ltd; 2005. pp. 65–88. [Google Scholar]
- 79.Baumbach JI, Eiceman GA. Ion mobility spectrometry: Arriving on site and moving beyond a low profile. Appl Spectrosc. 1999;53:338A–55. doi: 10.1366/0003702991947847. [DOI] [PubMed] [Google Scholar]
- 80.Collins DC, Lee ML. Developments in ion mobility spectrometry-mass spectrometry. Anal Bioanal Chem. 2002;372:66–73. doi: 10.1007/s00216-001-1195-5. [DOI] [PubMed] [Google Scholar]
- 81.Makas AL, Troshkov ML. Field gas chromatography-mass spectrometry for fast analysis. J Chromatogr B Anal Technol Biomed Life Sci. 2004;800:55–61. doi: 10.1016/j.jchromb.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y, Ong KY. Detection Technologies for Chemical Warfare Agents and Toxic Vapors. Boca Raton FL: CRC Press; 2005. [Google Scholar]
- 83.Musa SR, Banderet LE, Cadarette B. In Chapter 36: Protective uniforms for nuclear, biological, and chemical warfare: Metabolic, thermal, respiratory and psychological issues. In: Pandolf KB, Burr RE, editors. Textbook of Military Medicine: Medical Aspects of Harsh Environments. Vol. 2. United States of America Falls Church, Virginia VA: Office of the Surgeon General Department of the Army; 2002. pp. 1084–27. [Google Scholar]
- 84.Ellison DH. Handbook of Chemical and Biological Warfare Agents. Boca Raton, FL: CRC Press; 2000. p. 472. [Google Scholar]
- 85.Gander TJ, editor. Janes NBC protection equipment hand book. 10th ed. New York, USA: Janes Information Group; 1997. p. 280. [Google Scholar]
- 86.Chan JT, Yeung RS, Tang SY. Hospital preparedness for chemical and biological incidents in Hong Kong. Hong Kong Med J. 2002;8:440–6. [PubMed] [Google Scholar]
- 87.Boopathi M, Singh B, Vijayaraghavan R. A review on NBC body protective clothing. Open Textile J. 2008;1:1. [Google Scholar]
- 88.Seto Y. Decontamination of chemical and biological warfare agents. Yakugaku Zasshi. 2009;129:53–69. doi: 10.1248/yakushi.129.53. [DOI] [PubMed] [Google Scholar]
- 89.Sidell FR. Management of chemical casualties: A handbook for emergency medical services. Bel Air, MD: HB Publishing; 1995. [Google Scholar]
- 90.Cox RD. Decontamination and management of hazardous materials exposure victims in the emergency department. Ann Emerg Med. 1994;23:761–70. doi: 10.1016/s0196-0644(94)70312-4. [DOI] [PubMed] [Google Scholar]
- 91.Levitin HW, Siegelson HJ, Dickinson S, Halpern P, Haraguchi Y, Nocera A, et al. Decontamination of mass casualties: Re-evaluating existing dogma. Prehospital Disast Med. 2003;8:200–7. doi: 10.1017/s1049023x00001060. [DOI] [PubMed] [Google Scholar]
- 92.Yang YC, Baker JA, Ward JR. Decontamination of chemical warfare agents. Chem Rev. 1992;92:1729–43. [Google Scholar]
- 93.Devereaux A, Amundson DE, Parrish JS, Lazarus AA. Vesicants and nerve agents in chemical warfare: Decontamination and treatment strategies for a changed world. Postgrad Med. 2002;112:90–6. doi: 10.3810/pgm.2002.10.1334. [DOI] [PubMed] [Google Scholar]
- 94.Lazarus AA, Devereaux A. Potential agents of chemical warfare: Worst-case scenario protection and decontamination methods. Postgrad Med. 2002;112:133–40. doi: 10.3810/pgm.2002.11.1350. [DOI] [PubMed] [Google Scholar]
- 95.Raber E, McGuire R. Oxidative decontamination of chemical and biological warfare agents using L-Gel. J Hazard Mater. 2002;93:339–52. doi: 10.1016/s0304-3894(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 96.Amitai G, Murata H, Andersen JD, Koepsel RR, Russell AJ. Decontamination of chemical and biological warfare agents with a single multi-functional material. Biomaterials. 2010;31:4417–25. doi: 10.1016/j.biomaterials.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Wagner GW, Yang YC. Rapid nucleophilic/oxidative decontamination of chemical warfare agents. Ind Eng Chem Res. 2002;41:1925–8. [Google Scholar]
- 98.Venske BN, editor. First Responder Chem-Bio Handbook. Alexandria, Virginia: Tempest Publishing; 1998. [Google Scholar]
- 99.Clarke SF, Chilcott RP, Wilson JC, Kamanyire R, Baker DJ, Hallett A. Decontamination of multiple casualties who are chemically contaminated: A challenge for acute hospitals. Prehosp Disaster Med. 2008;23:175–81. doi: 10.1017/s1049023x00005811. [DOI] [PubMed] [Google Scholar]
- 100.Trapp R. The detoxification and natural degradation of chemical warfare agents. Stockholm: International Peace Research Institute; 1985. [Google Scholar]
- 101.Noll GG, Hildebrand MS, Yvorra JG. Hazardous Materials: Managing the Incident. 2nd ed. Oklahoma State University, Stillwater, OK: Fire Protection Publications; 1995. [Google Scholar]
- 102.Benz KG. NBC Defense-an overview, Part 2: Detection and Decontamination. Internat Def Rev. 1984:159–64. [Google Scholar]
- 103.National Disaster Management Guidelines: Management of Chemical (Terrorism) Disaster. National Disaster Management Authority, Government of India. 2009. Jun, Available from: http://www.ndma.gov.in .
- 104.OPCW: Report on the implementation of the convention on the prohibition of the development, production, stockpiling and use of chemical weapons and on their destruction. http://www.opcw.nl .
- 105.National Disaster Management Guidelines: Chemical Disaster. National Disaster Management Authority, Government of India. 2007. Apr, Available from: http://www.ndma.gov.in .
- 106.National Disaster Management Guidelines: Medical preparedness and mass casualty management. National Disaster Management Authority, Government of India. 2007. Oct, Available from: http”//www.ndma.gov.in .


