Abstract
The recent bioterrorist attacks using anthrax spores have emphasized the need to detect and decontaminate critical facilities in the shortest possible time. There has been a remarkable progress in the detection, protection and decontamination of biological warfare agents as many instrumentation platforms and detection methodologies are developed and commissioned. Even then the threat of biological warfare agents and their use in bioterrorist attacks still remain a leading cause of global concern. Furthermore in the past decade there have been threats due to the emerging new diseases and also the re-emergence of old diseases and development of antimicrobial resistance and spread to new geographical regions. The preparedness against these agents need complete knowledge about the disease, better research and training facilities, diagnostic facilities and improved public health system. This review on the biological warfare agents will provide information on the biological warfare agents, their mode of transmission and spread and also the detection systems available to detect them. In addition the current information on the availability of commercially available and developing technologies against biological warfare agents has also been discussed. The risk that arise due to the use of these agents in warfare or bioterrorism related scenario can be mitigated with the availability of improved detection technologies.
Keywords: Anthrax, biological warfare agents, botulism, detection of BW agents, plague
Several bacterial, viral agents and toxins can pose public health risk in the event of a bioterrorist or biological warfare attack.[1] These agents, if used in any such attacks, can pose a difficult public health challenge and can cause large number of causalities and will be difficult to contain. The alleged used of biological agents is a serious problem and the risk of using these agents in a bioterrorist attack is increasing. In the ancient history, the well-known attempt of use of biological warfare agent was during the 14th century medieval siege of Kaffa, Feodosiya, Ukraine.[2] In this incident, the Tartars (Mongols) who attacked Kaffa, tossed dead and dying plague victims into the city in an attempt to spread the disease. In another well-documented incident at Fort Pitt, Ohio River Valley, the British troops deliberately spread smallpox among native Indian population by presenting them with blankets and linens used by smallpox victims.[3] There is reference in history of attempts to spread smallpox via infected British soldiers during the American Revolutionary War (1776–1781) and by contaminated clothing during the American Civil War (1980–1864). The importance of biological weapons was significantly advanced in the present century due to several wars and multiple threats. The accidental release of anthrax from a military testing facility in the former Soviet Union in 1979 and possession of anthrax, botulinum toxin and aflatoxin in Iraq in 1995 point out to research and development of these agents in spite of the 1972 Biological Weapons Convention. The factors that are important for the use of biological warfare agents in a bioterrorism related incident are the accessibility to the agent, scientific expertise to handle and mass produce them and deliver in the proper size for dissemination as aerosol.[4] In Dalles, Oregon, USA, the rajhneeshees attempted to influence local elections by contaminating salad bars with Salmonella typhimurium, the bacteria that can cause food poisoning.
Biological Warfare agents are microorganisms like virus, bacteria, fungi, protozoa or toxins produced by them, that give rise to diseases in man, animals or plants, when deliberately dispersed in an area [Table 1]. These agents can cause large-scale mortality, morbidity and can incapacitate a large number of people in the shortest possible time and have adverse effects on human health. The use of biological warfare (BW) agents can be covert or overt and they differ from conventional weapons by way of several unique properties. The effects of these agents are not instantaneous and require few hours to weeks before the symptoms appear in the affected population. These attacks require a release of small quantity of viable material and are capable of self-replication and can cause a disease outbreak in an area. Viruses are capable of replication only inside a living cell and are pathogenic to man, animals and plants. They consist of proteins and nucleic acids (DNA and RNA) and multiply and spread much faster. Bacteria are single-celled prokaryotic organisms and with a definite cell wall. Fungi are unicellular or multicellular, eukaryotic organisms and have no chlorophyll. Several fungal species are known to cause diseases in plants and few of them in humans as well. Toxins are secondary metabolites produced by bacteria, fungi, algae, plants, fishes, crustaceans and molluscs and are known to act in very low concentrations and can affect the functioning of cells [Table 2].
Table 1.
Agents that can be used as biological warfare or bioterrorism-related incidents
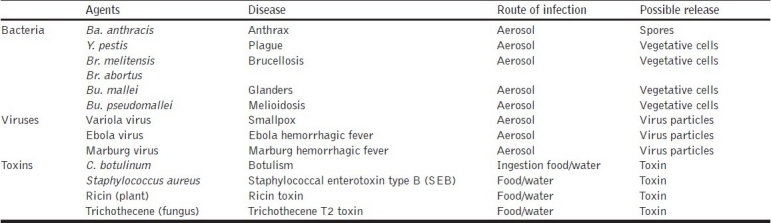
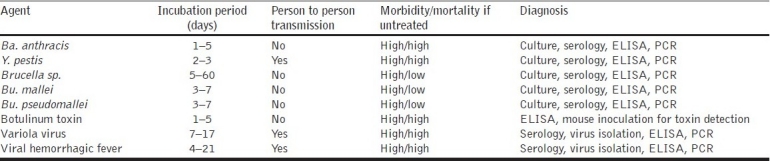
Table 2.
Summary of characteristics of selected biological warfare agents
Anthrax
Anthrax is a disease of humans and animals, caused by the bacterium Bacillus anthracis, a gram-negative, facultative, anaerobic, nonmotile and spore forming bacterium. It derives its name from the Greek word for coal “anthrakis” because it causes black coal like skin lesions. This disease has been known since antiquity and affects humans who tend animals or come in contact with animals or their infected products. The bacterium often penetrates the body via wounds in the skin and may also infect humans as aerosol or ingestion. The ability of sporulation and resistance of the spores to harsh environmental conditions like heat and humidity, disinfectants and UV radiation makes anthrax the most important biological warfare agent. It is reported that the spores can survive up to 40 years in water or soil. Spores are formed only in the presence of oxygen. Experiments involving detonation of bombs containing Ba. anthracis spores were conducted by US and its allies in the Gruinard Island during the period 1943. After the experiments, live spores were detected in the soil at various depths and took 40 years and tons of formaldehyde to disinfect the island and declare it free of anthrax spores.[5]
Anthrax is prevalent in many parts of the world, and historical accounts have documented outbreaks of the disease in livestock and humans. Herbivores can become infected with anthrax by grazing in pastures that are contaminated with spores. The animals develop bacteremia and contaminate the environment with vegetative cells, which can subsequently sporulate and form spores. When the animal dies due to anthrax, the animal carcasses become infectious, and when the animal decomposes, the spores can again mix with the soil and the transmission cycle continues. Human anthrax naturally occurs in three clinical forms: cutaneous anthrax when spores come in contact with the skin and develop into black lesions, this occurs mostly with the handling of infected animals; gastrointestinal anthrax that occurs by the consumption of infected animal products and undercooked/raw meat, respiratory anthrax that is caused by the inhalation of spores through respiration. The virulence factors of Ba. anthracis consist of toxin and an antiphagocytic capsular polypeptide containing D-glutamic acid.[6] The toxin is composed of three proteins: protective antigen (PA), edema factor (EF) and lethal factor (LF). In India, the incidence of the disease in animals is extremely low and few districts of Tamil Nadu, Andhra Pradesh and Karnataka report cases annually and are considered endemic to this disease. Sporadic cases from humans are also reported from this region. Anthrax in all forms can lead to septicemia and death if untreated; early treatment of cutaneous anthrax is usually cured but in all other forms the recovery depends on the early detection and treatment. The case fatality rates range from 25 to 75% for gastrointestinal anthrax and can reach up to 90–100% in case of inhalation anthrax. The natural source of infection for humans, in naturally acquired disease, is infected livestock and wild animals or contaminated animal products. Human to human transmission is extremely rare and reported only with cutaneous anthrax. Cutaneous anthrax is the most common manifestation of infection with Ba. anthracis. Inhalation anthrax occurs in persons working in certain occupations where spores may be released into the air from contaminated animal products, such as wool industry. The occupation high-risk groups include those who domesticate animals, workers who come in contact with livestock, e.g., veterinarians, animal handlers, abattoir workers and laboratory technicians who handle infected specimens. Anthrax can be treated in its earliest stages with penicillin (often delivered intravenously), tetracycline, ciprofloxacin or doxycycline. Evidence of antibiotic effectiveness comes from use in human and animals infected with Ba. anthracis. Antibiotics related to ciprofloxacin including chloramphenicol, erythromycin and some cephalosporins were also found to be effective. The antibiotic treatment is highly effective in early stage of the disease and becomes largely ineffective in later stages with severe complications. Attenuated spore based vaccine is available for use in animals. Recently, recombinant proteins, mainly the recombinant protective antigen, are used effectively to protect human anthrax and are widely available in many countries. The drawbacks with the recombinant protein based vaccines are short protective efficacy, need of yearly booster doses, side effects and poor tolerance in individuals.
Plague
Plague is an infectious disease of animals and humans caused by the bacteria, Yersinia pestis.[7] Y. pestis is a gram-negative, nonmotile, facultative anaerobe, on Wayson or Giemsa staining shows a bipolar or safety pin-like appearance. It is a zoonotic infection involving the rodents and humans. Millions of people in Europe, Asia and African continents died of plague in the earlier centuries due to flea infested rats inhabiting human homes and ports. Several port towns and cities were devastated by plague outbreak and epidemics used to spread across countries and continents. Today, the modern antibiotics and better sanitary conditions have effectively reduced the natural outbreaks of plague although small numbers of cases are reported from rural communities. World Health Organization (WHO) annually reports around 1000–3000 cases of plague from different countries around the world. In India, active plague foci are known to exist in Gujarat, Himachal Pradesh, Maharashtra, Karnataka and Tamil Nadu. Humans are infected from plague from the bite of rodent flea that carries the plague bacteria or by handling an infected animal. In the natural infection involving rodent flea bites, a person develops bubos or swelling of lymph nodes in groin and underarms. Further, after the appearance of bubos, the hematogenous spread of bacteria results in septicemia and infection of lungs and results in pneumonic plague. In a biological warfare or bioterrorist event, the plague bacilli are inhaled as aerosol and directly result in the pneumonic plague without the involvement of bubonic plague. The pneumonic plague is highly contagious and spreads from one individual to other by airborne droplets. The incubation period of conventional plague is 2–10 days, which may be considerably reduced to 8–12 hours in the case of pneumonic plague. The clinical sign of plague is a very painful, usually swollen and often hot to touch lymph node called a bubo. The bubos are accompanied by fever, extreme exhaustion and a history of possible exposure to rodents, rodent fleas, wild rabbits, sick or dead carnivores. Initial clinical manifestations include fever, headache, and general illness, followed by development of bubos. The disease progresses rapidly and the bacteria invade the blood stream, producing severe illness and further develop into overwhelming pneumonia with high fever, cough bloody sputum and chills. In untreated cases, the mortality rate can reach more than 50%.
Brucellosis
Brucellosis is one of the world's major zoonosis, caused by bacteria of the genus Brucella and can be transferred from animals to human. Brucellosis is a an important human disease in many parts of the world, especially in the Mediterranean countries of Europe, North and East Africa, Middle East, South and Central Asia, Central and South America. It is a serious public health problem in India as well as in other developing countries. In India, it is a neglected disease and closely associated with the agrarian society and mostly linked to the animal husbandry. The genus Brucella consists of eight species, designated on the basis of host preference, biochemical and molecular characteristics as follows: Brucella melitensis (goat and sheep), Brucella abortus (cattle), Brucella suis (pigs, reindeer), Brucella canis (dogs), Brucella ovis (sheep), Brucella neotomae (desert wood rats), Brucella pinnipediae (seal/otter) and Brucella cetaceae (porpoise/whale).[8] Br. pinnipediae and Br. cetaceae have been reported from marine mammals. Recently, one more species of Brucella, namely, Brucella mocroti has been isolated from common voles, Microtus arvalis.[9] The disease has been known by several terms, including Malta fever, undulant fever, Gibraltar fever, Bang's disease typhomalarial fever due to its resemblance to malaria and typhoid fevers.
The causative organisms are gram-negative, facultative intracellular bacteria in the shape of cocci, coccobacilli or short rods, 0.5–0.7 μm in diameter and 0.6–1.5 μm in length. It is a partially acid fast bacterium that lacks endospores or native plasmids. Optimal pH condition for growth of this bacterium is between 6.6 and 7.4. All Brucella strains lose viability when incubated at 56°C; however, a minimum of 1 hour incubation at a temperature over 70°C may be required to ensure complete inactivation. It is a systemic infection that can involve any organ or organ system of the body; symptoms are nonspecific, generally occurring within 2–3 weeks of infection. It affects people of all age groups and sex. The infection in humans usually manifests itself as an acute febrile illness which may persist and progress to a chronically incapacitating disease with severe complications. The transmission of Brucella infection and its prevalence in a region depends upon several factors like food habits, methods of processing milk and milk products, social customs, husbandry practices, climatic conditions, socioeconomic status and environment hygiene. The disease has been recognized as one of the most common laboratory acquired infections; it has been reported to occur in clinical research and production laboratories. Brucella spp. is also considered as important biothreat agent and is characterized as category B pathogens in the bacterial list of biological warfare agents. Its significance as a potential agent of bioterrorism was acknowledged early, and the pathogen remains on the category B bio-defence research lists of both Center for Disease Control and Prevention (CDC) and the National Institute of Allergy and Infectious Diseases (NIAID).[10]
Human brucellosis is a multi-system disease that may present with a broad spectrum of clinical manifestations and diagnosis requires microbiological confirmations by means of isolation from blood culture or demonstration of the presence of specific antibodies by serological tests. Blood culture provides definite proof of brucellosis but may not provide a positive result for all patients even under ideal conditions. Brucella is a slow growing organism and cultures are rarely positive and should be kept at least 45 days before the culture can be concluded negative. Serological tests are used in the diagnosis of brucellosis, which can be classified depending on the antigens used, as conventional tests (i.e., those using suspensions of whole cells as antigens) and tests using antigenic extracts (i.e., outer membrane proteins, cytoplasmic and periplasmic proteins). Outer membrane proteins (OMPs) are structural constituents of the cell and are unlikely to function as virulence factors. They induce immune responses useful in diagnosis[11] and in conferring protective immunity.
Glanders
Burkholderia mallei is the etiological agent of glanders, a disease of equines, i.e., horses, mules and donkeys. Bu. mallei is a nonmotile, aerobic gram-negative coccobacillus, which may or may not be oxidase positive and grow on Macconkey Agar. During World War I, the German military used this agent as a biological weapon against horses and mules that were primary forms of transport during the war.[2] The disease has been eradicated from most part of the developed world due to compulsory slaughter of infected or seropositive horses and mules. Reports on naturally acquired cases are mostly those of infections acquired from laboratory and many cases of laboratory acquired human glanders have been reported. Equines are the primary reservoirs of this rare illness and natural infection has been reported from eastern Europe, Middle East, Asia including India and Africa. The disease can be present as either a cutaneous or systemic disease. The incubation period ranges from 1 to 14 days depending on the virulence of the strain. Patients with cutaneous infection have nodules with accompanying localized lymphadenitis. The systemic illness usually manifests itself either as brancho or lobar pneumonia. Bacteremia may also occur, resulting in lesions being seen in liver and spleen. Infection in humans with Bu. mallei is often fatal without antimicrobial therapy. There is little or no clinical experience in the treatment of Bu. mallei infection in humans with the modern antimicrobial agents due to the rareness of this disease. The antimicrobial susceptibility studies with various strains and limited animal experiments suggest that ciprofloxacin ad doxycycline could be used for prophylaxis.[12] No vaccine against glanders is available for human use.
Melioidosis
Burkholderia pseudomallei is a recognized biothreat agent and the causative agent of melioidosis in animals and humans.[13] Melioidosis is prevalent in Southeast Asia, Northern Australia, and some other tropical and subtropical regions and also endemic in few states of India (Kerala, Maharashtra, Tamil Nadu, Orissa, Assam, West Bengal and Tripura). Bu. pseudomallei is an oxidase-positive, aerobic gram-negative bacillus that is straight or slightly curved. The organism grows on most standard laboratory media, such as sheep blood and chocolate and MacConkey agars, and it produces a characteristic musty odor. It is a resilient bacterium that can survive in disinfectant and antiseptic solution and extreme temperatures and grows very slowly in salt medium. The presence of Bu. pseudomallei in soil is an important factor in determining disease incidence in a given endemic area. This organism was first reported as causing human infections in 1911 by Whitmore from individuals living in Rangoon, Myanmar, and in earlier medical literature was called Whitmore's Disease. The diagnosis of this disease is very important for effective treatment and the populations at risk within endemic areas rarely have access to appropriate health care. Bu. pseudomallei is a natural inhabitant of soil and water in the tropics and subtropics, but can also survive in dry atmospheric conditions. It is present in rubber plantations, cleared fields, cultivated and irrigated agricultural sites as well as drains and ditches. There are several established modes of transmission with the patient population. The possible modes are ingestion of contaminated drinking water, inhalation and inoculation through the skin lesions from the contaminated soil. The rural poor and agricultural workers are likely to be at greatest risk of infection. Person to person transmission of Bu. pseudomallei, especially between patient and siblings or one of their playmates, is common; vertical transmission from mother to child is also possible. It can be transmitted by direct contact with infected rodents or infected food, soil, water, excreta; transmission is also possible through use of contaminated injection needle. Factors that may influence the distribution of Bu. pseudomallei in the environment may include physical factors such as rainfall, humidity, UV radiation, temperature, soil composition, vegetation, use of fertilizers, soil disturbance such as excavation and ploughing. The disease prevalence in nature has a significant seasonal and a strong linear correlation with rainfall. It is recognized as the most common cause of severe community-acquired sepsis in parts of northeast Thailand, where melioidosis accounts for 20% of community-acquired bacteremias and associated with a mortality of approximately 50%.[14]
The clinical symptoms of melioidosis are so varied that the disease has been termed “the great mimicker”. The clinical manifestation of melioidosis can be divided into asymptomatic, chronic, acute and subacute infections.[15] Chronic melioidosis is characterized by osteomyelitis (inflammation of the bone) and pus-filled abscesses in the skin, lungs or other organs. Acute melioidosis takes one of three forms: a localized skin infection that may spread to nearby lymph nodes; an infection of the lungs associated with high fever (102°F/38.9°C), headache, chest pain, coughing, septicemia characterized by disorientation, difficulty in breathing, severe headache, and an eruption of pimples on the head or trunk; the third form is most common among drug addicts and may be rapidly fatal. The clinical manifestation of melioidosis may be observed as in apparent infection, asymptomatic pulmonary infiltration, acute localized supportive infection, acute pulmonary infection, acute septicemic infection or chronic supportive infection. The highest mortality occurs in patients with the septicemic form of melioidosis, which is characterized by dissemination of the bacteria from blood to various organs. The clinical course of septicemic melioidosis is associated with liver and spleen and often includes rapid deterioration and death that occur within a few days after hospitalization. The host determinants such as diabetes mellitus, chronic alcoholism and renal failure represent Bu. pseudomallei. Diversity of the clinical manifestations necessitates the isolation and identification of causative organism for a definitive diagnosis of melioidosis. The causative organism, Bu. pseudomallei, is amenable to antibiotic treatment. For acute or chronic infections, parenteral administration of imipenem or ceftazidime for 2–4 weeks, followed by oral therapy with amoxicillin, clavulanate or a combination of doxycycline and trimethoprim-sulfamethoxazole for 3–6 months is recommended. Due to development of resistance with ceftazidime therapy, combination therapy is usually recommended for initial treatment. Bu. pseudomallei organisms are frequently resistant to aminoglycosides, first and second generation cephalosporins, and fluoroquinolone antimicrobials in vitro, and so, drugs in these classes are not likely to be good prophylactic agents. There are no data published on prophylactic agents for Bu. pseudomallei although it is likely that the agents used for oral therapy would be useful prophylactically.
Botulism
Botulism is a rare, naturally occurring disease caused by the bacteria Clostridium botulinum. It produces seven immunologically distinct toxins, which are designated by the letters A–G. Similar toxins are also produced by other closely related bacterial species, Clostridium baratii and Clostridium butyricum. These toxins can produce clinical syndromes such as food borne botulism (as intoxication), wound botulism (infection and toxin production) and botulism caused by toxin production after clostridial colonization of the intestine of infants (infant botulism) or older children and adults.[16] These toxins spread through the blood stream and affect the neuromuscular junctions, where they inhibit the release of acetylcholine. Their action on the cholinergic system at the presynaptic motor neuron terminal by blocking acetylcholine transmission across the neuromuscular junction causes neuromuscular blockade, resulting in flaccid paralysis. The toxins also affect the adrenergic system without any significant consequences. The lethal dose of the botulinum toxin is very low and is also the most potent toxin known to mankind; in humans, the exact dose is calculated based on the extrapolations made from the primate experiments. The lethal dose for the crystalline purified toxin type A is 0.009–0.01 mg for a man of 70 kg, when introduced intravenously; 0.80–0.90 mg when injected intravenously; 0.80–0.90 mg when introduced inhalationally; and 70 mg when introduced orally.[17] The toxins are highly heat sensitive and most of the toxins get inactivated by heating to 85°C for 5 minutes. C. botulinum forms spores under stress and these spores can survive standard cooking and food processing procedures. However, the conditions that can allow the spores to germinate, i.e., anaerobic condition, nonacidic pH, low salt and sugar content, are not commonly encountered in the food and may be the reason for few food borne botulism cases.
Home canned foods have long been associated with botulism and actual incidence of botulism is not available in most of the countries. In case of the food borne botulism, the presumptive evidence can be obtained by collecting 3–5 day food history from the patient, evidence of consumption of home canned food, as the preformed toxins are ingested in the food borne infection, and the patient rapidly progresses to respiratory failure and mechanical ventilation despite administration of supportive and specific therapy. Wound botulism is caused by the contamination of wound with C. botulinum spores from the environment and further germination of the spore and production of toxin in the anaerobic condition prevalent in the wounds. This type of botulism is very common among the drug addicts who inject black tar heroin, a specific preparation of heroin into tissues. Infant botulism results from the adsorption of the toxin produced by the bacteria that have colonized the intestine of infants aged below 1 year. The main reason for the colonization of infant intestine was suspected to be use of contaminated honey. The clinical presentation resembles that of adult, with inability to suck and swallow, weakened voice, ptosis and floppy neck and it progresses to generalized flaccidity and respiratory compromise. A licensed vaccine with human source antitoxin reduces the median hospitalization from 6 to 3 weeks; speedy recovery is possible with intensive care and antitoxin therapy. The adult intestinal botulism is produced from adsorption of toxin produced in situ by rarely occurring intestinal colonization. The patients have anatomical or functional bowel abnormality or due to using antimicrobials, which may permit protection of fastidious C. botulinum from competitive normal bowel microbial flora. The botulinum toxin is also used for cosmetic or therapeutic purpose, and the dose of toxin used is very low that it cannot cause systemic disease. The licensed toxins contain very low concentration of toxin, which cannot produce any symptoms. With respect to inhalation botulism, only one case was reported among German laboratory workers handling this bacterial toxin in 1962, and mostly the symptoms resemble those of food borne botulism. The deliberate dissemination of botulism is possible through aerosol route and can produce outbreak of inhalation botulism. In the event of a bioterrorist attack, the most likely event is the contamination of food and water source with the purified or partially purified botulinum toxin.
In the earlier days, the mortality due to botulism was very high even after the administration of equine antitoxin. The current mortality rates of 3–5% in natural infection is mostly attributed to the use of highly effective modern intensive care techniques, mainly the mechanical ventilation, and most of the deaths result from respiratory failure. The only specific treatment available for botulism is antitoxin. The antitoxin can arrest the progression of paralysis and decrease the duration of paralysis and needs good ventilation support system. The ideal antitoxin treatment should start within 24 hours of onset of symptoms and the antitoxin binds only to the toxin molecule that has not reached the nerve endings. There are many adverse effects like anaphylaxis, hypersensitivity reaction and serum sickness associated with the use of antitoxin. The sensitivity of the serum or antitoxin should be ascertained before administration; one vial of botulism antitoxin produces serum levels of toxin type specific antibodies capable of neutralizing serum toxin concentrations many fold in excess of those reported in the patients. When large numbers of persons are infected simultaneously from single food source, it has been observed that the appearance of symptoms and the susceptibility to the toxin varies with the host response and the concentration of toxin ingested. The botulinum toxin cannot be absorbed through intact skin, but can be absorbed through mucosal surfaces, eye and nonintact skin. Person to person transmission has never been reported for these bacteria and no licensed vaccine is available for human use. In laboratories equipped and capable of diagnosing botulism, serum and other body fluids are examined for the presence of toxin. The most commonly used method for toxin detection is enzyme-linked immunosorbent assay (ELISA) and mouse inoculation bioassay. The vomitus, stool or debrided tissue from infected wounds are subjected to culture for the isolation of these bacteria and the isolates are further confirmed based on biochemical and molecular characters and tested for the protection of toxin.
Variola Virus
The smallpox (variola) virus is the largest of the animal viruses. The virus particles are brick-shaped to ovoid and morphologically indistinguishable from the less pathogenic, closely related vaccinia virus. The variola virus contains double-stranded DNA and has a complex structure. Two lipoprotein membrane layers surround the dumbbell-shaped nucleoid. The nucleoid is embedded in an ellipsoid body, forming the thick center of the virion and double membrane surrounds the virus particle. The variola virus is highly contagious and very virulent, with a case fatality rate of 30% in unvaccinated persons. As extreme biohazards involved in working with the smallpox virus have limited research on this organism, the WHO decided to destroy the virus and the virus has been stored only in two places, one each in the United States and former Soviet Union, for research purpose, if need arises in the future.[18] Poxviruses are divided into four different groups. Group 1 comprises variola, vaccinia, cowpox, ectromelia, rabbitpox and monkeypox viruses. The variola virus exists as one of these two strains: variola major causing severe smallpox and variola minor causing mild smallpox or alastrim. Both these strains cannot be differentiated based on immunologic methods and vary only in the clinical presentation. Vaccination against smallpox is performed with the vaccinia virus, which has many antigenic structures in common with the smallpox agent. It was used worldwide in a live vaccine against smallpox and served as a laboratory model for the poxviruses. The origin of the vaccinia virus remains unclear. It is different from Jenner's cowpox virus and may be a mutant of the variola and alastrim viruses. At least seven distinct major variola virus antigens can be recognized by immunodiffusion techniques, and 17 polypeptide chains can be identified. Hemagglutinating, complement-fixing, and neutralizing antibodies may be produced in response to antigens of the smallpox virus. Neutralizing antibodies are directed against two antigens in the surface membrane of the virus particle. Complement-fixing antibodies react with a family antigen common to each subgroup of the unclassified poxviruses. The incubation period is 12 days and the clinical presentation is fever and headache. The infection causes pus-filled vesicles throughout the body, and the mortality rate is around 30% in the nonimmunized group with a mortality rate of about 3% in the immunized group.[19] Today, smallpox is considered a serious threat due to several reasons, the most important being that most of the human population is not immunized against this disease and susceptible to smallpox as the universal vaccination ended in 1980. Second, the spread of smallpox is rapid due to its high infectivity and the mode of spread through aerosol route can be used for the intentional spread of this disease.[4]
Detection
The use of anthrax spores in letters in a bioterrorist event in 2001 has emphasized the need for immediate detection and identification of biothreat agents from environmental samples as well as from affected persons.[20] The technologies so developed need to be rapid, accurate and have to unequivocally confirm the presence of these agents in varied matrices. An ideal detection system for a biothreat agent should not only be capable of detecting the agents in very low concentration, but also have the possibility to detect in various matrices. In addition, it should be portable, user-friendly and capable of detecting multiple threat agents. Among all the detection systems available for use, none of them satisfy all these criteria and the selection of methodology should be situation specific. Development of detection systems that can detect the biological agents in concentration at which they can cause disease in humans is a challenge, and due to lack of sensitivity of many of the available antigen and antibody based systems, research is focused on development of nucleic acid based sensors that are much more sensitive but need complex sample preparation. The Polymerase Chain Reaction (PCR) based assays can detect >10 microorganisms per sample but limitations are with the detection of toxins and other non nucleic acid containing prions, etc. Further, the nucleic acid based assays are to be performed in much cleaner environment as there is a possibility of DNA from laboratory and instrument contamination getting amplified and thus producing a false positive result. The immunologic assays based on the antigen and antibodies are highly specific due to the specificity of the antigen and antibody used but their specificity is low when compared with other assays. In addition to the sensitivity and specificity, the reproducibility of detection system or assay is very important as the data generated need to be reproducible under different environmental conditions. The reproducibility is decided by the stability and consistency of the reagents and assay conditions. The ideal detection system should have the capability to detect multiple threat agents as we expect the sample to contain a variety of bacteria, viruses and toxins. In the recent times, genetically modified bacterial and viral agents not covered under the list of probable agents may pose an additional threat and make the detection much more difficult.
In the scenario of detection of biological warfare agents, we expect not only human clinical samples like blood, sputum, urine, stool, CSF, etc., but also suspected powdery material, food and water samples and, most importantly, the environmental air sample. The pollutants present in the air, the anticoagulants, leukocyte DNA and blood heme compounds are known to inhibit the polymerase reaction. In the case of immunoassay, the food samples with high protein and lipid content and high bacterial load in the stool samples pose a challenge in the detection. Due to this reason, a good sample preparation is needed in most of the systems that have been developed with either the nucleic acid or antibody based detection systems. The sample preparation takes hours to days depending on the standardized protocols and often cannot be performed in the field conditions. In the case of bacteria, most of the time the sample that is obtained from field condition is not viable and is unsuitable for culture conditions.[21] The sample collection procedures, handling, transportation and preparation are very important and vital for correct identification. The air monitoring requires concentration of bacteria from large air volumes and resuspension of concentrated material into appropriate liquid as most of the detection systems currently developed can use only liquid samples. Although the sample preparation and efficacy of extraction procedures determine the concentration of the agent availability for detection, in some instances, the viability of virus or bacteria has to be confirmed by conventional culture methods. The conventional detection and identification of human pathogens mostly rely on cultivation on bacteriological media and biochemical tests that take minimum 3–7 days and in some cases even more than 15 days to provide results. The culture and biochemical tests are reliable but need highly trained manpower to perform. Moreover they do not provide results on real time basis and as such cannot be used in environmental detection or in situations that need immediate results. Efforts are currently on to develop methods that are rapid, accurate and can detect the agents in field as well as in laboratories that have limited or no instrumental support. The detection technologies can be broadly classified into biochemical test based assays, antibody based assays and nucleic acid based assay. Most of these assays are at various stages of development and test on limited scale, their use in case of emergency situation need to be further evaluated on a case to case basis.
Biochemical test based assays
The culture of bacteria in a routine microbiological laboratory and further identification based on biochemical tests is the conventional identification method mostly followed in most of the hospital setups even today. As this method is followed for various diseases, the biological warfare agents, mainly the bacterial agents, need to be identified through this platform on a routine basis, if not during an intentional outbreak, but during natural prevalence of the disease. Most of the laboratories have standardized media for isolation for Ba. anthracis, Y. pestis, Burkholderia sp. and Brucella sp., and growth in specific media followed by few of the conventional biochemical tests can lead to the presumptive identification of these agents. Many commercial automated biochemical test platforms are today available for performing the tests after growth in bacteriological media, but these systems are mostly developed for bacterial diseases that are clinically important. These databases do contain data of important bacterial biological warfare agents and can be used in case of emergency. The tests based on substrate utilization pattern have been developed by Becton Dickinson (USA), Vitek (BioMe’rieux, France) and Microlog (Biolog, Hayward, CA USA). These systems have both visual observation and reader based formats for analysis of results and a database for comparison purposes. The main disadvantage of these systems is the need for pure culture and trained manpower to perform and for all the analyses. The BioMe’rieux API series of cards containing specific substrates have been used with good results in identification of most of the bacterial agents.[22,23] In addition to these tests, the system developed by MIDI Inc. (USA), called the microbial identification system, coverts cellular fatty acids from pure culture of bacteria to fatty acid methyl esters and uses a gas chromatograph for the separation and identification. In this method, a tedious sample preparation step in involved in which the bacterial cells need to be harvested, saponified, methylated, extracted and washed before analysis. But a chromatographic result pattern allows signature storage and database based retrieval for easy computing and analysis. Many of the databases based on the fatty acid profile have been now routinely used in laboratories for the confirmation of bacterial isolates. The major disadvantage is the need of time consuming isolation procedures as well as the need of highly calibrated instruments for reproducible performance. The advantage of these systems is that they allow simultaneous analysis of many samples and generate a highly reliable result output in the form of a fingerprint. They also allow addition of additional input based on experience.[24]
Bioluminescence based detection
Bioluminescence is the monitoring of luciferin and luciferase, the enzyme, interaction in the presence of ATP. The basic principle is ATP is found in all the living cells like virus and bacteria and its amount corresponds to the microbial load in air, water and in other environmental samples.[25] The bioluminescence has been widely used mainly in the quality control testing of bacterial contamination in food industry. This principle has been used in the Profile-1 hand held system developed by New Horizon Diagnostics (Columbia, MD, USA), which uses a microluminometer to read the sample bioluminescence.[26] In the Filtravette system developed by New Horizon Diagnostics, care has been taken to remove non-bacterial ATP. The main disadvantage of the bioluminescence based systems is the ATP contamination from nonmicrobial sources. Moreover, as all of them detect ATP, the results are specific and cannot be pointed toward any biowarfare agents. Since these systems are cost effective can be used for the real time air monitoring as first line defence. The continous air quality monitoring will trigger alarm for any unusual raise in the microbial load in the environment.
Antigen and antibody based detection systems
The antigen and antibody based immunoassays are being developed for the detection of bacterial and viral biowarfare agents.[27] The immunologic detection of antigens or antibodies based on ELISA has been developed, evaluated and currently in use for the detection of anthrax, plague, botulism, brucellosis, glanders and melioidosis. In these immunoassays, the efficacy of the detection depends on the availability of antigen or antibody in the detection matrix, the quality of the antigen that needs to be detected and the quality of the antibodies used in the detection of the antigen.[28] In short, it depends upon the quality of the antigen and antibody complex formed and the ability of the detection method to detect the formed antigen and antibody complex. Many different formats employing the ELISA principle have been developed using different substrate labels, i.e., fluorescent, chemiluminescent, electrochemiluminescent and on various platforms like solid support ELISA plates, visual dot and lateral flow formats. In addition, instruments using the immunoassay principles like biosensors, flow cytometry and micro array are under different stages of development. The disadvantage of the ELISA based systems is its ability to perform detection of only one agent at a time and sequential assays need to be performed to detect more than one agent. The specificity of the immunoassays limits the detection to minimum 105 CFU per test and is considered much higher than the DNA based assay.
The immunoassay based on the sandwich antigen capture assay format is used in the Luminex xMAP system developed by Luminex Corp , Austin, Tx,USA, in which the polystyrene beads are coated with the specific antibodies and these beads are spectrally unique and are color coded into different sets; as each of these beads is coated with antibodies specific to an agent, it allows simultaneous detection of multiple agents in a liquid phase detection.[29] The laser in the detector detects the excitation of internal bead dyes of any capture agent and the signal intensity is proportional to the amount to agent present in the sample. This system allows to be hooked to a continuous environmental monitoring system for the detection and online monitoring. In another instrument the BV M-Series developed by BioVersis Corp. (USA), the reporter molecule uses electrochemiluminescence using paramagenetic beads as the support for the coating the capture antibodies. The electromagnetic beads are separated from the matrix by passing through a magnetic field and detected; this system has been modified as a kit for portable detection.[30] The bio-detector of Smiths Detection (USA) uses ELISA principle in a tape format and in which liquid samples are injected. In the reaction, the antigen available in the sample is mixed with biotin labeled and fluorescenin labeled antibodies. The system has a sensor that detects the signal from the biotin and fluorescenin labeled antibodies. Dissociation enhanced lanthanide fluorescence immunoassay (DELFIA) developed by Perkin Elmer Life Science (USA) is a unique system based on time resolved fluorescence.[31] This technique relies on lanthanide chelate labels which have long fluorescence decay time that allows measurements without background signals. Simply, in this system, the antibodies are labeled with lanthanide and tests are performed on standard plate ELISA format. After the antigen and antibody complex is formed, the label is disassociated from the antibody using a low pH enhancement solution and the free molecules rapidly form new stable highly fluorescent chelates that can be read by the system.
The latest system that has gained popularity in the ELISA format is the lateral flow system which is rapid and cost effective when compared with the instrument-based detection systems. These tests are based on single use, disposable cartridge tests in the form immunochromatographic (ICT) line assays that generate visual lines in the membrane. In this class of assay, the detector antibodies are coated with colloidal gold or micro particles which by forming an antigen and antibody complex form a visual line or dot. These tests incorporate a procedural control that acts as a negative control and gives a confidence of testing while performing them.[32] These tests are easy to perform and are rapid than most of the immunoassays, but the sensitivity is often questioned as high false positive results are encountered. This can be used as a presumptive test and can be further followed up with a confirmative lab-based test. Lateral flows have been developed for all the biothreat agents by different agencies, but information on the use in real time detection situation and comparative evaluation with other methods is not available for recommendation.
Nucleic acid based detection
The nucleic acids, DNA and RNA, based detection systems is the most researched and developed detection system for the detection of biowarfare and biothreat agents. In the conventional PCR, the specific region of the genome is amplified and checked on electrophoresis for the amplification of correct size of product. In the advance quantitative real time PCR or Q PCR assays, the PCR amplification is combined with real time detection based on reporter fluorescence dyes.[33,34] In the non specific Q-PCR, the amplified DNA is detected based on DNA intercalating fluorescence dyes (SYBR green), and in specific Q-PCR, the fluorescence probes that specifically bind to a sequence are used. In the biowarfare agent detection, the specific Q-PCR assays based on probes have been developed for all the agents. In all the Q-PCR assays, software monitors the progress of the reaction, and the presence of the agent can be detected online on a monitor and the data can be transferred over a long distance for action. Today, compact, faster and sensitive real time PCR systems are available, making this method the most preferred method of agent detection. The limitations of this assay are the variation that exists with the nucleic acid based amplification techniques, the availability of starting material and its quality, inhibitory substances present in the matrices, sensitivity and specificity based issues related to quality of primers, probes, enzymes used. The advantage being the ability to detect a few cells, mostly up to 100 cells per test, makes it an attractive alternative to all the detection systems available today. The portability and stability of the reagents, use of stored power supply, etc., will make this technology more practical in the years to come. The developers based on the Q-PCR have incorporated disposable testing cartridges for various types of samples like the GeneXpert Q-PCR of Cepheid (USA) and eliminating the need to extract DNA prior to processing makes them convenient to use in field situations. The light cycler system of Roche Diagnostic, GmbH, Mannheim, Germany has assays specific to Ba. anthracis, which use a glass capillary into which extracted DNA is placed instead of a cartridge, and is commercially available. The Applied Biosystems (ABI, Foster City, USA) has several Q-PCR kits developed for anthrax and other biothreat agents, which can be used with their ABI 7500 and 7300 real time PCR systems. In case of the Bioseeq developed by Smiths Detection (USA), all the reagents are freeze dried for better stability and storage and this commercial hand held version can analyze up to six agents. Several other firms have also started marketing readymade kits for use with various real time PCR platforms for the detection of biowarfare agents, but no comparative results are available for these kits for evaluation.
Sensor based detection systems
The sensor based detection systems have been developed based on all the three groups that have been discussed already. The sensors have been developed for biochemical, immunologic and nucleic acid based detection systems. In the sensor based detection systems, the basic principles of all these three groups are integrated with a transducer than can transform the response in to an analyzable signal.[35] All these systems that integrate the biological component with a physical transducer are termed as biosensors. The physical transducers that produce an analyzable signal could be of electrochemical, optical, mass, thermal or of high frequency. In the electrochemical systems, the products that are formed due to an enzymatic activity associated with microbial metabolism are monitored based on conducting polymers. Array of sensors, each specific for different vapors or gases, are constructed and used to detect multiple analytes. Mostly, this technique is used to detect volatile organic compounds produced during the growth of bacteria or fungi and is termed as electronic noses. This system is very complex as most of the compounds are produced by multiple bacteria and analysis of results is difficult. But this has application in mainly detecting toxins that are highly specific and produced during the growth of bacteria.
The biosensors that use antibodies are also under development; current research is oriented toward developing highly sensitive and specific antibodies, mostly monoclonal antibodies, which can be used. In addition to monoclonal antibodies, antibody fragments, recombinant antibodies or phage probes are also under development. The recombinant engineered antibodies can improve the binding kinetics to the antigen and can offer high sensitivity, specificity and stability, when compared with the conventional antibodies. The phage display libraries have improved the development of affinity probes that can be synthetically constructed, scanning across thousand of possible peptides of an agent, and decide on the best possible combination. The aptamers and peptide ligands are also alternative to antibodies. Aptamers are small DNA or RNA ligands that recognize a target by shape, not by sequence, and are mostly generated using combinatorial methods. Aptamers have been demonstrated to be useful in ricin detection based on bead based biochip sensor. Several short peptide sequences have also been tried as detection probes in the development of biosensors.
Flow cytometry has been used as a detector in one of the biosensors, which uses luminex color coded beads conjugated to specific antibodies against biowarfare agents. The Autonomous pathogen detection system (APDS) developed by Lawrence Livermore National Laboratory (USA) used this system for the detection of biowarfare agents. The multicoded beads provide multiplexing capability as several agents can be looked for simultaneously; also, PCR has been successfully integrated with this system so that the positive flow cytometry result can be further confirmed using a PCR-based assay.[36] Various methods for detecting biowarfare agents using biochip technology are under development and evaluation, out of which the dielectrophoresis, for concentration of the target and further identification using an electric field driven immunoassay, is a very novel approach. In the Surface Plasmon Resonance (SPR) based biosensor, the target is directly analyzed by measuring the refractive index changes that occur when the target binds to the surface metal coated surface. The SPR biosensors that are small and field usable are being developed and tested for detection of toxins.
Conclusion
The threat of use of biological warfare agents in a terrorism-related issue or in a warfare situation is real and looming before us. The highly unpredictable nature of any event involving biological warfare agents has given rise to the need for developing rapid and accurate detection systems. The bioterrorist events are difficult to predict and prevent; in the case of a release, accurate, easy deployable detection systems are needed to minimize the damage and to prevent further spread of these agents. The intentional release of spores of Ba. anthracis in the US postal service proved the need for these detection platforms. Although many such detection systems are under development and are at various stages of evaluation, a single system to detect all the known biowarfare agents is going to be a real challenge. The complex matrices that need to be processed at the time of detection and genetically modified or uncharacterized agents that may be available in the sample have to be resolved. In this review, attempts have been made to provide basic information on biological warfare agents. Also, a comprehensive review of the current available methods for their detection has been made. The current knowledge on the agents and their detection methodologies will be highly useful in developing a highly effective response system through systematic planning.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Atlas R M. Bioterrorism: From threat to reality. Annul Rev Microbiol. 2002;56:167–85. doi: 10.1146/annurev.micro.56.012302.160616. [DOI] [PubMed] [Google Scholar]
- 2.Christophe G, Cieslak T, Pavlin J, Eitzen E. Biological warfare: A historical perspective. JAMA. 1997;278:412–7. [PubMed] [Google Scholar]
- 3.Wheelis M. Biological warfare before 1914. In: Moon JE van Courtland., editor. Biological and toxin weapons: Research, development, and use from the middle ages to 1945. Vol. 1. Stockholm, Sweden: Stockholm International Peace Research Institute; 1991. pp. 8–34. [Google Scholar]
- 4.Klietmann WF, Ruoff KL. Bioterrorism: Implications for the clinical microbiologist. Clin Microbiol Rev. 2001;14:364–81. doi: 10.1128/CMR.14.2.364-381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logan NA, Turnbull PC. Bacillus and recently derived genera. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 7th ed. Washington, D.C: American Society for Microbiology; 1999. pp. 357–69. [Google Scholar]
- 6.Swartz MN. Aerobic spore-forming bacilli. In: Davis BD, Dulbecco R, editors; Eisen HN, Ginsberg HS, editors. Microbiology. 4th ed. Philadelphia, Pa: J. B. Lippincott Company; 1990. pp. 625–31. [Google Scholar]
- 7.Butler T. Yersinia species, including plague. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2406–14. [Google Scholar]
- 8.Cloeckaert A, Grayon M, Grepinet O, Boumedine KS. Classification of Brucella strains isolated from marine mammals by infrequent restriction site-PCR and development of specific PCR identification tests. Microbes Infect. 2003;5:593–602. doi: 10.1016/s1286-4579(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 9.Hubalek Z, Scholz HC, Sedlacek I, Melzer F, Sanogo YO, Esvadbova NJ. Brucellosis of common vole (Microtus arvalis) Vector Brone Zoonotic Dis. 2007;7:679–87. doi: 10.1089/vbz.2007.0143. [DOI] [PubMed] [Google Scholar]
- 10.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new gobal map of human Brucellosis. Lancet Infect Dis. 2006;6:91–9. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 11.Thavaselvam D, Kumar A, Tiwari S, Mishra M, Prakash A. Cloning and expression of immunoreactive Brucella melitensis 28 kDa outer membrane protein (Omp28) gene and evaluation of its potential for clinical diagnosis of brucellosis. J Med Microbiol. 2010;56:421–8. doi: 10.1099/jmm.0.017566-0. [DOI] [PubMed] [Google Scholar]
- 12.Gilligan PH. Therapeutic challenges posed by bacterial bioterrorism threats. Curr Opin Microbiol. 2002;5:489–95. doi: 10.1016/s1369-5274(02)00359-4. [DOI] [PubMed] [Google Scholar]
- 13.Dance DA. Melioidosis: The tip of the iceberg? Clin Microbiol. 1991;4:52–60. doi: 10.1128/cmr.4.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaowagul W, White NJ, Dance DA. Melioidosis: A major cause of community-acquired septicemia in North eastern Thailand. J Infect Dis. 1989;159:890–9. doi: 10.1093/infdis/159.5.890. [DOI] [PubMed] [Google Scholar]
- 15.Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: Acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–4. doi: 10.1016/s0035-9203(00)90333-x. [DOI] [PubMed] [Google Scholar]
- 16.Allen SD, Emery CL, Siders JA. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. Clostridium; pp. 654–71. [Google Scholar]
- 17.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, et al. Botulism toxin as a biological weapon: Medical and public health management. JAMA. 2001;285:1059–70. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 18.Breman JG, Henderson DA. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–9. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 19.Davis BD, Dulbecco R, Eisen HN, Ginsberg HS, Wood WG, Jr, McCarty M. In Microbiology. 2nd e-d. Hagerstown, Md: Harper & Row Publishers, Inc; 1973. Poxviruses; pp. 1258–77. [Google Scholar]
- 20.Lim DV, Simpson JM, Kearns EA, Kramer MF. Current and developing technologies for monitoring agents of bioterrorism and biowarfare. Clin Microbiol. 2005;18:583–607. doi: 10.1128/CMR.18.4.583-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buttner MP, Cruz P, Stetzenbach LD, Klima-Comba AK, Stevens VL, Cronin TD. Determination of the efficacy of two building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl Environ Microbiol. 2004;70:4740–7. doi: 10.1128/AEM.70.8.4740-4747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archer JR, Schell RF, Pennell DR, Wick PD. Identification of Yersinia spp. with the API 20E system. J Clin Microbiol. 1987;25:2398–9. doi: 10.1128/jcm.25.12.2398-2399.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inglis TJ, Chiang D, Lee GS, Chor-Kiang L. Potential misidentification of Burkholderia psuedomallei by API 20NE. Pathology. 1998;30:62–4. doi: 10.1080/00313029800169685. [DOI] [PubMed] [Google Scholar]
- 24.Lowe P, Engler C, Norton R. Comparison of automated and nonautomated systems for identification of Burkholderia pseudomallei. J Clin Microbiol. 2002;40:4625–7. doi: 10.1128/JCM.40.12.4625-4627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Deininger RA. A rapid screening method for the detection of viable spores in powder using bioluminescence. Luminescence. 2004;19:209–11. doi: 10.1002/bio.775. [DOI] [PubMed] [Google Scholar]
- 26.Cutle CN, Dorsa WJ, Sirogusa GR. A rapid microbial ATP bioluminescence assay for meat carcasses. Dairy Food Environ Sanit. 1996;16:726–36. [Google Scholar]
- 27.Andreotti PE, Ludwig GV, Peruski AH, Tuite JJ, Morse SS, Peruski LF., Jr Immunoassay of infectious agents. Biotechniques. 2003;35:850–9. doi: 10.2144/03354ss02. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal SS, Mayo MW, Bruno JG, Bronk BV, Batt CA, Chambers JP. A review of molecular recognition technologies for detection of biological threat agents. Biosensor Bioelect. 2000;15:549–78. doi: 10.1016/s0956-5663(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 29.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol. 2000;243:243–55. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 30.Gatto-Menking DL, Yu H, Bruno JG, Goode MT, Miller M, Zulich AW. Sensitive detection of biotoxoids and bacterial spores using an immunomagnetic electrochemiluminescence sensor. Biosensor Bioelect. 1995;10:501–7. doi: 10.1016/0956-5663(95)96925-o. [DOI] [PubMed] [Google Scholar]
- 31.Peruski AH, Peruski LF., Jr Immunological methods for detection and identification of infectious disease and biological warfare agents. Clin Diagn Lab Immunol. 2003;10:506–13. doi: 10.1128/CDLI.10.4.506-513.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King D, Luna V, Cannons A, Cattani, Amuso P. Performance assessment of three commercial assays for direct detection of Bacillus anthracis spores. J Clin Microbiol. 2003;41:3454–5. doi: 10.1128/JCM.41.7.3454-3455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak K, Flood S, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–62. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 34.Heid C, Stevens J, Livak K, Williams P. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 35.Aberl F, Kosslinger C. In Molecular diagnosis of infectious diseases. Vol. 13. Totowa, N.J.: Humana Press, Inc; 1998. Biosensor-based methods in clinical diagnosis. [DOI] [PubMed] [Google Scholar]
- 36.Ali MF, Kirby R, Goodey AP, Rodriguez MD, Ellington AD, Neikirk DP, McDevitt JT. DNA hybridization and discrimination of single-nucleotide mismatches using chip-based microbead arrays. Anal Chem. 2003;75:4732–9. doi: 10.1021/ac034106z. [DOI] [PubMed] [Google Scholar]


