Abstract
In recent years, a lot of interest has been generated world over in the area of radioprotection for first responders going to work in the hot zones at the incident site. A large number of molecular drugs have been screened for radioprotective efficacy, but with little success. The requirement of differential radioprotection necessitates a holistic approach, which can be realized using herbs in view of their multifaceted mode of action. Our earlier studies showed the radioprotective potential of Rhodiola imbricata, a Himalayan high-altitude plant. In this study, our focus has been to compare the pro-oxidant/antioxidant activities of three fractionated extracts of R. imbricata. The aqueous fraction exhibited significant (P < 0.05) pro-oxidant activity (up to 100 μg/ml) under metal ion-induced stress ± flux [transition metal (Fe/Cu) ± 0.25 kGy]. A decrease in the dielectric constant of the solvent system utilized for extraction, exhibited a significant (P < 0.05) negative correlation (–0.955) with mean protection potential of lipid against radiation flux. Such an effect was visualized as a significant shift from pro-oxidant to antioxidant activity in methanolic fraction (dielectric constant = 33), as compared to aqueous fraction (dielectric constant = 80). Aqueous fraction is predominantly pro-oxidant at maximal concentrations, indicating its anticancer potential. The presence of transition metals modulates such a biphasic activity differentially in various fractions, i.e., the conversion of Fe(III) or Cu(II) to Fe(II) or Cu(I), respectively, due to the presence of certain bioactive constituents (electron donation at lower concentrations), favors pro-oxidant activity. On the other hand, certain other active constituents involved in metal ion chelation contributed to the overall antioxidant activity. The methanolic fraction exhibited significant antioxidant activity up to 250 μg/ml, which contributed to its radioprotective efficacy. The aquo-methanolic fraction exhibited (disparate properties), i.e., concentration-dependant cytotoxicity (up to 250 μg/ml) and cytoprotection at 1000 μg/ml. R. imbricata, in general, exhibited a significant solvent-dependant variation in radioprotective efficacy. In conclusion, solvent extraction and dose are crucial in bioactivity modulation and R. imbricata could be developed as a potential prophylactic radiation countermeasure for use in nuclear and radiological emergencies.
Keywords: Antioxidant, nuclear and radiological emergencies, oxidative stress, pro-oxidant, radioprotection, Rhodiola
Nuclear and radiological emergencies, either due to energy release resulting from a nuclear chain reaction or from the decay of toxic radiological products of reaction involving contamination, radiation exposure or both, are a present day reality. The recent incidence of Mayapuri accidental radiation exposure involving one death has shown that any inadvertent lapse in the product life cycle of even sealed radioactive substances may lead to the release of open radiation source in the civilian domain. The prompt and effective response to any such emergencies requires that the responders be given adequate prophylactic biological protection in addition to physical protection to mitigate the consequences of response in hot zones. Ionizing radiation carries enough energy to break chemical bonds, leading to induction of cellular oxidative stress. The consequent cellular oxidative stress is the outcome of an imbalance between reactive oxygen species (pro-oxidant) and the antioxidant defense system. It has been now widely accepted that radiation induces oxidative stress and it is crucial to modulate oxidative stress to develop effective treatment modalities for free radical mediated ailments.[1] Most of the work reported earlier over the past few decades has relied on synthetic derivatives with radioprotective efficacy, but such agents are known to manifest toxic side effects.[2] The shift toward the usage of herbals as an alternative has given a ray of hope since they contain a wide spectra of secondary metabolites that enable them to combat the adverse stresses like pollution, radiation, high altitude, severe temperature and UV flux in their native environment, which result in oxidative damage. Several plant species with known ethno-pharmacological importance, including Podophyllum hexandrum, Hippophae rhamnoides, Ginkgo blioba, Panax ginseng, etc. have shown promising radioprotective potential during in vitro and in vivo screening.[3,4]
The genus Rhodiola sps., a member of the Family Crassulaceae, has been widely used in traditional/modern medicine for its ability to enhance physical endurance and to treat impotence, fatigue, gastrointestinal, cardiac and central nervous system disorders.[5,6] Medicinal properties like anticancer, antimutagenic, anti-inflammatory, anti-aging activities make it invaluable for further pharmacological investigation.[7–9] The adaptogenic potential and therapeutic efficacy of Rhodiola sps. against high-altitude sickness, sexual dysfunction and sleep disturbances (depression) has indicated its pharmacological importance in humans.[5,10] Rhodiola possesses flavonoids, tannins, phenolic glycosides, mono- and tri-terpenes, phenyl ethanol derivatives and phenyl-propanoids.[5] The content and type of bioactive compounds are known to significantly vary with species. One such species with cytoprotective/radioprotective properties is Rhodiola imbricata.[4,11] R. imbricata has been investigated for its post-stress recovery potential by virtue of its ability to shift anaerobic metabolism to aerobic metabolic in cases of hypothermia,[12,13] possessing adjuvant/immunopotentiating activity for humoral and cell-mediated immune response against strong antigens like tetanus toxoid and weak antigens like ovalbumin in rats, being able to stimulate innate immune responses, i.e., toll-like receptor 4, granzyme-B and Th1 cytokines,[14,15] its anticancerous potential attributing toward NK cell cytotoxicity[16] and wound healing potential attributing toward an increase in antioxidant and a decrease in lipid peroxide levels in the granulation tissue.[17]
Presently, the screening protocols, in general, for radioprotectors are confined to protect normal tissue from radiation exposure (antioxidant aspects). On the other hand, if a drug selectively kills cancer cells, the reason could be attributed to its pro-oxidant activity and this activity imparts immense clinical importance to the drug.[18] Earlier, we had reported the radioprotective efficacy of the crude extract of Rhodiola in mice model system.[19] In the present study, we have attempted to compare the pro/antioxidant aspects of three fractionated extracts of R. imbricata in lipid phase and study the effect of solvent system (utilized for fractionation) on the overall radioprotective efficacy, i.e., biological protection to first responders during nuclear and radiological emergencies.
Materials and Methods
Chemicals
Ferrous chloride, ferrous sulfate, tween 20, ferric sulfate, copper (II) sulfate were purchased from Sigma Chemicals (St. Louis, MO, USA), while ethanol was obtained from BDH Chemical Co. (Toronto, Ontario, Canada). The rest of the chemicals including ammonium thiocyanate, linoleic acid, etc. utilized for this study were of analytical reagent (AR) grade and were obtained from reputed local suppliers in India. Plasticware (micro-centrifuge tubes, pipette tips) and pipettes were obtained from Tarsons (Kolkata, India).
Plant material
Plant material (stem portion) of R. imbricata was procured from the Field Research Laboratory (now called Defence Institute of High Altitude Research), Leh (Jammu and Kashmir, India). A voucher specimen of the same is deposited. The material was authenticated and freed of all extraneous material.
Preparation of fractionated extracts
The plant material was then powdered and Soxhlet extraction was carried out in water (100%), methanol:water (50:50) and methanol (100%) at a temperature of 55°C for 12 hours ×4 times, after making it free of fats and chlorophyll. The overall yield of the fractions, viz., aqueous, aquo-methanolic, and methanolic was 1.2, 0.8 and 1.9%, respectively.
High performance liquid chromatography fingerprinting analyses
The fractionated extracts were dissolved in their respective solvent systems and separated on a 250 × 4.0 mm (internal diameter), RP-18 Merck column, using Shimadzu LC-10 AT VP high performance liquid chromatography (HPLC) machine with photodiode SPD M-10A VP/RF-10 AXL fluorescent detector and auto injector SIL-10 ADVP. In each case, 20 μl of sample was injected into the column. The flow rate for all the separations was 0.8 ml/minute at 254 nm, and the column temperature was maintained at 37°C.
Pro-antioxidant activity in lipid phase
Preparation of lipid phase
Lipid phase was prepared using the methodology of Asamari and co-workers.[20] A pre-emulsion of linoleic acid was prepared by mixing 3 volumes of linoleic acid in 200 volumes of 30% (v/v) ethanol. An equal volume of tween-20 (~linoleic acid) was also added as an emulsifying agent.
Stress induction
A modified methodology[21] was adopted using the basic protocol described by Kitts and co-workers.[22] Varied concentrations of each extract were mixed with a pre-emulsion of linoleic acid in 1:10 ratio. The various stresses included Fe(II) (100 μM), Fe(III) (100 μM) and Cu(II) (10 μM) in combination with 0.25 kGy supra-lethal exposure [using 60Co gamma chamber (Gamma cell 5000, Bhabha Radiation Isotope Technology, Mumbai, India) at a dose rate of 3.20 kGy/hour]. The final assay mixture was three times the volume of pre-emulsion used with an incubation period of 2 hours.
Monitoring of peroxyl radicals induced radiation flux
In the present investigation, “supra-lethal radiation (0.25 kGy)” was used as a “flux” with/without numerous “stresses”, including “transition metal ions”, i.e., Fe(II), Fe(III) and Cu(II). The peroxyl ions generated due to the stress ± flux-induced linoleic acid degradation was monitored using ammonium thiocyanate assay.[22] A modified microassay was used for simultaneously monitoring of variation in the levels of peroxyl radical. The assay mixture contained 5 μl each of aliquot of respective sample, 30% ammonium thiocyanate and 0.1% ferrous chloride in a total volume of 200 μl in 75% ethanol. Color development was measured at 500 nm against ethanol. Percent enhancement in absorbance with respect to respective control was considered as pro-oxidant activity, while the decrease in percentage absorbance reflected antioxidant activity at a particular concentration.
Correlation analysis: Solvent effect
The solvent systems (with varying dielectric constants, viz., 80, 56.5 and 33) used for extraction were aqueous, aquo-methanolic and methanolic, respectively. A correlation analysis between mean protective potential against radiation (considering all concentrations) under different stress ± flux states and decrease in dielectric constant of solvent system, was carried out using SPSS software version 10 in an attempt to optimize the solvent system required for obtaining maximal radioprotective effect in lipid phase.
Results and Discussion
Oxygen itself is a biradical as it has two unpaired electrons. When it oxidizes another biomolecule by accepting a pair of electrons, the resultant spin of electrons is in a parallel fashion. On the other hand, electronic configuration is said to be thermodynamically more stable, if the electrons are present in an anti-parallel fashion. An unfavorable state of parallel spin (termed as “spin restriction”) forces the delay in transfer of electron, leading to slow rate of oxygen reaction.[23,24] Further, such a situation favors one-electron transfer leading to the formation of an atom/molecule with an unpaired electron called as free radical.[25] The initiation step in the formation of free radicals is the hydrolysis of water molecules by direct energy deposition during radiation flux. These free radicals, even at a very low concentration, are able to initiate lipid peroxidation.[26] It is thus a particularly pernicious phenomenon which must be fought as a priority by defense mechanisms including the action of antioxidants.[27] In the presence of physiological amplifiers (transition metals, i.e., iron and copper), oxidative stress is induced. Iron is an essential trace element and more than >60% of iron is present in the form of heme, cytochromes and metalloenzymes and stored in a complex from with transferrin or ferritin.[28] Transferrin is a plasma iron-binding glycoprotein (80 kDa) and can bind to 2 g of iron.[28] Transferrin, being a source of redox-active iron, acts as pro-oxidant, while it acts as an antioxidant by the virtue of its ion-binding capacity.[29] Radiation-induced degradation of plasma proteins leads to release of redox-active iron.
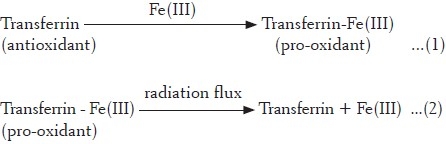
In the present study, three fractionated extracts of R. imbricata were assayed for their capacity to protect linoleic acid against the attack of peroxyl radicals in the presence of iron/copper ions and ±radiation flux. Aqueous fraction (dielectric constant = 80) exhibited a significant (P < 0.05) pro-oxidant activity in the case of untreated (–flux), which increased in a dose-dependant manner up to 100 μg/ml, beyond which a decrease in activity was observed [Table 1]. Further, the addition of Fe(II) as an inducer stress, did not cause any further increase in the pro-oxidant activity, while a gradual shift toward significant antioxidant activity (P < 0.05) at the highest concentration tested (64.66% inhibitor at 1000 μg/ml) was observed. The presence of Fe(III) (±flux) supported pro-oxidant activity, further validating the finding that the constituents exhibiting electron donation ability in lipid phase in the presence of Fe(III) act in the following manner:
Table 1.
Pro-oxidant–antioxidant activities of aqueous fraction in lipid phase

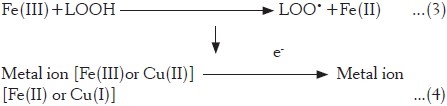
Reaction (4) is similar to the reaction occurring at physiological level, in which the free Fe(III) from transferrin or ferritin accepts an electron donated by superoxide ion (acting as pro-oxidant) and generates Fe(II) with higher oxidizing ability. Ascorbic acid, a known antioxidant,[30] in the presence of transition metal, acts as a pro-oxidant but at a lower concentration.[31–33] It indicates that the fraction also possesses a compound similar to ascorbic acid in terms of its pro-oxidant activity at specific concentrations. Such biphasic mechanisms (pro-oxidant-antioxidant) become more complex in herbal drugs and it is an outcome of a number of constituents acting in synergism/antagonism manner and these findings are further supported by our earlier observations in case of P. hexandrum.[21]
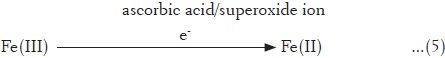
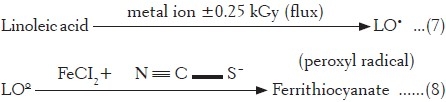
Such a transition of Fe(III) to Fe(II) amplifies the free radical flux[34] as Fe (II), redox active form of iron attacks lipids causing generation of peroxyl flux (Reaction 7). The constituents present in the fractionated extract also inducing linoleic acid degradation in the same way, thereby exhibiting increase in level of peroxyl radicals or prooxidant activity.
![]()
In the present study, such iron-induced peroxyl radicals were monitored to mimic the lipid peroxidation occurring in the living system [Reactions 7 and 8].
The antioxidant activity of aqueous fraction at 1000 μg/ml could be explained on the basis that the constituents with the ability to make coordinate complexes with metal ions have attained their bioeffective concentrations. Other workers have reported that in natural plant products containing polyphenolics,[19] antioxidant activity critically depends upon the presence of hydrogen donating constituents and these could have phenolic hydroxyl like structures whose number and position in the aromatic ring moieties of polyphenols gives them the ability to delocalize oxidant radicals and chelate transition metal ions.[35]
![]()
Aquo-methanolic fraction (dielectric constant = 56.5) exhibited a dose-dependant decrease in pro-oxidant activity in untreated (±flux) set up to 250 μg/ml [Table 2]. Above 500 μg/ml concentration, the aquo-methanolic fraction exhibited significant (P < 0.05) antioxidant activity (19.23%), which was further enhanced in the presence of Fe(II) to 36.05% and could be attributed to “metal ion chelators”, which got enriched due to decrease in dielectric constant of the solvent system. The presence of Fe(III) showed an increase in antioxidant activity, although a significant increase was not observed. This observation could be visualized as the higher level of compound possessing metal ion chelation activity as compared to the constituents having pro-oxidant activity at specific concentrations. It indicated the presence of some chain-breaking antioxidant moieties able to scavenge peroxyl radicals[36] at specific concentrations, playing a pivotal role in providing protection to lipid against the radiation flux. The ability of aquo-methanolic fraction to exhibit both antioxidant and pro-oxidant activities in a dose-dependent manner could be attributed to the presence of various metabolites, i.e., rhodioloside, rosarin, rosavin, rosin and cinnamic alcohol [Figure 1].
Table 2.
Pro-oxidant–antioxidant activity of aquo-methanolic fraction in lipid phase
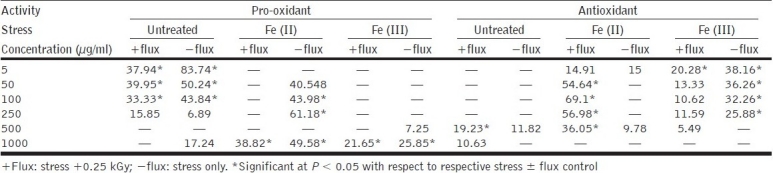
Figure 1.
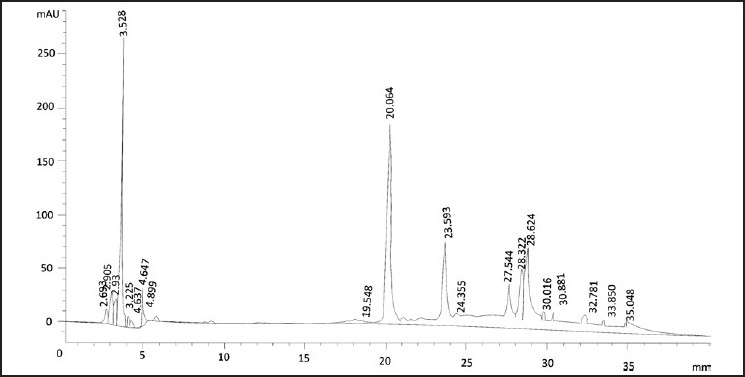
HPLC profile of aqueous methanolic extract of R. imbricata λ 340.8 nm
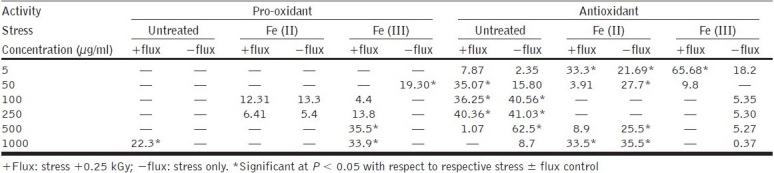
Methanolic extract (dielectric constant = 33) showed a significant (P < 0.05) increase in reductant power at all the concentrations tested as compared to the other two extracts. A dose-dependant increase in antioxidant activity up to 250 μg/ml in untreated (±flux) set was observed [Table 3]. The presence of Fe(II) promotes slight pro-oxidant activity at intermittent concentrations (100 and 250 μg/ml), and at higher concentrations (500–1000 μg/ml) it exhibited significant antioxidant activity which could be due to the metal chelation activity of flavonoids. In the case of Fe(III) + flux, a significant increase in pro-oxidant activity was observed [as compared to Fe(III) alone], indicating that loss/rate of conversion of methanol-soluble bioactive constituents, while encountering oxidant species, is rapid. Such an effect could also be due to the antagonistic interaction with other constituents or metal ion induced oxidation of the polyphenols. Polyphenols have been reported earlier to be present in R. imbricata.[19] Several workers have reported that the metal ion induced oxidation of polyphenols/flavonoids enhanced their pro-oxidant activity.[37] Flavonoids are strong chain-breaking antioxidants[38,39] in agreement with their fast reaction with lipid peroxyl radicals (antioxidant aspects), structurally attributed to di-hydroxyl groups (catechol moieties) of B-ring of basic structure of flavonoids,[40,41] while they are also able to act as chain initiators of radical-induced peroxidation (pro-oxidant aspects) and this has been reported as a flavonoid paradox.[27] Such a paradox favors the probability of fraction to act in dual manner, i.e., cytoprotective–cytotoxic in a concentration-dependant manner.
Table 3.
Pro-oxidant–antioxidant activity of methanolic fraction in lipid phase
In continuation, 10 μM of copper ions ± 0.25 kGy was also used as a stress inducer. Cu(II) and Zn(II) are the chief ions present in nucleus, serum and tissues.[42,43] Cu(II) is an essential constituent of chromatin and its levels are elevated during malignancies.[42,43] Copper ions from chromatin can be mobilized by metal chelating agents causing inter-nucleosomal DNA fragmentation, a signature mark for transformed cells undergoing apoptosis.[44] It is an essential trace element existing in two oxidation states [Cu(II) and Cu(I)], and at physiological level, it maximally remains bound to a copper-binding glycoprotein (130 kDa) called as ceruloplasmin. Ceruloplasmin has the ability to bind six or seven copper ions per molecule.[45] It also possesses ferroxidase activity and superoxide ion scavenging potential.[28,46] Radiation-mediated free radical flux acts as an initiating step in the degradation of proteins, thereby resulting in the release of free copper ions, which in the presence of pro-oxidants like superoxide ions/ascorbic acid reduce to Cu(I) state having higher oxidizing potential.[32]

In the present study, the aqueous fraction showed potent pro-oxidant activity at 2.5 μg/ml, while between 5 and 10 μg/ml, it showed antioxidant activity, beyond which no copper chelation effect was observed [Figure 2]. On the other hand, at 10 times higher concentration, i.e., 250 μg/ml, as compared to the aqueous fraction, the aquo-methanolic fraction exhibited significant pro-oxidant activity [Figure 3]. Beyond this concentration, a dose-dependant reduction in peroxyl flux was recorded. In the case of methanolic fraction [Figure 4] , antioxidant activity was found to increase in a concentration-dependant manner (50–500 μg/ml), indicating their switch over to antioxidant species enrichment with the usage of methanolic system.
Figure 2.
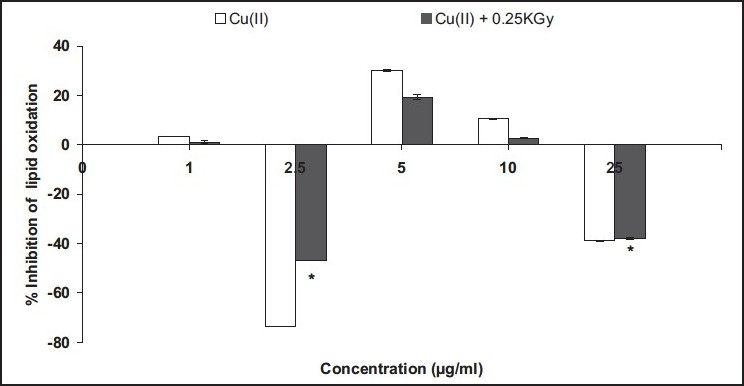
Antioxidant activity (in lipid phase) of aqueous fraction in linoleic acid pre-emulsion assay system. Cu (10 μM) was used to induce lipid peroxidative stress in combination with 0.25 kGy. %Inhibition of lipid oxidation was evaluated and compared to control (0% inhibition); *significant at P < 0.05 vs. control
Figure 3.
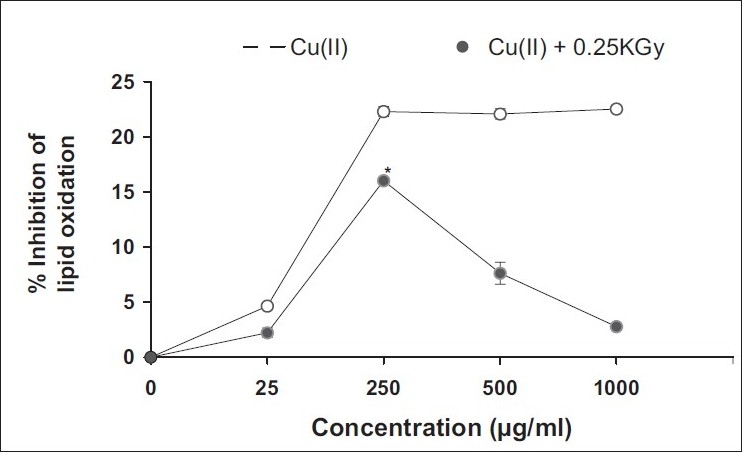
Antioxidant activity (in lipid phase) of aquo-methanolic fraction in linoleic acid pre-emulsion assay system. Cu (10 μM) was used to induce lipid peroxidative stress in combination with 0.25 kGy. %Inhibition of lipid oxidation was evaluated and compared to control (0% inhibition); *significant at P < 0.05 vs. control
Figure 4.
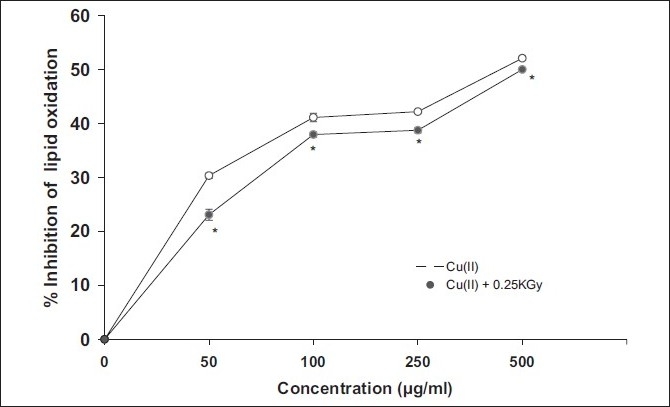
Antioxidant activity (in lipid phase) of methanolic fraction in linoleic acid pre-emulsion assay system. Cu (10 μM) was used to induce lipid peroxidative stress in combination with 0.25 kGy. %Inhibition of lipid oxidation was evaluated and compared to control (0% inhibition); significant at P < 0.05 vs. control
The pro-oxidant activity in the presence of copper ions could be attributed to either generation of Cu(I) by electron donation or copper ion mediated oxidation of active constituents to more pro-oxidant ones. On the other hand, methanol-soluble bioactive constituents could attribute antioxidant activity to chelation effect caused by polyphenolics or due to the direct scavenging of peroxyl radicals. It has been widely accepted that natural plant products like gallo-tannins such as epi-gallocatechin-3-gallate and gallic acid along with polyphenolics like curcumin and reservatrol were able to induce apoptosis in cancer cell lines, but not in normal cells.[47–50] Such differential anticancerous activity was mediated by mobilization of endogenous cooper ions and induction of copper-mediated oxidative damage to DNA of cancer cells.[51–54] The ability of fractions to act in differential ways against transition metals or radiation stresses indicates the probable role in achieving differential radioprotection in mammalian system, although it warrants further investigation. Recent studies on R. imbricata reporting on its immune-potentiating,[14,15] anticancerous[16] and wound healing abilities[17] support the observations of the present study to develop these fractions as potential safe prophylactics for biological radioprotection to first responders.
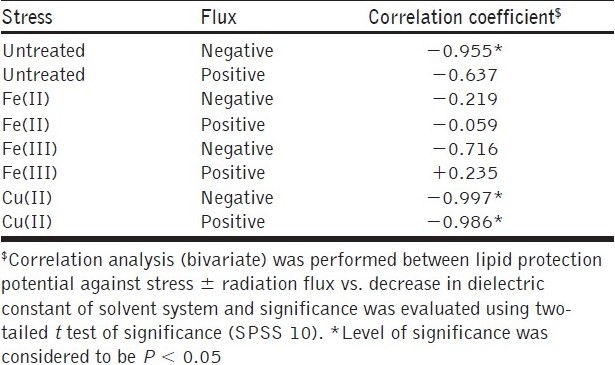
Further, the effect of solvent system on pro- and antioxidant activity was also studied and the correlation analysis also validated the above findings. A decrease in dielectric constant of solvent system exhibited a significant (P < 0.05) negative correlation (r = 0.955) with radioprotective efficacy, clearly indicating a shift from pro-oxidant to antioxidant activity. This finding was found to be in coherence with the higher antioxidant activity, which was exhibited by methanol-soluble bioactive constituents in all the cases of peroxidative stress. In case of Fe (III) + flux, induction of pro-oxidant activity was found to be positively correlated with decrease in dielectric constant. It indicates the possibility metal ion-induced oxidation of the constituents (soluble in methanol) leading to shift in their activity pattern towards prooxidant activity [Table 4]. In view of its biphasic disparate biological activity, it could be useful for cancer therapy and radioprotection. Further dose optimization studies are underway for achieving differential radioprotective efficacy in mammalian model system.
Table 4.
Effect of variation in dielectric constant of solvent system on radioprotective efficacy
Conclusion
We conclude that the R. imbricata is a potential prophylactic agent that can be used effectively as a biological radioprotector in nuclear and radiological emergencies. Previous studies indicate its nontoxicity in animal systems, revealing its safe usage up to significantly higher doses. Further studies are warranted to develop various formulations and comparative analysis of survival against lethal gamma radiation exposure in animal system.
Acknowledgments
Thanks are due to Director, INMAS, former and present Directors of Defence Institute of High Altitude Research (DIHAR), Leh, for facilitating the research activities and kindly providing the plant material of R. imbricate, respectively. This work was supported by research funds obtained from the Defence Research and Development Organization's thrust program on development of novel radiation countermeasure agents.
Footnotes
Source of Support: DRDO
Conflict of Interest: None declared.
References
- 1.Prasad KN. Rationale for using multiple antioxidants in protecting humans against low doses of radiation. Br J Radiat Biol. 2005;78:485–92. doi: 10.1259/bjr/87552880. [DOI] [PubMed] [Google Scholar]
- 2.Venkatachalam SR, Chattopadhyay S. Natural radioprotective agents: An overview. Curr Org Chem. 2005;9:389–404. [Google Scholar]
- 3.Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, et al. Radioprotection by plant products: Present status and Future prospects. Phytother Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 4.Chawla R, Arora R, Kumar R, Sharma A, Prasad J, Singh S, et al. Antioxidant activity of fractionated extracts of rhizomes of high-altitude Podophyllum hexandrum: Role in radiation protection. Molecul Cell Biochem. 2005;273:193–208. doi: 10.1007/s11010-005-0821-5. [DOI] [PubMed] [Google Scholar]
- 5.Brown RP, Gerbarg PL, Ramazanov Z. Rhodiola rosea: A phytomedicinal overview. Herbal Gram. 2002;56:40–52. [Google Scholar]
- 6.Ohwi J. Flora of Japan. Washington D.C: Smithsonian Institution; 1984. p. 495. [Google Scholar]
- 7.Narr H. Doctoral Dissertation, Faculty of Chemistry and Pharmacy. Munchen, Munchen, Germany: Ludwig-Maxmillans University; 1993. Phytochemical and Pharmacological investigation of the adaptogens: Eleutherococcus senticosus, Ocimum sanctum, Codonopsis pilosula, Rhodiola crenulata. [Google Scholar]
- 8.Saratikov AS, Krasnov EA. Rhodiola rosea is a valuable medicinal plant (golden root) Tomsk, Russia: Tomsk State University Press; 1987. pp. 194–215. [Google Scholar]
- 9.Germano C, Ramazanov Z, Bernal Suarez M. Arctic Root (Rhodiola rosea): The Powerful New Ginseng Alternative. New York, NY: Kensington Publishing Corp; 1999. [Google Scholar]
- 10.Ganzera M, Yayla Y, Khan IA. Analysis of the marker compounds of Rhodiola rosea L. (golden root) by reversed phase high performance liquid chromatography. Chem Pharm Bull. 2001;49:465–7. doi: 10.1248/cpb.49.465. [DOI] [PubMed] [Google Scholar]
- 11.Kanupriya, Prasad D, Sairam M, Kumar R, Sawhney RC, Sharma SK, et al. Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide injury in U-937 human macrophages. Mol Cell Biochem. 2005;275:1–6. doi: 10.1007/s11010-005-7637-1. [DOI] [PubMed] [Google Scholar]
- 12.Gupta V, Lahiri SS, Sultana S, Kumar R. Mechanism of action of Rhodiola imbricata Edgew during exposure to cold, hypoxia and restraint (C-H-R) stress induced hypothermia and post stress recovery in rats. Food Chem Toxicol. 2009;47:1239–45. doi: 10.1016/j.fct.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Gupta V, Lahiri SS, Sultana S, Tulsawani RK, Kumar R. Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C-H-R) exposure and post-stress recovery. Food Chem Toxicol. 2010;48:1019–25. doi: 10.1016/j.fct.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Mishra KP, Ganju L, Chanda S, Karan D, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome stimulates Toll-like receptor 4, granzyme-B and Th1 cytokines in vitro. Immunobiology. 2008;214:27–31. doi: 10.1016/j.imbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Mishra KP, Chanda S, Shukla K, Ganju L. Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on the immune responses to tetanus toxoid and ovalbumin in rats. Immunopharmacol Immunotoxicol. 2010;32:141–6. doi: 10.3109/08923970903503668. [DOI] [PubMed] [Google Scholar]
- 16.Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, Banerjee PK, et al. Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiology. 2008;213:125–31. doi: 10.1016/j.imbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Kumar R, Upadhyay NK, Pal K, Kumar R, Sawhney RC. Effects of Rhodiola imbricata on dermal wound healing. Planta Med. 2007;73:774–7. doi: 10.1055/s-2007-981546. [DOI] [PubMed] [Google Scholar]
- 18.Coleman NE, Blakely WF, Fike JR, Mac Vittie TJ, Metling NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1-10Gy) radiation and potential mechanisms of radiation protection: Report of a workshop (Bethesda, Maryland, USA, December, 17-18, 2001) Radiat Res. 2003;159:812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 19.Arora R, Chawla R, Sagar R, Prasad J, Singh S, Kumar R, et al. Evaluation of radioprotective activities of Rhodiola imbricata Edgew - A high altitude plant. Molecul Cell Biochem. 2005;273:209–23. doi: 10.1007/s11010-005-0822-4. [DOI] [PubMed] [Google Scholar]
- 20.Asamari AM, Addis PB, Epley RJ, Krick TP. Wild rice hull antioxidants. J Agric Food Chem. 1996;44:126–30. [Google Scholar]
- 21.Chawla R, Arora R, Puri SC, Sagar RK, Singh S, Kumar R, et al. 3-O-β-D- galactopyranoside of quercetin as an active principle from high altitude Podophyllum hexandrum and evaluation of its radioprotective properties. Z Naturforsch C. 2005;60:728–38. doi: 10.1515/znc-2005-9-1012. [DOI] [PubMed] [Google Scholar]
- 22.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 23.Floyd RA. CRC Press. Boca Raton, Florida: CRC Press; 1993. Free Radicals in Aging; pp. 39–55. [Google Scholar]
- 24.Pryor WA. Free Radicals in Molecular Biology, Aging an Diseases. New York: Raven Press; 1984. pp. 13–42. [Google Scholar]
- 25.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–80. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 26.Diplock AT. Free Radical Damage and Its Control, New Comprehensive Biochemistry. Vol. 28. Amsterdam: Elsevier; 1994. p. 113. [Google Scholar]
- 27.Dangles O, Dufour, Fargeix G. Inhibition of lipid per oxidation by quercetin and derivatives: Antioxidant and prooxidant effects. J Chem Soc Perkin Trans. 2000;2:1215–22. [Google Scholar]
- 28.Boyer RF, Schori BE. The incorporation of iron into apo-ferritin as mediated by ceruloplasmin. Biochem Biophys Res Commun. 1983;116:244–50. doi: 10.1016/0006-291x(83)90407-2. [DOI] [PubMed] [Google Scholar]
- 29.Osaki S, Johnson DA, Frieden E. The significance of the ferrous oxidase activity of Ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–51. [PubMed] [Google Scholar]
- 30.Frei B. Ascorbic acid protects lipid in human plasma and LDL against oxidative damage. Am J Cln Nutr. 1991;54:11113S–8. doi: 10.1093/ajcn/54.6.1113s. [DOI] [PubMed] [Google Scholar]
- 31.Beuttner GR, Jurkiewicz BA. Catalytic metals, Ascorbate and free radicals: Combinations to Avoid. Rad Res. 1996;145:532–41. [PubMed] [Google Scholar]
- 32.Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical reactions. Free Radical Biol Med. 1985;21:3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 33.Girotti AW. Mechanisms of lipid per oxidations. Free Radical Biol Med. 1985;1:87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 34.Aruoma OI, Halliwell B. Superoxide dependent and ascorabte-dependant formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl ion generation? Biochem J. 1985;241:273–8. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans CR. Antioxidant Food Supplements in Human Health. San Diego, CA(USA): Academic Press; 1999. pp. 239–53. [Google Scholar]
- 36.Rosa A, Deiana M, Corona G, Atzeri A, Incani A, Appendino G. Antioxidant properties of extracts and compounds from Psoralea morisiana. Eur J Lipid Sci Technol. 2005;107:521–9. [Google Scholar]
- 37.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol in Vitro. 2003;18:555–61. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Harborne JB. The Flavonoids, Advances in Research Since 1986. London: Chapman and Hall; 1994. [Google Scholar]
- 39.Pedrielli P, Pedulli GF, Skibsted LH. Antioxidant Mechanism of Flavonoids.Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J Agric Food Chem. 2001;49:3034–40. doi: 10.1021/jf010017g. [DOI] [PubMed] [Google Scholar]
- 40.Bors W, Michel C, Stettmaier K, Heller W. Health Effects and Applications. Champaign: AOCS Press; 1997. Natural Antioxidants: Chemistry; pp. 346–57. [Google Scholar]
- 41.Yamasaki H, Sakihama Y, Ikehara N. Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol. 1997;115:1405–12. doi: 10.1104/pp.115.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebadi E, Swanson S. The status of zinc, copper, and metallothionein in cancer patients. Prog Clin Biol Res. 1988;259:161–75. [PubMed] [Google Scholar]
- 43.Yoshida D, Ikeda Y, Nakazawa S. Quantitative analysis of copper, zinc, and copper/zinc ratio in selected human brain tumours. J Neurooncol. 1993;16:109–15. doi: 10.1007/BF01324697. [DOI] [PubMed] [Google Scholar]
- 44.Burkitt MJ, Milne L, Nicotera P, Orrenius S. 1,10-Phenathroline stimulates inter-nucleosomal DNA fragmentation in isolated rat liver nuclei by promoting redox activity of endogenous copper ions. Biochem J. 1996;313:163–9. doi: 10.1042/bj3130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutteridge JM, Stocks J. Ceruloplasmin: Pharmacological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14:257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- 46.Gutteridge JM. Lipid per oxidation initiated by superoxide ion dependant hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984;172:245–9. doi: 10.1016/0014-5793(84)81134-5. [DOI] [PubMed] [Google Scholar]
- 47.Inoue M, Suzuki R, Koide T. Antioxidant, gallic acid induces apoptosis in HL60RG cells. Biochem Biophys Res Commun. 1994;204:898–904. doi: 10.1006/bbrc.1994.2544. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad N, Feyes DK, Nieminen Al. Green Tea constituents epigallocatechin –3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Nat Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 49.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signalling-dependant apoptosis in human tumour cells. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 50.Piwocka K, Zablocki K, Weichowski MR. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp Cell Res. 1999;249:299–307. doi: 10.1006/excr.1999.4480. [DOI] [PubMed] [Google Scholar]
- 51.Mukhtar H, Das M, Khan WA. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethyl benz(a) anthracene-benzo (a) pyrene-, 3-methyl cholanthrene-and N-methyl-N-nitrsourea-induced skin tumorigenesis in mice. Cancer Res. 1988;48:2361–5. [PubMed] [Google Scholar]
- 52.Khan NS, Hadi SM. Structural features of tannic acid important for DNA degradation in the presence of Cu(II) Mutagenesis. 1998;13:271–4. doi: 10.1093/mutage/13.3.271. [DOI] [PubMed] [Google Scholar]
- 53.Singh S, Asad SF, Ahmed A, Khan NU, Hadi SM. Oxidative DNA damage by capasaicin and dihydro-capsaicin in the presence of Cu(II) Cancer Lett. 2001;169:139–46. doi: 10.1016/s0304-3835(01)00544-4. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad A, Asad SF, Singh S, Hadi SM. DNA breakages by resveratrol and Cu(II): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000;154:29–37. doi: 10.1016/s0304-3835(00)00351-7. [DOI] [PubMed] [Google Scholar]


