Abstract
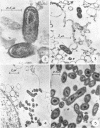
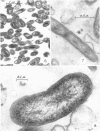
Anacker, R. L. (Rocky Mountain Laboratory, Hamilton, Mont.), K. Fukushi, E. G. Pickens, and D. B. Lackman. Electron microscopic observations of the development of Coxiella burnetii in the chick yolk sac. J. Bacteriol. 88:1130–1138. 1964.—Yolk sac material, obtained daily over a period of 1 week from embryos inoculated with seed of phase I Coxiella burnetii strain Ohio 314 containing 250 units of penicillin, was examined by electron microscopy and other techniques for the presence of rickettsiae. The concentration of rickettsiae in the yolk sac, as determined by electron microscopy, light microscopy, the complement-fixation test, recovery of organisms, and mouse infectivity, was low for the first 3 days, increased rapidly 3 to 5 days after infection, and then remained relatively constant. Rickettsiae in 3- to 7-day cultures, when observed by electron microscopy, had dense fibrillar centers surrounded by less-dense cytoplasmic material containing granules approximately 15 mμ in diameter. The whole was enclosed by multiple external layers. Many appeared to be in various stages of binary fission, and one form which contained a cross-wall was observed. These forms readily combined with ferritin-labeled specific antibody. In rare instances, several kinds of ”atypical” forms which did not combine with ferritin-labeled antibody were found in the cytoplasm of yolk-sac cells 4 to 5 days after inoculation; it is not certain whether these forms are artifacts or normal stages in the maturation of C. burnetii. These atypical forms were not observed in subsequent experiments in which embryonated eggs were inoculated with doses of penicillin varying from 0 to 4,000 units per egg.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES G. A., MORGAN C., HSU K. C., RIFKIND R. A., SEEGAL B. C. Electron microscopic studies of experimental nephritis with ferritin-conjugated antibody. The basement membranes and cisternae of visceral epithelial cells in nephritic rat glomeruli. J Exp Med. 1962 May 1;115:929–936. doi: 10.1084/jem.115.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZEMAN F. M., HOPPS H. E., DANAUSKAS J. X., JACKSON E. B., SMADEL J. E. Study on the growth of Rickettsiae. I. A tissue culture system for quantitative estimations of Rickettsia tsutsugamushi. J Immunol. 1956 Jun;76(6):475–488. [PubMed] [Google Scholar]
- CARO L. G., JACKSON E. B., SMADEL J. E., WISSIG S. L. Electron microscopic observations on intracellular rickettsiae. Am J Pathol. 1956 Nov-Dec;32(6):1117–1133. [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISET P., SMITH K. M., STOKER M. G. Internal structure of Rickettsia burnetii as shown by electron microscopy of thin sections. J Gen Microbiol. 1956 Dec;15(3):632–635. doi: 10.1099/00221287-15-3-632. [DOI] [PubMed] [Google Scholar]
- FREEMAN J. A., SPURLOCK B. O. A new epoxy embedment for electron microscopy. J Cell Biol. 1962 Jun;13:437–443. doi: 10.1083/jcb.13.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORDOVA N. Filterable particles of Coxiella burneti. Acta Virol. 1959 Jan;3(1):25–36. [PubMed] [Google Scholar]
- KORDOVA N., REHACEK J. Experimental infection of ticks in vivo and their organs in vitro with filterable particles of Coxiella burneti. Acta Virol. 1959 Oct;3:201–209. [PubMed] [Google Scholar]
- KRAVCHENKO A. T., GUDINA O. S., MULIUTIN V. N. [A study of the effect of antibiotics and specific sera on the development of viruses and rickettsia in tissue culture with the use of microcinematography. I. The effect of penicillin on psittacosis virus and Rickettsia burneti in tissue culture]. Vopr Virusol. 1961 May-Jun;7:300–306. [PubMed] [Google Scholar]
- LUOTO L. A capillary agglutination test for bovine Q fever. J Immunol. 1953 Oct;71(4):226–231. [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., LACKMAN D. B., PICKENS E. G. Relationships among complement-fixing values, infectious endpoints, and death curves in experimental Coxiella burnetii infection. J Immunol. 1951 Oct;67(4):257–264. [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG M., KORDOVA N. Multiplication of Coxiella burneti in Detroit-6 cell cultures. An electron microscope study. Acta Virol. 1962 Mar;6:176–180. [PubMed] [Google Scholar]
- ROSENBERG M., KORDOVA N. Study of intracellular forms of Coxiella burneti in the electron microscope. Acta Virol. 1960 Jan;4:52–55. [PubMed] [Google Scholar]
- SCHAECHTER M., BOZEMAN F. M., SMADEL J. E. Study on the growth of Rickettsiae. II. Morphologic observations of living Rickettsiae in tissue culture cells. Virology. 1957 Feb;3(1):160–172. doi: 10.1016/0042-6822(57)90030-2. [DOI] [PubMed] [Google Scholar]
- SIDWELL R. W., GEBHARDT L. P. Q fever antibody response in experimentally infected wild rodents and laboratory animals. J Immunol. 1962 Sep;89:318–322. [PubMed] [Google Scholar]
- TAJIMA M., NOMURA Y., KUBOTA Y. Structure and development of viruses of the psittacosis-lymphogranuloma group observed in the electron microscope. J Bacteriol. 1957 Nov;74(5):605–620. doi: 10.1128/jb.74.5.605-620.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAJIMA M., SAMEJIMA T., NOMURA Y. Morphology of meningopneumonitis virus exposed to penicillin as observed with the electron microscope. J Bacteriol. 1959 Jan;77(1):23–34. doi: 10.1128/jb.77.1.23-34.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAWDE S. S., RAM J. S. Conjugation of antibody to ferritin by means of p,p'-difluoro-m, m'-dinitrodiphenylsulphone. Arch Biochem Biophys. 1962 May;97:429–430. doi: 10.1016/0003-9861(62)90102-9. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]