Abstract
Gastric volvulus has been documented in several species of animals and is associated with high morbidity and mortality. We report 2 cases of gastric volvulus in guinea pigs that died without detection of prior clinical signs. Both guinea pigs were adult female guinea pigs in a breeding colony and had given birth to multiple litters; one was pregnant at the time of death. Gastric rotations of 540° and 360° were identified at necropsy examination. These cases include the first known report of gastric rotation greater than 360° in any species. Although gastric volvulus has been reported to occur in guinea pigs, little is known about its risk factors, etiology, and pathogenesis. We conducted a literature review to compare gastric volvulus between guinea pigs and other species.
Gastric volvulus occurs sporadically in many species but is reported most frequently in canines, humans, and swine. In most species gastric volvulus is associated with high morbidity and mortality.3,21,27-29 The rotation of the stomach results in functional obstruction of the esophageal and pyloric sphincters and is associated with pronounced gastric distention with gas. Physiologic consequences of gastric volvulus can be severe. Canines may experience hypovolemic shock, gastric perforation, splenic torsion, disseminated intravascular coagulation, cardiac arrhythmias, and death as a result of the condition.30 The mortality rate of acute gastric volvulus treated medically (without surgery) in humans is reported to be as high as 80%, and even with appropriate treatment, mortality remains high, at 30% to 50%.16 Spontaneous cases of gastric volvulus occasionally develop in guinea pigs and most often present as acute death.8,15,17 Here we describe 2 cases of fatal gastric volvulus in guinea pigs; and review risk factors, etiology, and clinical aspects of the condition, and compare the condition in guinea pigs with that in other species.
Case Report
Two cases of gastric volvulus occurred over a 3-y period in a Hartley guinea pig (Hilltop Lab Animals, Scottsdale, PA) breeding colony maintained in an AAALAC-accredited facility. Guinea pigs were negative for common pathogenic agents on arrival at the facility, including lymphocytic choriomeningitis virus, pneumonia virus of mice, reovirus 3, Sendai virus, Encephalitozoon cuniculi, mouse adenovirus, and Bordetella bronchiseptica. On average, 36 adult guinea pigs were housed in the facility daily, at a ratio of approximately 4 female to 1 male guinea pig. All animals were assigned to a breeding protocol approved by Wright State University's Laboratory Animal Care and Use Committee and housed under constant environmental conditions (temperature, 70° ± 1 °F (21.1 ± 0.6 °C); humidity, 40% to 60%; 12:12-h light:dark cycle). Guinea pigs were housed in solid-bottom cages on corncob bedding (Harlan Teklad, Madison, WI), with complete bedding changes and cage sanitization 3 times weekly. Water and a pelleted diet (7006.15, Harlan Teklad) were available ad libitum, and jointed sections of PVC pipes (diameter, 5 in.) were provided as cage enrichment. A polygamous breeding system was used, housing 1 male with as many as 4 female guinea pigs, and adults were weighed at least monthly. Pregnant female guinea pigs were removed from group housing approximately 1 wk prior to parturition and remained with the litter until weaning.
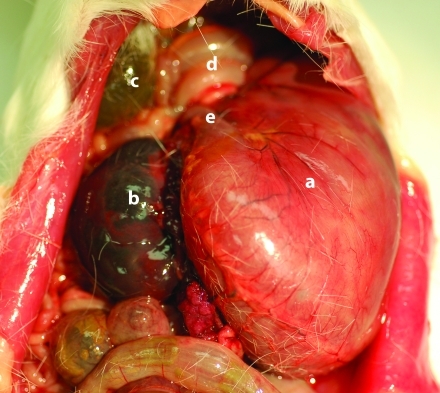
No symptoms were noted prior to death in either guinea pig. The animal in case 1 was an 11-mo-old nongravid female guinea pig that died while housed with 1 male and 1 female guinea pig. The guinea pig had given birth to 2 previous litters without incident. During necropsy examination, a 540° clockwise rotation of the stomach was present, resulting in esophageal and pyloric obstruction, gastric distention, and reversal of the anatomic position of the spleen and pylorus (Figure 1). Additional abnormalities included severe splenomegaly, gallbladder dilation, mild hyperemia of the serosal stomach surface, and mild serous ascites. A loss of 9% body weight had occurred within the 7 d prior to death.
Figure 1.
540° clockwise rotation of the stomach (a) with accompanying splenomegaly (b) and gallbladder dilation (c). The twisted duodenum (d) and esophagus (e) are in close proximity.
The animal in case 2 was an 18-mo-old female housed with 1 male and 2 female guinea pigs. The guinea pig had given birth to 3 previous litters and was approximately 3 wk pregnant at the time of death. A 360° clockwise rotation and moderate gaseous distention of the stomach were noted at necropsy. Other abnormalities included mild splenomegaly and gallbladder dilation. A weight loss equivalent to 30% of body weight had occurred in the 7 d prior to death. Five fetuses were present, and their death was presumed to be secondary to maternal death. Moderate autolysis of all tissues had occurred.
Discussion
Gastric volvulus is well documented in humans and canines and is reported sporadically in numerous other species, including monkeys, cats, and swine.4-6,21,29 First described in 1904,2 the classical triad of symptoms associated with acute gastric volvulus in humans includes gastric distention and upper abdominal pain, nonproductive retching, and difficulty or inability to pass a tube into the stomach. A chronic form of gastric volvulus occurs in humans and may be asymptomatic or associated with upper abdominal pain, nausea, and early satiety.1,16,18,25,28
By comparison, significant gas dilation accompanies rotation of the stomach in dogs, and the condition is referred to as gastric dilatation–volvulus. Overt clinical signs of gastric dilatation–volvulus in dogs include abdominal distention and pain and nonproductive retching. In addition, the dilated stomach of affected dogs causes respiratory difficulty and impedes venous blood return to the heart, leading to dyspnea, hypotension, and cardiovascular shock.3,20,30
Sudden death is the most common clinical presentation of gastric volvulus in swine.21,29 Clinical signs reported in swine have included anorexia, abdominal distention, respiratory compromise, and hypersalivation.21 Clinical signs of gastric volvulus reported in cats include acute-onset respiratory distress, abdominal distention, anorexia, and vomiting.6 Sudden death is the most common clinical presentation of gastric volvulus in laboratory guinea pigs; however, clinical signs have been reported to include dyspnea, cyanosis, tachycardia, and abdominal distention with an absence of peristaltic sounds.8,17,19 One of the current authors (ED) diagnosed gastric volvulus through necropsy in a pet guinea pig exhibiting clinical signs of lethargy, anorexia, and lack of feces. Lethargy, complete anorexia, and lack of feces were the presenting clinical signs in another 2 reported cases of gastric volvulus in pet guinea pigs.19,31 This apparent difference in clinical signs of gastric volvulus in laboratory-reared and privately owned guinea pigs may be due to the ability to monitor appetite and fecal production in individual pets, in contrast with guinea pigs housed in the laboratory under colony conditions. Table 1 summarizes the commonly reported clinical signs of acute gastric volvulus by species.
Table 1.
| Canines | Humans | Swine | Felines | Guinea pigs | |
| Gastric or abdominal distention | + | + | + | + | + |
| Respiratory distress | + | – | + | + | + |
| Upper abdominal pain | + | + | – | – | – |
| Nonproductive retching | + | + | – | – | – |
| Anorexia | – | – | + | + | + |
| Lack of Feces | – | – | – | – | + |
| Sudden death | – | – | + | – | + |
| Hypersalivation | + | – | + | – | – |
In many cases, gastric volvulus is strongly suspected based on clinical signs alone, and diagnostic imaging is commonly used to confirm the condition. Survey radiography may be helpful in diagnosing gastric volvulus in humans; confirmation is often based on barium studies, computed tomography scan, or laparotomy.16,18,25 Diagnosis of gastric dilatation–volvulus in dogs is confirmed radiographically with a right lateral recumbent view. When a 180° gastric volvulus is present, the pylorus is displaced dorsally and to the left, and the stomach has a typical ‘double bubble’ appearance due to the relatively narrow and tubular shape of the gas-filled pylorus.20,30 In addition, radiography has been effective during diagnosis of gastric volvulus in cats.6 Previous reports of gastric volvulus in guinea pigs have indicated varying degrees of rotation to 360°,14,17,19,31 and one case in the current report involved a 540° rotation. The radiographic appearance of gastric volvulus varies with the degree of rotation present; however, a gas-distended structure often filling more than 50% of the abdominal cavity typically is present. Gastric volvulus should be considered based on this radiographic finding in a guinea pig exhibiting symptoms consistent with the condition.19,31 Confirmation of gastric volvulus could occur through exploratory surgery or postmortem examination, if death occurs.
Acute and chronic forms of gastric volvulus occur in humans, and the etiology most often is attributed to anatomic abnormalities.1,16,18,28 Cases are classified as approximately 30% primary, caused by abnormal lengthening of gastric ligaments, and 70% secondary, associated with congenital or acquired upper abdominal defects.1,16,18,28 Defects commonly predisposing to secondary gastric volvulus include hernias (paraesophageal, diaphragmatic) or eventrations—elevations of the diaphragm resulting in thinning and atrophy.1,16,28 These congenital defects are more common in males, accounting for the higher incidence of gastric volvulus in males than females.28 Dietary and behavioral risk factors have not been identified and are not believed to play a role in the development of gastric volvulus in humans. Chronic recurring gastric volvulus develops in humans when the torsed stomach spontaneously corrects, and this form of the condition is likely more common than is the acute form.18
Gastric volvulus in cats similarly is associated with anatomic abnormalities, most often trauma-related.6 One group of authors reported 3 cases of gastric volvulus in cats with recent histories of being hit by a car.6 Gastric volvulus was diagnosed 24 h to 6 mo after the traumas occurred, and diaphragmatic hernias or diaphragmatic lacerations were present in all cases.6
The pathogenesis of gastric dilatation–volvulus in dogs is unknown; however, many risk factors have been identified. In the dog, most risk factors are categorized as anatomic, dietary, or physiologic. Anatomic or physiologic risk factors include large-size breed, high thoracic depth-to-width ratio, older age, and underweight body condition.9-11,24,26 Sex was not found to be a risk factor.10,26 Dietary-related risk factors include feeding a single meal daily, feeding large meals,11,23 feeding a diet that lists a fat or oil as 1 of the first 4 ingredients,22 and feeding a diet with kibble size less than 30 mm.26 Cereal-based diets have been excluded as risk factors contributing to the development of gastric dilatation–volvulus, and no link between level of dietary carbohydrates and the condition has been identified.22,23 Dogs that are fed by using a raised food bowl were found to have an increased risk,10 however rapid eating behavior has not consistently been shown to increase the risk of developing gastric dilatation–volvulus in dogs.10,11,23,26 Although disorders of gastric motility have been suspected to be risk factors for gastric volvulus in dogs, this remains unproven.13,20 Stress appears to have a role in the development of gastric dilatation–volvulus, in that dogs described by their owners as having a ‘happy’ personality were at lower risk of developing gastric dilatation–volvulus, whereas dogs experiencing a stressful event (such as boarding, grooming, or veterinary exams) were at higher risk.9-11
Gastric torsion in swine has been associated with feeding practices that encourage the intake of large amounts of food and water very rapidly.21,29 One group of authors reported 14 cases of gastric torsion in sows and suggested that feeding large amounts of food every 24 to 48 h was an inciting cause.21 Excessive excitement at feeding time is suspected of being a possible factor of the development of gastric torsion in swine;21,29 however, such infrequent feeding schedules may cause stress, which leads to the development of gastric volvulus.
The pathogenesis of gastric volvulus in guinea pigs is unknown, although the condition has been associated with breeding.8,15,17 One report of 6 cases in a breeding colony of guinea pigs did not report the sex of the affected animals,17 another report involved a single case of the condition in a nonpregnant female breeder,15 and another case also involved a female guinea pig, although breeding history was not mentioned.31 One group of investigators reported 5 cases of gastric volvulus that occurred within 1 y in a breeding colony of complement-4-deficient guinea pigs8 and postulated that the colony's feeding behavior (consuming large amounts of dry food and water within a short time) predisposed the guinea pigs to the condition. A recent report suggested that gastric volvulus may develop secondary to conditions resulting in delayed gastric emptying, such as gastric stasis disorder.19 Gastric stasis typically occurs secondary to anorexia or pain, and guinea pigs are predisposed by dental disease and a diet deficient in fiber.12 Clinical signs of gastric stasis are similar to those reported for gastric volvulus and often include anorexia, reduced or absent fecal material, abdominal pain, and reduced or absent gut sounds.12 One case of gastric volvulus occurred in the acute postoperative period after placement of a jugular venous cannula in a pregnant guinea pig.14 The guinea pig was anesthetized with ketamine and xylazine and was not fasted prior to surgery. The authors hypothesized that xylazine's inhibitory effect on gastrointestinal motility, the weight of the ingesta-filled stomach, and the frequent positional changes during the procedure contributed to the volvulus.14 In addition, stress may be a risk factor for the development of gastric volvulus in guinea pigs, as it is in dogs. Intensive breeding systems commonly used in laboratory guinea pig colonies may be physiologically stressful and contribute to gastric volvulus development.
Comparative pathogenesis of gastric volvulus demonstrates both similarities and differences. Table 2 summarizes the etiology and risk factors associated with the development of gastric volvulus by species. Humans and cats develop the condition most commonly due to diaphragmatic defects or other anatomic abnormalities.1,6,16,18,28 Although the shape of the thoracic cavity affects the development of gastric volvulus in dogs, anatomic abnormalities are not known to be associated with the condition in dogs, swine, or guinea pigs. In these species, risk factors include physiologic and dietary habits.3,8-11,13,17,20-24,26,29 Feeding practices including meal size and frequency are associated with gastric volvulus development in dogs and pigs.3,11,20,21,23,29 Diet composition has been found to be a risk factor for gastric volvulus in dogs only;11,20,22,23,26 however, it has not been thoroughly evaluated as a risk factor in other species. Physiologic factors including advanced age and malnourishment in dogs and breeding status in guinea pigs also increase the risk of developing gastric volvulus.9-11,15,17,20 In addition, stress, rapid ingestion of large amounts of food and water, and gastric stasis may be risk factors in guinea pigs.15,19
Table 2.
Etiology and risk factors in species in which gastric volvulus has been documented1,6,8-11,14-18,21-24,26,28,29,31
| Canines | Humans | Swine | Felines | Guinea pigs | ||
| Anatomic | ||||||
| Diaphragmatic abnormality | – | + | – | + | – | |
| Gastric ligament abnormality | – | + | – | – | – | |
| Thoracic depth:width | + | – | – | – | – | |
| Dietary | ||||||
| Composition | + | – | – | – | – | |
| Meal size | + | – | ± | – | – | |
| Eating frequency | + | – | ± | – | – | |
| Eating speed | ± | – | ± | – | ± | |
| Physiologic | ||||||
| Stress | + | – | ± | – | ± | |
| Breeding | – | – | – | – | + | |
| Sex | – | + | – | – | + | |
| Gastric motility disorder | – | – | – | – | ± | |
| Age | + | – | – | – | – | |
| Malnourishment | + | – | – | – | – | |
+ Confirmed or well-documented etiology or risk factor; ±, suspected etiology or risk factor, −, not associated as etiology or risk factor
The 2 cases of gastric volvulus that we describe here occurred in a breeding colony of guinea pigs. The affected animals were group-housed mature female breeders, and although only one was pregnant at the time of gastric volvulus, each had successfully delivered multiple pregnancies in the past. The gastric ligaments were lax due to the current state of gastric volvulus, but whether this laxity was present prior to volvulus was unknown. Neither guinea pig had any physical defect that could have predisposed it to developing the condition. Published cases of gastric volvulus in guinea pigs have affected female breeders (both pregnant and nonpregnant), with the exception of a single case in a complement-4-deficient male8 and one case in a 3-y-old female with no history of pregnancy.19 We observed this sex-associated predilection as well, although our facility houses more female than male guinea pigs. The relatively high incidence in female breeding guinea pigs suggests a causal relationship between pregnancy or postpregnancy and the development of gastric volvulus. Speculating on the cause of gastric volvulus, it is noteworthy that there are no reports of the condition in more commonly used laboratory rodents such as rats or mice. Unlike rat and mouse pups, guinea pig pups are born precocious, with the weight of the gravid uterus reported to be as much as 50% of the dam's total body weight.7 Under these circumstances, displacement of abdominal organs during late pregnancy is inevitable. Gastric displacement during late pregnancy may result in lengthening of gastric ligaments and increased gastric mobility, both of which conditions predispose guinea pigs to developing gastric volvulus.
In conclusion, we here report 2 cases of gastric volvulus in female breeding guinea pigs, including the first known report of 540° gastric rotation. Clinical signs were not apparent prior to death in either case, but weight loss and gastric dilation were identified during necropsy examination. Gastric volvulus should be considered as a potential cause of acute death in adult female breeding guinea pigs or those exhibiting respiratory compromise with abdominal distention, particularly when anorexia is present and peristaltic sounds or feces are absent. In some guinea pigs death is preceded by a period of anorexia, lethargy, and reduced or absent feces production. Gastric volvulus should be differentiated from the more common gastric stasis so that appropriate treatment can be administered. Survey radiographs should be performed to support the diagnosis when gastric volvulus is suspected, and surgical exploration may be necessary for confirmation.
Gastric volvulus in guinea pigs is more common in female breeders, and etiology is most likely multifactorial. In addition to the suspected pregnancy-induced changes to gastric mobility, other factors that might play a role include thoracic cavity anatomy, stress, and dietary factors. To clarify the pathogenesis of gastric volvulus, future research could evaluate gastric ligament lengthening associated with pregnancy in guinea pigs, as well as predisposing thoracic anatomic features. In addition, evaluating the effect of breeding colony management on stress might explain why female breeder guinea pigs appear to be at an increased risk of developing gastric volvulus.
Acknowledgments
We thank Andrea Hoffmann and Matthijs Amelink for their assistance in the translation of foreign literature used in the preparation of this report.
References
- 1.Askew AR. 1978. Treatment of acute and chronic gastric volvulus. Ann R Coll Surg Engl 60:326–328 [PMC free article] [PubMed] [Google Scholar]
- 2.Borchardt M. 1904. Zun pathologie und therapie des magnevolvulus. Arch Klin Chir 74:243–260 [Google Scholar]
- 3.DeHoff WD, Greene RW. 1973. Gastric dilatation and the gastric torsion complex. Vet Clin North Am 2:141–153 [DOI] [PubMed] [Google Scholar]
- 4.Elwell MR, DePaoli A. 1978. Gastric dilatation and volvulus in a squirrel monkey. J Am Vet Med Assoc 173:1235–1236 [PubMed] [Google Scholar]
- 5.Farah IO, Chege GK, Riday AM. 1993. Acute gastric dilatation in 2 black-and-white colobus monkeys. J Med Primatol 22:278–279 [PubMed] [Google Scholar]
- 6.Formaggini L, Schmidt K, De Lorenzi D. 2008. Gastric dilatation–volvulus associated with diaphragmatic hernia in 3 cats: clinical presentation, surgical treatment, and presumptive aetiology. J Feline Med Surg 10:198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganaway JR, Allen AM. 1971. Obesity predisposes to pregnancy toxemia (ketosis) of guinea pigs. Lab Anim Sci 21:40–44 [PubMed] [Google Scholar]
- 8.Gialamas VJ, Höger HH, Adamiker D. 1985. [Acute stomach dilatation and stomach torsion in the guinea pig]. Zentralbl Veterinarmed A 32:772–777 [Article in German] [PubMed] [Google Scholar]
- 9.Glickman LT, Glickman NW, Schellenberg DB, Raghavan M, Lee TL. 2000. Incidence of and breed-related risk factors for gastric dilatation–volvulus in dogs. J Am Vet Med Assoc 216:40–45 [DOI] [PubMed] [Google Scholar]
- 10.Glickman LT, Glickman NW, Schellenberg DB, Raghavan M, Lee TL. 2000. Nondietary risk factors for gastric dilatation–volvulus in large- and giant-breed dogs. J Am Vet Med Assoc 217:1492–1499 [DOI] [PubMed] [Google Scholar]
- 11.Glickman LT, Glickman NW, Schellenberg DB, Simpson K, Lantz GC. 1997. Multiple risk factors for the gastric dilatation–volvulus syndrome in dogs: a practitioner–owner case-control study. J Am Anim Hosp Assoc 33:197–204 [DOI] [PubMed] [Google Scholar]
- 12.Hawkins MG, Graham JE. 2007. Emergency and critical care of rodents. Vet Clin North Am Exot Anim Pract 10:501–531 [DOI] [PubMed] [Google Scholar]
- 13.Hosgood G. 1994. Gastric dilatation–volvulus in dogs. J Am Vet Med Assoc 204:1742–1747 [PubMed] [Google Scholar]
- 14.Keith JC, Jr, Rowles TK, Warwick KE, Yau ET. 1992. Correspondence. Lab Anim Sci 42:331–332 [PubMed] [Google Scholar]
- 15.Kunstyr I. 1981. Torsion of the uterus and the stomach in guinea pigs. Z Versuchstierkd 23:67–69 [PubMed] [Google Scholar]
- 16.Laurent S, Grayet D, Lavigne ChM. 2010. Acute and chronic gastric volvulus: radical different prognosis and management. Case report. Acta Chir Belg 110:76–79 [DOI] [PubMed] [Google Scholar]
- 17.Lee KJ, Johnson WD, Lang CM. 1977. Acute gastric dilatation associated with gastric volvulus in the guinea pig. Lab Anim Sci 27:685–686 [PubMed] [Google Scholar]
- 18.Mayo A, Erez I, Lazar L, Rathaus V, Konen O, Freud E. 2001. Volvulus of the stomach in childhood: the spectrum of the disease. Pediatr Emerg Care 17:344–348 [DOI] [PubMed] [Google Scholar]
- 19.Mitchell EB, Hawkins MG, Gaffney PM, MacLeod AG. 2010. Gastric dilatation–volvulus in a guinea pig (Cavia porcellus). J Am Anim Hosp Assoc 46:174–180 [DOI] [PubMed] [Google Scholar]
- 20.Monnet E. 2003. Gastric dilatation–volvulus syndrome in dogs. Vet Clin North Am Small Anim Pract 33:987–1005 [DOI] [PubMed] [Google Scholar]
- 21.Morin M, Sauvageau R, Phaneuf JB, Teuscher E, Beauregard M, Lagace A. 1984. Torsion of abdominal organs in sows: a report of 36 cases. Can Vet J 25:440–442 [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavan M, Glickman NW, Glickman LT. 2006. The effect of ingredients in dry dog foods on the risk of gastric dilatation–volvulus in dogs. J Am Anim Hosp Assoc 42:28–36 [DOI] [PubMed] [Google Scholar]
- 23.Raghavan M, Glickman NW, McCabe G, Lantz G, Glickman LT. 2004. Diet-related risk factors for gastric dilation–volvulus in dogs of high-risk breeds. J Am Anim Hosp Assoc 40:192–203 [DOI] [PubMed] [Google Scholar]
- 24.Schellenberg D, Yi Q, Glickman NW, Glickman LT. 1998. Influence of thoracic conformation and genetics on the risk of gastric dilatation–volvulus in Irish setters. J Am Anim Hosp Assoc 34:64–73 [DOI] [PubMed] [Google Scholar]
- 25.Shivanand G, Seema S, Srivastava DN, Pande GK, Sahni P, Prasad R, Ramachandra N. 2003. Gastric volvulus acute and chronic presentation. Clin Imaging 27:265–268 [DOI] [PubMed] [Google Scholar]
- 26.Theyse LF, van de Brom WE, van Sluijs FJ. 1998. Small size of food particles and age as risk factors for gastric dilatation volvulus in great danes. Vet Rec 143:48–50 [DOI] [PubMed] [Google Scholar]
- 27.Van Kruiningen HJ, Gregoire K, Meuten DJ. 1974. Acute gastric dilatation: a review of comparative aspects, by species, and a study in dogs and monkeys. J Am Anim Hosp Assoc 10:294–324 [Google Scholar]
- 28.Wasselle JA, Norman J. 1993. Acute gastric volvulus: pathogenesis, diagnosis, and treatment. Am J Gastroenterol 88:1780–1784 [PubMed] [Google Scholar]
- 29.Wendt M. 1997. [Stomach torsion in swine] Tierarztl Prax 15:375–376 [Article in German] [PubMed] [Google Scholar]
- 30.Willard MD. 1995. Diseases of the stomach, p 1143–1168 : Ettinger SJ, Feldman ED. Textbook of veterinary internal medicine, vol 2 Philadelphia (PA): WB Saunders [Google Scholar]
- 31.Willemse A. 1975. [Gastric torsion in a guinea pig (author's translation)]. Tijdschr Diergeneeskd 100:840–841 [Article in Dutch] [PubMed] [Google Scholar]