Abstract
Echocardiography is a widely used evaluation tool in cardiovascular research. Although rats are a common model in such research, normal echocardiographic values for young, developing rats have not been established. Furthermore, whether exercise during the developmental phase of the lifespan affects the structure or function of the heart is unclear. Male Sprague–Dawley rat pups (21 d) were assigned randomly to a nonexercise or voluntary exercise group for 12 wk. Echocardiograms were obtained before and at weekly intervals during the 12-wk observation period. Maturation resulted in changes in many echocardiographically derived variables, whereas voluntary exercise failed to alter the development of cardiac structure or function. This study provides normal echocardiographic variables for developing male rats and provides evidence that exercise during the developmental phase of the lifespan has little effect on cardiac morphology and function as assessed by echocardiography.
Abbreviation: d, diastole; ET, ejection time; FS, fractional shortening; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; LVD, left ventricular dimension; MPI, myocardial performance index; NEX, nonexercised; PW, posterior wall thickness; RWT, relative wall thickness; s, systole; SW, septal wall thickness; TVI, time velocity integral; Vcf, velocity of circumferential shortening; VEX, voluntary exercise; Vmax, maximal flow velocity; Vmean, mean flow velocity
Echocardiography is a reliable, valid, and safe method for the examination of cardiac structure and function in both humans and animals. It has become an invaluable tool for serial measures of cardiac structure and function in research using animal models due to the fact that echocardiography potentially can replace highly invasive or terminal procedures. As a result, the implementation of echocardiography in research using laboratory animals has increased steadily.
Over the past century, laboratory rats have been used as a standard research model. Their size and physical characteristics make them an effective model to study human physiology and disease. Because rats frequently are used in cardiovascular research, the echocardiographic alterations that occur in young rats need to be understood to evaluate possible changes that may occur during this period of development. Furthermore, rats have proven to be a well-accepted model for investigating the effects of physical activity. With a growing emphasis on the effect of lifelong exercise on cardiovascular health, rats could serve as an effective model to investigate the effects of exercise during the developmental phase of the lifespan.
Although baseline echocardiographic values for adult rats have been reported,33 little is known regarding the time course of changes in cardiac morphology and function in developing rats. Likewise, whether exercise initiated several days after weaning has an effect on the normal development of the rat heart, either in morphology or function, is unknown. The purpose of the current study was to characterize changes in cardiac structure and function in developing Sprague–Dawley rats and to investigate the effects of exercise training on these developmental changes. We hypothesized that exercise training during the developmental stages of life would cause maturational changes in cardiac structure and function in male Sprague–Dawley rats.
Materials and Methods
All procedures were approved by the University of Northern Colorado Institutional Animal Care and Use Committee and were in compliance with Animal Welfare Act1 guidelines and the AALAS Position Statement on the humane care and use of laboratory animals. Male Sprague–Dawley rat pups were purchased from Harlan (Indianapolis, IN), arrived at our facility at 14 d of age, and were allowed to acclimate 1 wk before being assigned to an experimental group. Pups were housed in an environmentally controlled facility on a 12:12-h light:dark cycle and were provided chow and water ad libitum.
Exercise training.
At 21 d of age, pups were assigned randomly to either a nonexercise (NEX; n = 10) or voluntary exercise (VEX; n = 10) group. Rats assigned to the NEX group were free-moving young rats without the opportunity to exercise. They were housed individually in standard cages for the duration of the experimental protocol. Rats assigned to the VEX group were free-moving young rats with the opportunity to voluntarily exercise. They were housed individually in cages fitted with commercially available voluntary running wheels (MiniMitter, Bend, OR) and were allowed 24-h access to the wheels for 12 wk. No acclimation or initial training period was provided to rats in the running group. Running-wheel activity data were collected by using a data-acquisition system (Vital View, MiniMitter). All rats underwent echocardiography immediately before and at weekly intervals during the 12-wk exercise protocol. At 12 h before each echocardiogram, VEX rats were removed from their cages to negate any effects of acute exercise.
Echocardiography.
For transthoracic echocardiography (Nemio 30, Toshiba, Tustin, CA), rats were sedated with ketamine (40 mg/kg IP), their anterior and left lateral thoracic regions were shaved, and rats were placed in the left lateral decubital position. Ultrasound gel was placed on the thorax to optimize visibility. The probe was positioned to obtain short-axis, long-axis, and 4-chamber views. From the short-axis view, an M-mode tracing of the left ventricle (LV) was obtained for measures of septal wall thickness during systole (SWs) and diastole (SWd), posterior wall thickness during systole (PWs) and diastole (PWd), and LV dimension during systole (LVDs) and diastole (LVDd). For all cardiac dimensions, we used a leading–edge-to-leading–edge technique as described by the American Society of Echocardiography.17 Aortic flow was assessed from the 4-chamber apical view by using pulsed-wave Doppler ultrasonography, with the smallest possible sample volume placed at the level of the aortic annulus. Mitral flow was assessed from a 4-chamber apical view by using pulsed-wave Doppler with the smallest possible sample volume placed at the tips of the mitral valve. LV mass was calculated as 1.04[(LVDd + PWd + SWd)3 – LVDd3], relative wall thickness (RWT) was calculated as [PWd + SWd] / LVDd, and fractional shortening (FS) was calculated as [LVDd – LVDs] / LVDd. The velocity of circumferential shortening (Vcf) was calculated as FS / ET, where ET is ejection time; ET was obtained from Doppler measures of aortic flow and measured as the time between aortic valve opening and aortic valve closure. The myocardial performance index (MPI), an index used to assess combined systolic and diastolic function,31 was calculated as [IVCT + IVRT] / ET, where IVCT is isovolumic contraction time, and IVRT is isovolumic relaxation time. From pulsed Doppler mitral and aortic flow images, the time velocity integral (TVI), maximal flow velocity (Vmax), and mean flow velocity (Vmean) were measured. For measures of cardiac dimensions, time intervals, and flow velocities, data from 3 consecutive cardiac cycles were obtained and averaged for each rat at each time point. Echocardiography was completed within 10 to 15 min.
Statistical analyses.
Data are reported as mean ± SE. Differences over time were determined by one-way ANOVA using GraphPad Prism software (La Jolla, CA). When differences were noted over time, Dunnett posthoc analyses were conducted comparing time course values to their corresponding baseline values at 3 wk of age. An independent t test was used to determine significant differences in mean values between exercise groups and control groups for each echocardiographic parameter. A significant level of P < 0.05 was used for all statistical analyses.
Results
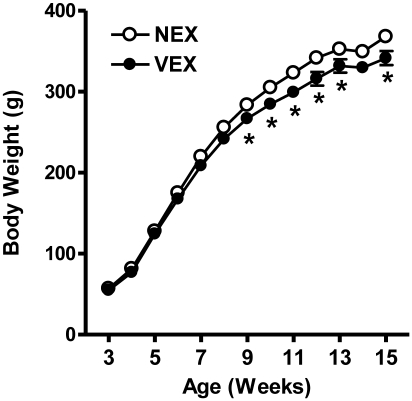
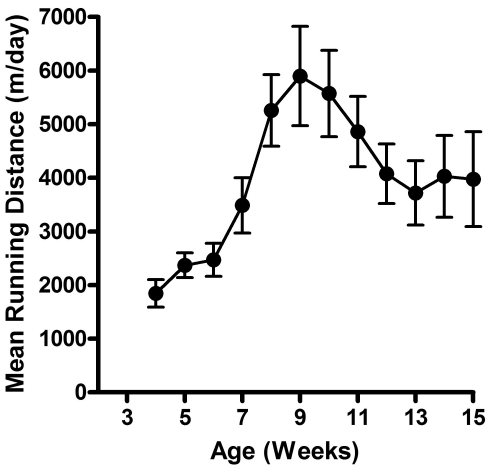
Male Sprague–Dawley rats were monitored from 3 to 15 wk of age. Body weight measurements during that period closely followed the growth curve reported by the vendor. Although growth curves for NEX and VEX rats had similar time-course responses (Figure 1), significant (P < 0.05) differences between groups were observed at weeks 9 through 13 and 15. At these intervals, VEX rats weighed significantly (P < 0.05) less than did NEX rats. LV mass, as calculated by using echocardiographic measures, responded in a similar fashion. Both NEX and VEX groups showed continuous increases in LV mass throughout the study. No significant differences in LV mass were noted between groups except at the 10-wk interval, with NEX rats demonstrating a significantly (P < 0.05) higher mean mass at that time. Figure 2 summarizes mean weekly running distances. Rats in the voluntary exercise group showed progressive increases in running behavior during the first 6 wk of wheel exposure. Running volume then declined progressively during weeks 9 through 12 and remained stable from weeks 13 to 15.
Figure 1.
Body weight (mean ± SE) is significantly lower in voluntarily exercised compared with nonexercised male Sprague–Dawley rats. NEX, nonexercised; VEX, voluntarily exercised. *, P < 0.05 compared with value for NEX.
Figure 2.
Weekly voluntary running distances (mean ± SE) in male Sprague–Dawley rats.
Each cardiac parameter (mean ± SE) measured by echocardiography appears in Tables 1 through 3. NEX rats showed distinct progressive increases in all cardiac dimensions (that is, chamber dimensions, wall thicknesses) throughout the 12-wk observation period (Table 1), consistent with increases in body weight and LV mass of the developing animal. No significant differences between NEX and VEX groups were observed for any cardiac geometry measure at any time point, with the exception of PWd at 3 wk and PWs at weeks 3 and 15 (P < 0.05). In addition to measures of cardiac geometry and morphology, several additional standardized echocardiographic measurements were obtained and are summarized in Table 2. Although mean resting heart rate in NEX rats ranged from 422 bpm (week 14) to 513 bpm (week 5), values did not significantly differ from the week 3 heart rate at any time point. In VEX rats, resting heart rate significantly (P < 0.05) increased from week 3 to week 5 and was significantly (P < 0.05) decreased at weeks 12 to 15 when compared with the week 3 resting heart rate. In NEX rats, RWT did not change throughout the course of the study, whereas significant (P < 0.05) decreases in RWT were noted for the VEX group at weeks 6 through 8 and 13 wk. However, no differences in RWT were noted between groups at any time point. In comparison to week 3 values, we noted no significant differences for Vcf during the course of this study, and no significant differences were observed between NEX and VEX groups. Both NEX and VEX groups showed a significant decline in IVRT by week 6 (P < 0.05) and showed an upward trend for the remainder of the observation period. No significant differences in IVRT were observed between groups at any time point. NEX rats showed a trend toward a decrease in FS over time, with significantly lower FS at weeks 13, 14, and 15 (P < 0.05). Whereas VEX rats showed a similar trend as that in NEX rats, FS did not significantly change during the 12-wk observation period. At week 3, mean FS values were above 64% for both groups but at or below 60% by 15 wk. This pattern occurred in both NEX and VEX groups, with no significant differences between groups at any observation interval.
Table 1.
Cardiac geometry (mm; mean ± SE)
| 3 wk |
4 wk |
5 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| SWs | 2.26 ± 0.08 | 2.11 ± 0.07 | 2.49 ± 0.11 | 2.40 ± 0.09a | 2.70 ± 0.13a | 2.87 ± 0.10a |
| SWd | 1.09 ± 0.06 | 1.13 ± 0.08 | 1.30 ± 0.08a | 1.20 ± 0.08 | 1.30 ± 0.06a | 1.36 ± 0.05a |
| PWs | 2.31 ± 0.09 | 2.26 ± 0.10b | 2.39 ± 0.08 | 2.61 ± 0.08 | 2.49 ± 0.12 | 2.79 ± 0.13 |
| PWd | 1.30 ± 0.09 | 1.44 ± 0.08b | 1.37 ± 0.09 | 1.56 ± 0.11 | 1.29 ± 0.09 | 1.42 ± 0.08 |
| LVDs | 1.34 ± 0.10 | 1.45 ± 0.09 | 1.70 ± 0.15a | 1.67 ± 0.12 | 1.85 ± 0.10a | 1.63 ± 0.13 |
| LVDd | 2.98 ± 0.14 | 4.06 ± 0.08 | 4.61 ± 0.11 | 4.63 ± 0.19a | 4.90 ± 0.17a | 4.88 ± 0.15a |
| 6 wk |
7 wk |
8 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| SWs | 2.89 ± 0.16a | 2.64 ± 0.08a | 3.01 ± 0.13a | 3.00 ± 0.08a | 3.20 ± 0.12a | 3.16 ± 0.07a |
| SWd | 1.43 ± 0.08a | 1.36 ± 0.07a | 1.62 ± 0.10a | 1.54 ± 0.07a | 1.57 ± 0.06a | 1.53 ± 0.06a |
| PWs | 2.94 ± 0.17a | 2.73 ± 0.12a | 2.83 ± 0.18a | 3.04 ± 0.12a | 3.15 ± 0.27a | 3.05 ± 0.14a |
| PWd | 1.70 ± 0.13a | 1.56 ± 0.08 | 1.53 ± 0.09a | 1.52 ± 0.08 | 1.76 ± 0.11a | 1.70 ± 0.11a |
| LVDs | 2.09 ± 0.21a | 2.16 ± 0.13a | 2.25 ± 0.19a | 1.97 ± 0.16a | 2.12 ± 0.22a | 2.00 ± 0.13a |
| LVDd | 5.51 ± 0.19a | 5.43 ± 0.18a | 5.66 ± 0.18a | 5.54 ± 0.12a | 5.80 ± 0.12a | 5.75 ± 0.12a |
| 9 wk |
10 wk |
11 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| SWs | 3.01 ± 0.10a | 3.08 ± 0.14a | 3.33 ± 0.12a | 3.17 ± 0.09a | 3.28 ± 0.11a | 3.27 ± 0.11a |
| SWd | 1.63 ± 0.17a | 1.55 ± 0.11a | 1.84 ± 0.010a | 1.65 ± 0.11a | 1.69 ± 0.16a | 1.80 ± 0.15a |
| PWs | 3.04 ± 0.25a | 3.20 ± 0.12a | 3.21 ± 0.13a | 3.08 ± 0.16a | 3.52 ± 0.11a | 3.34 ± 0.14a |
| PWd | 1.79 ± 0.08a | 1.66 ± 0.12 | 1.71 ± 0.08a | 1.75 ± 0.10a | 2.01 ± 0.16a | 1.84 ± 0.12a |
| LVDs | 1.94 ± 0.15a | 1.85 ± 0.16a | 2.34 ± 0.20a | 2.02 ± 0.12a | 2.16 ± 0.18a | 2.19 ± 0.12a |
| LVDd | 5.92 ± 0.12a | 5.76 ± 0.20a | 6.24 ± 0.14a | 5.79 ± 0.22ab | 6.35 ± 0.21a | 6.18 ± 0.23a |
| 12 wk |
13 wk |
14 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| SWs | 3.34 ± 0.13a | 3.27 ± 0.12a | 3.15 ± 0.14a | 3.11 ± 0.13a | 3.42 ± 0.17a | 3.27 ± 0.14a |
| SWd | 1.92 ± 0.09a | 1.89 ± 0.11a | 1.79 ± 0.08a | 1.74 ± 0.11a | 2.01 ± 0.13a | 1.86 ± 0.08a |
| PWs | 3.33 ± 0.18a | 3.14 ± 0.14a | 3.26 ± 0.18a | 3.50 ± 0.06a | 3.36 ± 0.14a | 3.36 ± 0.10a |
| PWd | 1.81 ± 0.11a | 1.73 ± 0.10a | 1.89 ± 0.10a | 1.82 ± 0.07a | 1.93 ± 0.10a | 1.87 ± 0.09a |
| LVDs | 2.66 ± 0.30a | 2.45 ± 0.15a | 2.78 ± 0.19a | 2.52 ± 0.18a | 2.57 ± 0.17a | 2.60 ± 0.27a |
| LVDd | 6.36 ± 0.22a | 6.35 ± 0.21a | 6.57 ± 0.18a | 6.57 ± 0.21a | 6.31 ± 0.15a | 6.37 ± 0.24a |
| 15 wk |
||||||
| NEX | VEX | |||||
| SWs | 3.45 ± 0.15a | 3.37 ± 0.13a | ||||
| SWd | 2.03 ± 0.14a | 2.04 ± 0.11a | ||||
| PWs | 3.34 ± 0.09a | 3.05 ± 0.08ab | ||||
| PWd | 1.83 ± 0.10a | 1.67 ± 0.11a | ||||
| LVDs | 2.63 ± 0.19a | 2.58 ± 0.15a | ||||
| LVDd | 6.34 ± 0.15a | 6.48 ± 0.20a | ||||
d, diastole; s, systole; NEX, nonexercised; VEX, voluntary exercise; SW, septal wall thickness; PW, posterior wall thickness; LVD, left ventricular dimension.
P < 0.05 compared with week 3 value
P < 0.05 compared with NEX
Table 2.
Other echocardiographic variables (mean ± SE)
| 3 wk |
4 wk |
5 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| HR (bpm) | 453 ± 29 | 487 ± 11 | 496 ± 11 | 484 ± 25 | 513 ± 18 | 544 ± 03a |
| LVM (mg) | 207 ± 18 | 235 ± 08 | 301 ± 19a | 319 ± 19a | 319 ± 17a | 346 ± 12a |
| RWT | 0.60 ± 0.02 | 0.64 ± 0.02 | 0.58 ± 0.04 | 0.61 ± 0.04 | 0.53 ± 0.04 | 0.57 ± 0.02 |
| Vcf (circ/s) | 1.42 ± 0.15 | 1.29 ± 0.06 | 1.28 ± 0.08 | 1.22 ± 0.06 | 1.38 ± 0.06 | 1.34 ± 0.09 |
| IVRT (ms) | 16.9 ± 0.8 | 17.8 ± 1.1 | 15.8 ± 0.6 | 15.5 ± 0.9 | 15.4 ± 1.1 | 14.6 ± 0.7a |
| MPI | 0.88 ± 0.08 | 0.74 ± 0.06 | 0.69 ± 0.07 | 0.61 ± 0.07 | 0.77 ± 0.08a | 0.53 ± 0.06b |
| FS (%) | 66 ± 2 | 64 ± 2 | 63 ± 3 | 64 ± 3 | 62 ± 1 | 67 ± 3 |
| 6 wk |
7 wk |
8 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| HR (bpm) | 504 ± 10 | 513 ± 13 | 484 ± 14 | 490 ± 08 | 489 ± 08 | 485 ± 09 |
| LVM (mg) | 498 ± 34a | 440 ± 12a | 527 ± 37a | 488 ± 26a | 590 ± 41a | 558 ± 26a |
| RWT | 0.58 ± 0.04 | 0.55 ± 0.03a | 0.56 ± 0.02 | 0.56 ± 0.02a | 0.58 ± 0.03 | 0.56 ± 0.02a |
| Vcf (circ/s) | 1.27 ± 0.09 | 1.24 ± 0.09 | 1.34 ± 0.12 | 1.42 ± 0.04 | 1.39 ± 0.10 | 1.43 ± 0.12 |
| IVRT (ms) | 13.9 ± 1.0a | 14.0 ± 0.7a | 16.5 ± 0.8 | 14.9 ± 0.8 | 16.7 ± 1.1 | 16.8 ± 0.9a |
| MPI | 0.63 ± 0.05a | 0.63 ± 0.10 | 0.96 ± 0.11 | 0.82 ± 0.06 | 0.78 ± 0.12 | 0.85 ± 0.19 |
| FS (%) | 63 ± 3 | 61 ± 2 | 61 ± 2 | 65 ± 2 | 63 ± 4 | 65 ± 2 |
| 9 wk |
10 wk |
11 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| HR (bpm) | 461 ± 12 | 453 ± 14 | 461 ± 09 | 464 ± 10 | 417 ± 16 | 445 ± 12 |
| LVM (mg) | 633 ± 38a | 557 ± 48a | 718 ± 21a | 608 ± 38ab | 792 ± 67a | 736 ± 29a |
| RWT | 0.58 ± 0.04 | 0.56 ± 0.04 | 0.58 ± 0.02 | 0.59 ± 0.03 | 0.59 ± 0.06 | 0.60 ± 0.05 |
| Vcf (circ/s) | 1.32 ± 0.06 | 1.41 ± 0.07 | 1.22 ± 0.08 | 1.34 ± 0.05 | 1.17 ± 0.07 | 1.24 ± 0.05 |
| IVRT (ms) | 15.1 ± 0.7 | 15.2 ± 0.8 | 16.6 ± 0.9 | 15.8 ± 0.9 | 18.9 ± 1.6 | 16.0 ± 0.8 |
| MPI | 0.70 ± 0.06 | 0.83 ± 0.05 | 0.67 ± 0.03a | 0.73 ± 0.02 | 0.74 ± 0.04 | 0.67 ± 0.04 |
| FS (%) | 67 ± 2 | 68 ± 3 | 63 ± 3 | 65 ± 1 | 65 ± 3 | 64 ± 1 |
| 12 wk |
13 wk |
14 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| HR (bpm) | 434 ± 06 | 440 ± 13a | 424 ± 08 | 428 ± 07a | 422 ± 03 | 415 ± 15a |
| LVM (mg) | 800 ± 30a | 764 ± 30a | 825 ± 32a | 788 ± 47a | 855 ± 41a | 806 ± 41a |
| RWT | 0.59 ± 0.04 | 0.58 ± 0.03 | 0.56 ± 0.03 | 0.55 ± 0.03a | 0.63 ± 0.04 | 0.59 ± 0.03 |
| Vcf (circ/s) | 1.12 ± 0.07 | 1.21 ± 0.03 | 1.09 ± 0.07 | 1.16 ± 0.06 | 1.17 ± 0.05 | 1.14 ± 0.07 |
| IVRT (ms) | 18.5 ± 1.1 | 15.9 ± 0.6 | 17.2 ± 0.8 | 16.3 ± 0.8 | 18.9 ± 0.8 | 18.4 ± 1.3 |
| MPI | 0.73 ± 0.07 | 0.71 ± 0.04 | 0.73 ± 0.05 | 0.66 ± 0.03 | 0.66 ± 0.04 | 0.78 ± 0.06 |
| FS (%) | 59 ± 4 | 62 ± 1 | 58 ± 2a | 62 ± 2 | 59 ± 2a | 59 ± 0 |
| 15 wk |
||||||
| NEX | VEX | |||||
| HR (bpm) | 432 ± 13 | 423 ± 10a | ||||
| LVM (mg) | 842 ± 54a | 817 ± 42a | ||||
| RWT | 0.61 ± 0.03 | 0.58 ± 0.04 | ||||
| Vcf (circ/s) | 1.19 ± 0.07 | 1.17 ± 0.05 | ||||
| IVRT (ms) | 19.2 ± 1.6a | 17.8 ± 1.1 | ||||
| MPI | 0.86 ± 0.02 | 0.79 ± 0.05 | ||||
| FS (%) | 58 ± 3a | 60 ± 2 | ||||
HR, heart rate; LVM, left ventricular mass; RWT, relative wall thickness; Vcf, velocity of circumferential (circ) shortening; IVRT, isovolumetric relaxation time; MPI, myocardial performance index; FS, fractional shortening.
P < 0.05 compared with week 3 value.
P < 0.05 compared with value for NEX.
Table 3.
Aortic and mitral Doppler measures (mean ± SE)
| 3 wk |
4 wk |
5 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| Aortic TVI (cm) | 2.6 ± 0.2 | 3.2 ± 0.2* | 3.2 ± 0.3 | 3.8 ± 0.2a | 3.4 ± 0.2a | 3.6 ± 0.2a |
| Aortic Vmean (cm/s) | 53 ± 5 | 63 ± 3 | 64 ± 4 | 102 ± 4a | 72 ± 3a | 74 ± 4a |
| Aortic Vmax (cm/s) | 78 ± 7 | 91 ± 4 | 92 ± 5 | 102 ± 4 | 100 ± 3a | 108 ± 3a |
| Mitral TVI (cm) | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.9 ± 0.1a | 3.2 ± 0.1a | 3.1 ± 0.1a | 3.0 ± 0.1 |
| Mitral Vmean (cm/s) | 65 ± 4 | 71 ± 2 | 78 ± 2a | 78 ± 4 | 80 ± 2a | 82 ± 2a |
| Mitral Vmax (cm/s) | 90 ± 6 | 98 ± 3 | 106 ± 3a | 105 ± 4 | 110 ± 3a | 110 ± 3a |
| 6 wk |
7 wk |
8 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| Aortic TVI (cm) | 4.2 ± 0.2a | 3.9 ± 0.2a | 3.7 ± 0.2a | 3.7 ± 0.2 | 3.5 ± 0.3a | 3.4 ± 0.2 |
| Aortic Vmean (cm/s) | 84 ± 2a | 78 ± 3a | 81 ± 5a | 80 ± 4a | 76 ± 3a | 74 ± 4a |
| Aortic Vmax (cm/s) | 112 ± 2a | 108 ± 4a | 109 ± 5a | 112 ± 3a | 105 ± 3a | 100 ± 5 |
| Mitral TVI (cm) | 3.3 ± 0.1a | 3.2 ± 0.1a | 3.1 ± 0.1a | 3.2 ± 0.1a | 3.4 ± 0.2a | 3.2 ± 0.2a |
| Mitral Vmean (cm/s) | 86 ± 3a | 85 ± 2a | 83 ± 3a | 80 ± 3a | 80 ± 2a | 80 ± 2a |
| Mitral Vmax (cm/s) | 113 ± 3a | 114 ± 2a | 109 ± 2a | 110 ± 3a | 109 ± 3a | 106 ± 3a |
| 9 wk |
10 wk |
11 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| Aortic TVI (cm) | 4.2 ± 0.2a | 4.1 ± 0.2a | 4.3 ± 0.2a | 4.3 ± 0.2a | 4.4 ± 0.2a | 3.9 ± 0.1a |
| Aortic Vmean (cm/s) | 82 ± 4a | 86 ± 5a | 84 ± 3a | 85 ± 5a | 81 ± 4a | 75 ± 2 |
| Aortic Vmax (cm/s) | 125 ± 5a | 129 ± 5a | 121 ± 5a | 131 ± 4a | 118 ± 5a | 116 ± 3 |
| Mitral TVI (cm) | 3.9 ± 0.1a | 3.8 ± 0.1a | 3.7 ± 0.2a | 3.9 ± 0.1a | 3.9 ± 0.2a | 3.8 ± 0.2a |
| Mitral Vmean (cm/s) | 88 ± 3a | 84 ± 4a | 83 ± 3a | 85 ± 5a | 77 ± 3 | 82 ± 5a |
| Mitral Vmax (cm/s) | 121 ± 5a | 113 ± 6a | 113 ± 3a | 117 ± 5a | 105 ± 6a | 111 ± 7a |
| 12 wk |
13 wk |
14 wk |
||||
| NEX | VEX | NEX | VEX | NEX | VEX | |
| Aortic TVI (cm) | 4.1 ± 0.2a | 3.5 ± 0.2 | 4.0 ± 0.3a | 4.0 ± 0.2a | 3.5 ± 0.3a | 3.9 ± 0.2 |
| Aortic Vmean (cm/s) | 78 ± 3a | 70 ± 4 | 75 ± 4a | 75 ± 3a | 71 ± 4a | 73 ± 4 |
| Aortic Vmax (cm/s) | 118 ± 4a | 107 ± 4a | 111 ± 5a | 115 ± 5a | 109 ± 7a | 114 ± 5a |
| Mitral TVI (cm) | 3.7 ± 0.2a | 4.0 ± 0.1a | 3.6 ± 0.1a | 4.1 ± 0.2a* | 3.6 ± 0.2a | 4.0 ± 0.2a |
| Mitral Vmean (cm/s) | 80 ± 3a | 80 ± 6 | 74 ± 3 | 79 ± 3a | 71 ± 3 | 78 ± 5 |
| Mitral Vmax (cm/s) | 109 ± 3a | 109 ± 6 | 103 ± 2 | 108 ± 4 | 100 ± 3 | 105 ± 7 |
| 15 wk |
||||||
| NEX | VEX | |||||
| Aortic TVI (cm) | 3.5 ± 0.3a | 3.9 ± 0.3 | ||||
| Aortic Vmean (cm/s) | 71 ± 5a | 76 ± 4 | ||||
| Aortic Vmax (cm/s) | 106 ± 8a | 112 ± 6a | ||||
| Mitral TVI (cm) | 3.4 ± 0.2a | 3.9 ± 0.2a | ||||
| Mitral Vmean (cm/s) | 78 ± 4 | 77 ± 4 | ||||
| Mitral Vmax (cm/s) | 108 ± 6 | 105 ± 7 | ||||
TVI, time velocity integral; Vmean, mean flow velocity; Vmax, maximal flow velocity.
P < 0.05 compared with week 3 value
P < 0.05 compared with NEX
We noted a high degree of similarity in the response of all Doppler blood flow measures (Table 3). Each of these variables showed progressive increases from week 3 until approximately week 9, followed by a plateau or a slight decline in mean values between weeks 9 and 15. No significant differences between NEX and VEX groups were noted for any variable at any time point, with the exception of aortic TVI at 3 wk and mitral TVI at 13 wk.
Discussion
Rats in the current study demonstrated a pattern of increased running during the first 5 wk of exposure to the wheel, followed by a decline in running activity over the next 6 wk. We previously reported in several other studies a similar pattern of running activity in mature male Sprague–Dawley rats.13-15 Although dozens of internal and external factors have been reported to influence running behavior in rats (see reference 30 for review), this pattern of running behavior may be related to the novelty or complexity of the running activity. When given the choice, most laboratory rats will choose more complex and novel activities.30 Therefore, a plausible explanation for the increased running activity followed by a decline and plateau may be that the running behavior is driven by the level of novelty or complexity.
Data presented in the current study indicate that both cardiac morphology and function changed during weeks 3 through 15 of the lifespan in male Sprague–Dawley rats. However, voluntary exercise during this same period had little effect on this developmental process. Although exercise did alter body weight, this effect did not translate into changes in cardiac morphology and function.
Rats engaged in voluntary exercise demonstrated a similar growth pattern to that of nonexercised animals, but body weight was less in VEX when compared with NEX between 9 and 15 wk of age. It has long been purported that forced exercise will slow the growth rate of male rats and that this effect is due to the combination of increased caloric expenditure without a commensurate increase in caloric intake.22 Others have shown that voluntary exercise by young male rats results in a negative energy balance that may account for the differences in body weight.8 Blunting of the growth curve by voluntary exercise appears to occur in male but not female rats, even when exercise is conducted during the developmental phases of the lifespan.8,22 This discrepancy in body weight between NEX and VEX groups did not translate into differences in LV mass. Although LV mass in VEX rats tended to be lower than that of NEX, no statistically significant differences between groups were present at any time point.
Here, we report that chronic exercise results in virtually no change in any cardiac morphologic or functional characteristic in young, developing Sprague–Dawley rats. In fact, of the 247 possible comparisons between NEX and VEX groups (19 echocardiographic variables, 13 separate time intervals), only 6 significant between-group differences (PWs at 3 and 15 wk; LVDd at 10 wk; MPI at 5 wk; aortic TVI at 3 wk; mitral TVI at 13 wk) occurred during the course of the current study. Although statistical differences occurred sporadically across different variables over many weeks, there was little evidence overall for comprehensive, systematic differences between exercised and nonexercised rats. In mature rats ranging in age from approximately 12 to 112 wk of age, exercise training does not significantly change LVDd, LVDs, or FS.9,10,14,15,21,29,34,35 Likewise, exercise training has no significant effect on SWs,14,15,21,34 SWd,3,14,15,21,34 PWs,14,15,21,34 or PWd.3,14,15,21,34,35 In the present study, our results indicate that exercise training had no significant effect on mitral inflow or aortic outflow during the developmental phase of the lifespan. These findings are in agreement with several other studies in adult rats indicating that exercise training does not significantly affect mitral3,10,14,15,21,34 or aortic blood flow.14,15
Voluntary wheel running has been used by our lab and others as an effective means of exercise training in rats. Wheel-running activity in rats improves oxygen consumption,16 increases citrate synthase activity,7,16,28 enhances force production in cardiomyocytes,22 and results in hypertrophy of the whole heart and individual myocytes.23,24 Although similar adaptations are observed with treadmill exercise, there is little doubt that voluntary exercise is a less stressful means by which animals exercise19,20 and may possibly better mimic the exercise practices of most people. One study36 compared voluntary wheel running with forced treadmill running at comparable speeds in rats and reported that the pattern of cardiovascular adjustment between the 2 forms of exercise was similar, yet mean arterial pressure, heart rate, and mesenteric blood flow responses were significantly greater (33% to 66%) with treadmill exercise. Although running velocities were similar, wheel-running activity produced a much more moderate cardiovascular response. Even with these differences in the cardiovascular response to voluntary exercise when compared with forced treadmill exercise, there is insufficient evidence to suggest that these exercise modalities (that is, voluntary exercise compared with forced treadmill exercise) have different effects on the morphologic or functional characteristics of the heart. Several studies have failed to show a significant effect of either forced treadmill11,15,21,29,34 or voluntary wheel-running exercise14,15 on cardiac morphology and function as assessed by echocardiography.
Other studies have evaluated cardiac structure and function following exercise training using modalities that do not require running. One study9 found that swimming exercise in rats did not significantly affect cardiac morphology, whereas another32 found that moderate- and high-intensity swimming significantly increased both PWd and SWd. Furthermore, a 3-mo resistance training program in rats had no effect on LVDd, FS, Vcf, or IVRT but significantly increased PWd, SWd, and RWT.2 Far fewer studies use swimming or resistance exercise than treadmill or wheel-running activity, and as a result, drawing conclusions is more difficult. However, exercise modalities such as swimming and resistance training may provide a different stimulus than does running-type exercise and may elicit changes in selected cardiac variables measured echocardiographically.
Why exercise during the developmental phase does not have a substantial effect on cardiac development or function is unknown. Clearly, several different interventions profoundly affect cardiac structure and function in Sprague–Dawley rats, including ischemia–reperfusion,18 hypertension,4 and anthracycline exposure.12 Theoretically, exercise has the capability of stimulating concentric ventricular hypertrophy (that is, increased wall thickness) in response to the increase in wall stress that is associated with higher ventricular pressures (pressure overload), and the capability of stimulating eccentric hypertrophy (that is, chamber dilation) as a result of a volume overload that accompanies the increase in venous return (volume overload). However, neither the pressure nor the volume overload associated with voluntary exercise in the current model was sufficient to cause any change in cardiac structure or function. Therefore, although there appears to be a threshold at which such stimuli can elicit structural or functional changes, what that threshold is remains unclear.
Although not a focus of the current study, different results might have been observed in female rats. Several studies have demonstrated an influence of gender on cardiac function and sex-associated differences in how the cardiovascular system responds to exercise training in male and female rats.5,6 However, in our laboratory, 8 wk of voluntary wheel running did not significantly change any echocardiographic measure of cardiac morphology or function in either adult male or adult female rats. Whether similar results would be obtained in young developing female rats is unclear.
The protocol used in the current study incorporated repeated exposure of growing rats to sedatives and other stress-related factors (for example, handling, injections, sedative exposure). Chronic exposure to adverse factors during the early phases of development has been shown to permanently affect the cardiovascular system of mammals.25,27 In light of our observations, exposure to these factors early in life did not appear to have a significant effect on the investigated variables. The growth curve of NEX rats was within approximately 10 g of the vendor-reported growth curve at all weekly intervals; this result suggests that these stress-related factors did not affect overall growth rate during the developmental phase. Furthermore, the data obtained in the present study between 10 and 15 wk of age are similar to the data obtained in our laboratory from nonexercise controls in other studies at a comparable age that have not been handled on a weekly basis.12 Rats were not observed beyond 15 wk of age, and differences in cardiac structure or function may have occurred at later time points.
Although we hypothesized that voluntary exercise during the developmental phase of the lifespan would significantly alter the cardiac morphology and function, this does not appear to be the case. Several studies11,14,15,21,29,34 have indicated that exercise training itself does not significantly affect cardiac structure or function in the adult rat. Likewise, data from the present study suggest that exercise does not significantly affect cardiac structure or function during developmental life stages in rats. The maturation process clearly resulted in significant changes in several echocardiographic variables, yet voluntary exercise appeared to have very little effect on cardiac structure or function in young, developing rats. Although exercise can elicit both structural and functional changes in the heart, voluntary exercise during the early developmental phase of life does not provide a sufficient stimulus to change either structure or function beyond that observed during the normal maturation process. All of our echocardiographic assessments were conducted during the resting state and differences between nonexercised and exercised rats may occur when the heart is stressed, such as during exercise or after dobutamine exposure.26 Nonetheless, voluntary exercise during the developmental phase of the lifespan did not stunt or augment cardiac development as assessed by echocardiography in male Sprague–Dawley rats.
References
- 1. Animal Welfare Act as Amended. 2007.7 USC §2131-2159.
- 2.Barauna VG, Rosa KT, Irigoyen MC, de Oliveira EM. 2007. Effects of resistance training on ventricular function and hypertrophy in a rat model. Clin Med Res 5:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boissiere J, Eder V, Machet MC, Courteix D, Bonnet P. 2008. Moderate exercise training does not worsen left ventricle remodeling and function in untreated severe hypertensive rats. J Appl Physiol 104:321–327 [DOI] [PubMed] [Google Scholar]
- 4.Cantor EJ, Babick AP, Vasanji Z, Dhalla NS, Netticadan T. 2005. A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. J Mol Cell Cardiol 38:777–786 [DOI] [PubMed] [Google Scholar]
- 5.Chen CY, DiCarlo SE. 1996. Daily exercise and gender influence arterial baroreflex regulation of heart rate and nerve activity. Am J Physiol 271:H1840–H1848 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Chandler MP, DiCarlo SE. 1997. Daily exercise and gender influence postexercise cardiac autonomic responses in hypertensive rats. Am J Physiol 272:H1412–H1418 [DOI] [PubMed] [Google Scholar]
- 7.Chicco AJ, Schneider CM, Hayward R. 2005. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol 289:R424–R431 [DOI] [PubMed] [Google Scholar]
- 8.Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. 1997. Daily exercise reduces fat, protein, and body mass in male but not female rats. Physiol Behav 62:105–111 [DOI] [PubMed] [Google Scholar]
- 9.Dayan A, Feinberg MS, Holbova R, Deshet N, Scheinowitz M. 2005. Swimming exercise training prior to acute myocardial infarction attenuates left ventricular remodeling and improves left ventricular function in rats. Ann Clin Lab Sci 35:73–78 [PubMed] [Google Scholar]
- 10.Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. 2008. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci 63:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. 2009. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120:42–49 [DOI] [PubMed] [Google Scholar]
- 12.Hayward R, Hydock DS. 2007. Doxorubicin cardiotoxicity in the rat: an in vivo characterization. J Am Assoc Lab Anim Sci 46:20–32 [PubMed] [Google Scholar]
- 13.Hydock DS, Iwaniec UT, Turner RT, Lien CY, Jensen BT, Parry TL, Schneider CM, Hayward R. 2008. Effects of voluntary wheel running on goserelin acetate-induced bone degeneration. Pathophysiology 15:253–259 [DOI] [PubMed] [Google Scholar]
- 14.Hydock DS, Lien CY, Schneider CM, Hayward R. 2007. Effects of voluntary wheel running on cardiac function and myosin heavy chain in chemically gonadectomized rats. Am J Physiol Heart Circ Physiol 293:H3254–H3264 [DOI] [PubMed] [Google Scholar]
- 15.Hydock DS, Lien CY, Schneider CM, Hayward R. 2008. Exercise preconditioning protects against doxorubicin-induced cardiac dysfunction. Med Sci Sports Exerc 40:808–817 [DOI] [PubMed] [Google Scholar]
- 16.Lambert MI, Noakes TD. 1990. Spontaneous running increases VO2max and running performance in rats. J Appl Physiol 68:400–403 [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MJ, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography 2005. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 18.Migrino RQ, Zhu X, Pajewski N, Brahmbhatt T, Hoffmann R, Zhao M. 2007. Assessment of segmental myocardial viability using regional 2-dimensional strain echocardiography. J Am Soc Echocardiogr 20:342–351 [DOI] [PubMed] [Google Scholar]
- 19.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. 2000. Treadmill running produces both positive and negative physiological adaptations in Sprague–Dawley rats. Am J Physiol Regul Integr Comp Physiol 279:R1321–R1329 [DOI] [PubMed] [Google Scholar]
- 20.Moraska A, Fleshner M. 2001. Voluntary physical activity prevents stress-induced behavioral depression and antiKLH antibody suppression. Am J Physiol Regul Integr Comp Physiol 281:R484–R489 [DOI] [PubMed] [Google Scholar]
- 21.Morris RT, Fine DM, Lees SJ, Booth FW, Link CD, Ferrario CM, Stump CS, Sowers JR. 2007. Exercise training prevents development of cardiac contractile dysfunction in hypertensive TG (mREN2)27 rats. J Am Soc Hypertens 1:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nance DM, Bromley B, Barnard RJ, Gorski RA. 1977. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiol Behav 19:155–158 [DOI] [PubMed] [Google Scholar]
- 23.Natali AJ, Turner DL, Harrison SM, White E. 2001. Regional effects of voluntary exercise on cell size and contraction-frequency responses in rat cardiac myocytes. J Exp Biol 204:1191–1199 [DOI] [PubMed] [Google Scholar]
- 24.Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. 2002. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes. J Physiol 541:863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuyt AM. 2008. Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 114:1–17 [DOI] [PubMed] [Google Scholar]
- 26.Plante E, Lachance D, Drolet MC, Roussel E, Couet J, Arsenault M. 2005. Dobutamine stress echocardiography in healthy adult male rats. Cardiovasc Ultrasound 3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porrello ER, Widdop RE, Delbridge LM. 2008. Early origins of cardiac hypertrophy: does cardiomyocyte attrition programme for pathological ‘catch-up’ growth of the heart? Clin Exp Pharmacol Physiol 35:1358–1364 [DOI] [PubMed] [Google Scholar]
- 28.Sexton WL. 1995. Vascular adaptations in rat hindlimb skeletal muscle after voluntary running-wheel exercise. J Appl Physiol 79:287–296 [DOI] [PubMed] [Google Scholar]
- 29.Shao CH, Wehrens XH, Wyatt TA, Parbhu S, Rozanski GJ, Patel KP, Bidasee KR. 2009. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol 106:1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherwin CM. 1998. Voluntary wheel running: a review and novel interpretation. Anim Behav 56:11–27 [DOI] [PubMed] [Google Scholar]
- 31.Tei C, Nishimura RA, Seward JB, Tajik AJ. 1997. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 10:169–178 [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Ma JZ, Zhu SS, Xu DJ, Zou JG, Cao KJ. 2008. Swimming training can affect intrinsic calcium current characteristics in rat myocardium. Eur J Appl Physiol 104:549–555 [DOI] [PubMed] [Google Scholar]
- 33.Watson LE, Sheth M, Denyer RF, Dostal DE. 2004. Baseline echocardiographic values for adult male rats. J Am Soc Echocardiogr 17:161–167 [DOI] [PubMed] [Google Scholar]
- 34.Wisloff U, Loennechen JP, Currie S, Smith GL, Ellingsen O. 2002. Aerobic exercise reduces cardiomyocyte hypertrophy and increases contractility, Ca2+ sensitivity and SERCA2 in rat after myocardial infarction. Cardiovasc Res 54:162–174 [DOI] [PubMed] [Google Scholar]
- 35.Wisloff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. 2001. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance-trained rats. Cardiovasc Res 50:495–508 [DOI] [PubMed] [Google Scholar]
- 36.Yancey SL, Overton JM. 1993. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol 75:1334–1340 [DOI] [PubMed] [Google Scholar]