Abstract
A 5.5-y-old spayed female ferret (Mustela putorius furo) with a history of adrenal disease, respiratory disease, and chronic obesity was evaluated for progressive lethargy and ataxia, diminished appetite, and possible polyuria and polydipsia. Physical examination revealed obesity, lethargy, tachypnea, dyspnea, a pendulous abdomen, significant weakness and ataxia of the hindlimbs, prolonged skin tenting, and mild tail-tip alopecia. Clinicopathologic analysis revealed severe hyperglycemia, azotemia, an increased anion gap, glucosuria, ketonuria, proteinuria, and hematuria. Abdominal ultrasonography showed hyperechoic hepatomegaly, bilateral adrenomegaly, splenic nodules, mild peritoneal effusion, and thickened and mildly hypoechoic limbs of the pancreas with surrounding hyperechoic mesentery. Fine-needle aspirates of the liver were highly suggestive of hepatic lipidosis. In light of a diagnosis of concurrent diabetic ketoacidosis and pancreatitis, the ferret was treated with fluid therapy, regular and long-acting insulin administration, and pain medication. However, electrolyte derangements, metabolic acidosis, dyspnea, and the clinical appearance of the ferret progressively worsened despite treatment, and euthanasia was elected. Necropsy revealed severe hepatic lipidosis, severe suppurative pancreatitis and vacuolar degeneration of pancreatic islet cells, a pancreatic β islet cell tumor, bilateral adrenal cortical adenomas, and myocardial fibrosis. To our knowledge, this case represents the first report of concurrent diabetes mellitus, pancreatitis, pancreatic β islet cell tumor (insulinoma), and adrenal disease in a domestic ferret. The simultaneous existence of 3 endocrine diseases, pancreatitis, and their associated complications is a unique and clinically challenging situation.
Endocrine diseases are extremely common in domestic ferrets in North America. The 2 most commonly encountered endocrine diseases are adrenal gland disease (with a spectrum of causes ranging from benign hyperplasia to malignant carcinoma14) and pancreatic β islet cell tumors (insulinomas).7 Unlike dogs and cats, ferrets rarely are diagnosed with diabetes mellitus, and when the disease does occur, it is most commonly a postoperative sequela to pancreatectomy performed to remove an insulinoma.14 Factors that predispose ferrets to the development of diabetes mellitus are not currently known because of the lack of reported cases, but risk factors for ferret diabetes likely will be similar to those identified in dogs and cats, such as obesity, excess glucocorticoids, and pancreatitis. As with all species, diabetes mellitus in ferrets can result in the more serious metabolic disturbance of diabetic ketoacidosis.
Case Report
A 5.5-y-old spayed female ferret (Mustela putorius furo) presented for evaluation of progressive lethargy and ataxia, diminished appetite, and possible polyuria and polydipsia. The ferret had extensive history of previously documented medical problems including chronic obesity, intermittent gastroenteritis, mild vacuolar hepatopathy, intermittent mild hypoglycemia, bilateral adrenomegaly, bilateral elbow osteochondromatosis, and long-standing pleural effusion of unknown cause. At the time of the current presentation, the ferret's adrenal disease was being managed by using a subcutaneous deslorelin implant (Suprelorin, Peptech Animal Health, New South Wales, Australia),which had been placed approximately 4 mo prior to the current presentation. The ferret also was receiving oral prednisolone (1.5 mg/kg PO every 24 h) for chronic respiratory signs; the prednisolone had been given for approximately 1 mo on a tapering course. Obesity had been a long-standing problem in the ferret prior to the start of prednisolone administration. Vaccinations for rabies and canine distemper were current. The ferret's diet consisted of variable mixtures of dry ferret food, dry kitten food, commercial cat food (a/d, Hill's Pet Nutrition, Topeka, KS), baby food, and Nutrical supplement (Vetoquinol USA, Forth Worth, TX).
On physical examination, the ferret was quiet and alert, had tacky pale-pink mucus membranes with a capillary refill time of 2 s, and increased respiratory rate and effort. The ferret was obese (1.2 kg; reference range for female ferret body weight, 0.5 to 1.0 kg4), with a pendulous abdomen, decreased muscle mass, significant weakness and ataxia of the hindlimbs, prolonged skin tenting, and mild tail-tip alopecia.
A plasma chemistry panel obtained at admission showed severe hyperglycemia (935 mg/dL; reference range, 63 to 134 mg/dL4), azotemia (blood urea nitrogen: 103 mg/dL; reference range, 12 to 43 mg/dL; creatinine: 4.5 mg/dL; reference range, 0.2 to 0.6 mg/dL), mild hyponatremia (143 M; reference range, 146 to 160 M), mild hypokalemia (4.2 M; reference range, 4.3 to 5.3 M), mild hypochloremia (100 M; reference range, 102 to 121 M), mild hypobicarbonemia (12 M; no established reference range for ferrets), an elevated anion gap (35.6 mEq/L; feline reference range, 8 to 12 mEq/L), and marked lipemia. A CBC showed a normal leukocyte count (5400 cells/µL; reference range, 2500 to 8600 cells/µL) with mild neutrophilia (4400 cells/µL), and mild anemia (45%; reference range, 47% to 51%). A voided urine sample had a specific gravity of 1.021 (measured on a feline scale by refractometer) despite clinical dehydration, and a urine dipstick test was positive for glucose (2 g/dL), ketones (80 mg/dL), blood (1+), and protein (100 mg/dL). Laboratory results are summarized in Table 1. The combined findings of hyperglycemia and low bicarbonate with concurrent glucosuria and ketonuria were consistent with a diagnosis of diabetic ketoacidosis.
Table 1.
Serial chemistry and urinalysis values from a ferret that presented with concurrent diabetic ketoacidosis and pancreatitis
| After admission |
|||
| At admission | 24 h | 48 h | |
| Plasma Chemistry (reference range) | |||
| Glucose (63–134 mg/dL) | 935 | 484 | 194 |
| Urea nitrogen (12–43 mg/dL) | 103 | 57 | 49 |
| Creatinine (0.2–0.6 mg/dL) | 4.5 | 1.1 | 1.0 |
| Protein, total (5.3–7.2 g/dL) | 7.1 | 4.8 | 4.8 |
| Albumin (3.3–4.1 g/dL) | 3.1 | 2.1 | 1.7 |
| Globulin, calculated (2.0–2.9 g/dL) | 4.0 | 2.7 | 3.1 |
| Calcium, total (8.6–10.5 mg/dL) | 11.1 | 9.0 | 9.3 |
| Phosphorus (5.6–8.7 mg/dL) | 7.7 | 3.8 | 5.8 |
| Sodium (146–160 mmol/L) | 143 | 157 | 157 |
| Potassium (4.3–5.3 mmol/L) | 4.3 | 3.6 | 4.1 |
| Chloride (102–121 mmol/L) | 100 | 132 | 134 |
| Bicarbonate (mmol/L) | 12 | 6 | 8 |
| Anion gap, calculated (mmol/L) | 36 | 24 | 20 |
| Alanine aminotransferase (82–289 U/L) | 132 | 105 | 218 |
| Alkaline phosphatase (30–120 U/L) | 48 | 40 | 17 |
| Creatine kinase (U/L) | 150 | 393 | 219 |
| Bilirubin, total (<1 mg/dL) | 0.2 | 1.3 | 0.6 |
| Cholesterol (102–121 mg/dL) | 296 | 273 | 191 |
| Plasma appearance | marked lipemia | marked lipemia | normal |
| Urinalysis | |||
| Urine specific gravity | 1.021 | ||
| Glucose (g/dL) | 2 | 1 | 0 |
| Ketones (g/dL) | 80 | 40-80 | 0 |
| Hemoglobin (g/dL) | 1+ | 3+ | not detectable |
| Protein (mg/dL) | 100 | 30 | not detectable |
A ferret reference range was unavailable for plasma bicarbonate, anion gap, and creatine kinase and all urinalysis parameters. Reference intervals were obtained from reference 4.
After placement of an intravenous catheter, intravenous administration of 0.9% NaCl supplemented with 40 mEq/L potassium chloride was provided. A few hours after starting fluid therapy, the ferret's blood glucose still exceeded the upper limit of the glucometer (AccuChek, Roche Diagnostics, Indianapolis, IN) and regular insulin therapy was started (0.25 U/kg IM every 4 h as long as the blood glucose was greater than 250 mg/dL). Three hours after insulin was begun, the blood glucose had decreased to 204 mg/dL.
A plasma chemistry panel obtained 24 h after admission showed persistent hyperglycemia but improving azotemia. However, hypophosphatemia had developed, and hypokalemia and hypobicarbonemia had worsened (Table 1). Intravenous fluid additives were modified to a 1:1 combination of potassium chloride (20 mEq/L) and potassium phosphate (20 mEq/L) to provide potassium and phosphate supplementation. Glucosuria, ketonuria, proteinuria, and hematuria persisted (Table 1). The presence of hematuria suggested urinary tract infection. Because results from a urine sediment examination were not available, empirical therapy using amoxicillin–clavulanate (13 mg/kg PO every 12 h; Clavamox, Pfizer, New York, NY) was begun. Regular insulin administration (0.25 U/kg IM every 4 h) was continued as indicated by frequent blood glucose determinations. After 24 h, insulin therapy was modified to include insulin glargine (0.5 U SC every 12 h; Lantus, Sanofi–Aventis US, Bridgewater Township, NJ), a long-acting synthetic insulin, along with regular insulin (0.25 U/kg IM every 4 h), which was supplemented as needed for short-term control of hyperglycemia.
Abdominal ultrasonography obtained after the patient was stabilized showed hyperechoic hepatomegaly with distended hepatic and portal vasculature, bilateral adrenomegaly, splenic nodules, mild peritoneal effusion, thickened and mildly hypoechoic limbs of the pancreas with surrounding hyperechoic mesentery, and mild bilateral pyelectasia. The sonographic findings were most suggestive of acute, severe pancreatitis. The sonographic appearance of the liver suggested hepatic lipidosis, which was confirmed by cytology of tissue obtained by fine-needle aspiration.
Once the diagnosis of pancreatitis was established, the ferret was given buprenorphine (0.01 mg/kg IV every 6 h) to treat abdominal pain and discomfort. After 48 h of insulin therapy and supportive care, the ferret was normoglycemic and no longer had glucosuria or ketonuria. On the third day of treatment, a heart murmur was ausculted. The volume of intravenous fluid was decreased from 100 mL/kg daily to 60 mL/kg daily due to the concern that aggressive fluid therapy might have compromised cardiac function. However the ferret's condition steadily deteriorated over the next 12 h. The heart murmur persisted, respiratory rate and effort increased considerably, abdominal discomfort worsened, and the ferret developed anuria. Plasma chemistry panel revealed mild hyperglycemia, normal phosphorus, and hypokalemia, but persistently low bicarbonate (Table 1). Because of concerns about possible congestive heart failure and the poor prognosis of treating concurrent heart disease, diabetic ketoacidosis, and pancreatitis, the ferret's owners elected euthanasia.
On gross necropsy, the ferret was severely obese with abundant fat deposits within the thoracic and abdominal cavities. The liver was tan and friable and bulged on cut surface. The kidneys were also tan and friable. The heart was rounded and lacked a prominent apex. The pancreas and abundant peripancreatic fat appeared grossly normal.
Histopathologic examination of tissues with hematoxylin and eosin and oil red O stains revealed diffuse swelling of hepatocytes, with numerous lipid vacuoles. Within the kidneys, the renal tubular epithelial cells contained intracytoplasmic lipid vacuoles. In addition, the renal interstitium had multifocal areas of low numbers of lymphocytes and plasma cells.
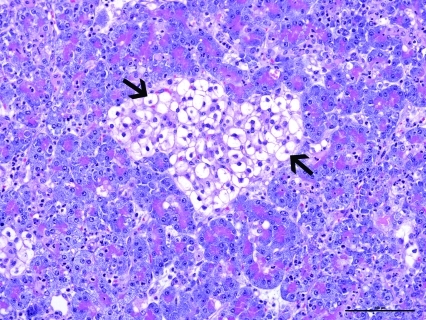
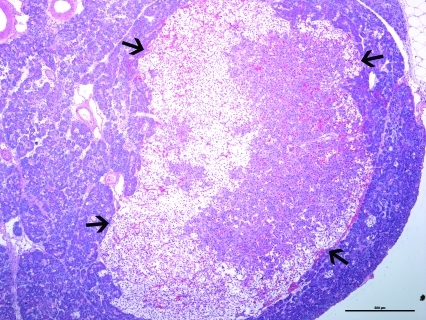
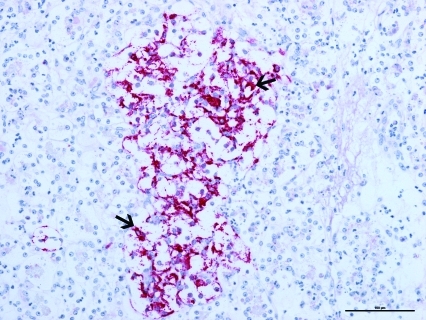
The pancreas was infiltrated by large numbers of intact and degenerate neutrophils surrounding the pancreatic acini and within the pancreatic ducts. The pancreatic islet cells had cytoplasmic vacuolation with glycogen accumulation (Figure 1). The mesenteric fat surrounding the pancreas had adipocyte necrosis, with infiltration by small numbers of intact and degenerate neutrophils. In addition, there was a poorly delineated, unencapsulated, moderately cellular pancreatic neoplasm (Figure 2). The neoplasm was composed of small clusters and nests of round to polygonal cells arranged on a thin fibrovascular stroma. The neoplastic cells contained moderate amounts of amphophilic granular cytoplasm with indistinct cell borders. The nuclei were round to oval, with finely granular chromatin, and prominent nucleoli. There was mild anisocytosis and anisokaryosis. Immunohistochemical staining of the mass was positive for insulin within the neoplastic cells (Figure 3).
Figure 1.
Photomicrograph of the pancreas from a ferret (Mustela putorius furo) that presented with concurrent diabetic ketoacidosis and severe pancreatitis. Note the vacuolar degeneration of the pancreatic islet cells (arrows). Hematoxylin and eosin stain; bar, 100 µm.
Figure 2.
Photomicrograph of the pancreatic mass from the ferret described in Figure 1. A well-delineated (arrows), expansile, moderately cellular neoplasm composed of nests and packets of neoplastic cells in a fine fibrovascular stroma was present. Hematoxylin and eosin stain; bar, 500 µm.
Figure 3.
Photomicrograph of the pancreatic mass from the ferret described in Figure 1. The neoplasm is strongly positive for insulin stain (arrows) in the cytoplasm of the neoplastic cells. Bar, 100 µm.
The left and right ventricles of the heart had multifocal to coalescing areas of myocardial degeneration characterized by sarcoplasmic fragmentation and hypereosinophilia with replacement by moderate amounts of fibrous connective tissue (confirmed by Masson trichrome stain). There were multifocal regions of cardiomyocyte hypertrophy adjacent to the areas of degeneration.
Each adrenal gland contained a partially delineated, moderately cellular, unencapsulated neoplasm composed of sheets of polygonal cells that compressed on the adjacent cortex and medulla. The neoplastic cells had indistinct cell borders with moderate amounts of eosinophilic granular cytoplasm. The nuclei were round to oval with finely granular chromatin and contained prominent nucleoli. There was mild anisocytosis and anisokaryosis. Mitotic figures were rare. One adrenal gland had multiple irregular cysts lined by flattened cuboidal to tall columnar epithelium that was positive for cytokeratin by immunohistochemistry, indicating epithelial origin. The cyst lumen contained pale eosinophilic homogenous material with occasional mineralization.
These histopathologic findings were consistent with severe diffuse hepatic lipidosis; moderate diffuse renal tubular epithelial lipidosis and mild multifocal interstitial nephritis; severe acute suppurative pancreatitis with multifocal pancreatic necrosis and peripancreatic fat necrosis; severe diffuse vacuolar degeneration of the pancreatic islet cells; a pancreatic β islet cell insulin-producing neoplasm (insulinoma); moderate multifocal to coalescing myocardial fibrosis; and bilateral adrenal cortical adenoma and unilateral adrenal cortical cysts.
Discussion
To date, the literature contains only a few reports of spontaneous diabetes mellitus in domestic ferrets,1,2,8 although a single case of diabetes mellitus in a black-footed ferret (Mustela nigripes) has been reported.5 The etiology of the diabetes mellitus in the current case is likely multifactorial. In general, diabetes mellitus is due to either failure of insulin production or failure of insulin action. Sometimes both mechanisms can occur concurrently. Deficient insulin production can be a result of genetic mutations, conditions that produce persistent hyperglycemia or hyperlipidemia (that is, glucose toxicity and lipotoxicity), or destruction of pancreatic β islet cells, such as with immune-mediated disease, pancreatitis, pancreatic trauma, or alteration of normal pancreatic architecture.6,11,13 In the present case, severe pancreatitis could have impaired insulin production by destroying endocrine tissue, leading to insulinopenia and clinical diabetes. We also hypothesize that the vacuolar degeneration in the pancreatic islet cells may have altered cell function and decreased insulin production, although whether the vacuolar change preceded the development of pancreatitis is unclear. Indeed it is also possible that diabetes may have preceded and ultimately lead to pancreatitis. One theory is that the hyperlipidemia often associated with diabetic ketoacidosis has a toxic effect on pancreatic acinar cells and capillary endothelium, thereby leading to pancreatic inflammation and necrosis.12 In humans, progression of pancreatitis to diabetic ketoacidosis and diabetic ketoacidosis to pancreatitis are thought both to occur. Acute pancreatitis has been recognized in 10% to 15% of human diabetic ketoacidosis cases.12 In a study of dogs with diabetic ketoacidosis, 41% also had acute pancreatitis, which was the most common concurrent disorder.9 Determining the order of development of these diseases is often not possible.
Insulin resistance at the receptor level can be caused by obesity, stress, high carbohydrate diets, glucocorticoid administration, and chronic hyperinsulinemia (as occurs with an insulinoma).1,6,11,15 The ferret in the current case had severe obesity, which might have increased the risk of developing diabetes considerably. Obesity in humans and cats has been linked to the onset of type II diabetes mellitus.11 In addition, the ferret was receiving prednisolone, which also may have contributed to both obesity and insulin resistance.
A prior history of clinical signs (alopecia and pruritus), significant adrenal enlargement on multiple abdominal ultrasonograms, and histologic evidence of bilateral adrenal cortical adenomas were all strongly suggestive of adrenal disease in this ferret. Treatment with leuprolide acetate and the deslorelin implant may have caused a reduction in the size of the adrenal glands, leaving only very small visible adenomas at necropsy. Previous case reports and studies have found variable responses in adrenal size to treatment with leuprolide and deslorelin. In one ferret assessed by using serial ultrasound examinations, the adrenal gland shrunk after treatment.16 In other studies, adrenal size either stabilized or eventually increased with treatment.19,20 The reason for the variability observed in adrenal size after treatment is unknown but may reflect the underlying adrenal pathology (hyperplasia compared with adenoma compared with adenocarcinoma) at the time of treatment.16,19,20
Adrenal disease in ferrets usually results in overproduction of sex hormones, rather than cortisol. However, more diffuse adrenal involvement can occur,16 in which situation an overproduction of corticosteroids may result and ultimately exacerbate insulin resistance.2 Like cortisol, gonadotropin-releasing hormone is an endogenous molecule that promotes insulin resistance. Therefore, treatment with gonadotropin-releasing hormone agonists such as leuprolide and deslorelin can foster the development of diabetes. In human prostate cancer studies, an association between men treated with gonadotropin-releasing hormone agonists and increased incidence of diabetes and cardiovascular disease has been documented.10,18 Gonadotropin-releasing hormone agonists can have diabetogenic consequences because of their effects to promote obesity, increase the fasting plasma insulin level (through induction of insulin resistance), and increase levels of serum cholesterol and triglycerides. Last, the ferret had a previously undiagnosed pancreatic β islet cell tumor discovered at necropsy (Figure 2). Prior to the onset of diabetes in this ferret, intermittent mild hypoglycemia was noted on routine blood glucose checks, but clinical episodes of hypoglycemia did not occur. Whether the presence of the insulinoma played a role in the development of diabetes is unknown. Concurrent diabetes mellitus and pancreatic β islet cell tumors are rare but have been documented in humans and dogs, although no cause and effect relationship has been proven.3,17
Just as there are many possible causes for diabetes mellitus, there are also numerous triggers for the development of diabetic ketoacidosis. Any infection, systemic disorder, or other malady that further inhibits insulin production or its effects can shift the diabetic patient toward a ketoacidotic state, in which increased hepatic delivery of free fatty acids results in excessive ketone production. In this particular patient, it is difficult to say what precipitated ketoacidosis, because the ferret had a multiple concurrent disorders and had been receiving steroids. The fatty changes observed in the ferret's liver are likely a combination of obesity, anorexia, and diabetes. Hepatic lipidosis has been reported to occur in ferrets and cats as a consequence of diabetes mellitus.1,5,6 Because the ferret was obese, it also had diffuse deposition of fat within the thoracic and abdominal cavities, which likely contributed to the dyspnea, onset of diabetes, and associated complications. Alternatively, the ferret's tachypnea and dyspnea may have been a reflection of underlying heart disease and attempted respiratory compensation for metabolic acidosis.
Here we document a case of concurrent diabetes mellitus, pancreatitis, and pancreatic β islet cell tumor (insulinoma) in a ferret; to our knowledge this constellation of diseases has not previously been described in this species. The clinical presentation in the current case was dominated by signs caused by complicated diabetes mellitus. In general, the prognosis for diabetes mellitus in ferrets is guarded to poor, and this outlook worsens with complications such as diabetic ketoacidosis.6,14 Concurrent pancreatitis likely made effective treatment more difficult. Simultaneous acute pancreatitis and diabetic ketoacidosis have not been associated with a poorer prognosis in dogs and humans.9,12 However, such studies have not yet been performed in ferrets, and experience managing both of these diseases is scarce. Treatment of diabetes ketoacidosis consists of aggressive fluid therapy, electrolyte corrections, and insulin therapy. Short-acting insulin (regular insulin in the current case) is used initially to gain glycemic control, after which long-acting insulin (insulin glargine in the current case) is used for maintenance glycemic control. Fluid therapy was challenging in the current case because of difficulty in establishing and maintaining venous access due to the patient's small size and obese body condition and because of her subsequent intolerance of the catheter once placed. Regular insulin was very well tolerated in this patient. Theoretically the long-acting insulin glargine might have proved an effective long-term insulin therapy option for this ferret; unfortunately the patient did not survive long enough for evaluation of long-term glycemic control. The use of long-acting insulins, such as glargine, in cases of ferret diabetes mellitus would be interesting area of future research. Multiple concurrent diseases including endocrine diseases, pancreatitis, and cardiac disease made effective treatment extremely difficult in the current case.
References
- 1.Benoit-Biancamano MO, Morin M, Langlois I. 2005. Histopathologic lesions of diabetes mellitus in a domestic ferret. Can Vet J 46:895–897 [PMC free article] [PubMed] [Google Scholar]
- 2.Boari A, Papa V, Di Silverio F, Aste G, Olivero D, Rocconi F. 2010. Type 1 diabetes mellitus and hyperadrenocorticism in a ferret. Vet Res Commun 34:S107–S110 [DOI] [PubMed] [Google Scholar]
- 3.Bryson ER, Snead ECR, McMillan C, MacDougall L, Allen AL. 2007. Insulinoma in a dog with preexisting insulin-dependent diabetes mellitus. J Am Anim Hosp Assoc 43:65–69 [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JW. 2005. Hematologic values of ferrets, p 464–465 : Exotic animal formulary, 3rd ed St Louis (MO): Elsevier/Saunders [Google Scholar]
- 5.Carpenter JW, Novilla MN. 1977. Diabetes mellitus in a black-footed ferret. J Am Vet Med Assoc 171:890–893 [PubMed] [Google Scholar]
- 6.Chen S. 2008. Pancreatic endocrinopathies in ferrets. Vet Clin North Am Exot Anim Pract 11:107–123 [DOI] [PubMed] [Google Scholar]
- 7.Fox JG, Dangler CA, Snyder SB, Richard MJ, Thilsted JP. 2000. C-cell carcinoma (medullary thyroid carcinoma) associated with multiple endocrine neoplasms in a ferret (Mustela putorius). Vet Pathol 37:278–282 [DOI] [PubMed] [Google Scholar]
- 8.Hillyer E. 1992. Ferret endocrinology, p 1185–1188 : Kirk RW, Bonagura JD. Current veterinary therapy XI: small animal practice. Philadelphia (PA): WB Saunders [Google Scholar]
- 9.Hume DZ, Drobatz KJ, Hess RS. 2006. Outcome of dogs with diabetic ketoacidosis: 127 dogs (1993–2003). J Vet Intern Med 20:547–555 [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, O'Malley AJ, Smith MR. 2006. Diabetes and cardiovascular diseases during androgen-deprivation therapy for prostate cancer. J Clin Oncol 24:4448–4456 [DOI] [PubMed] [Google Scholar]
- 11.Monroe WE, Rand JS, Greco DS. 2009. Endocrine and metabolic diseases, p 196–218 : Bonagura JD, Twedt DC. Kirk's current veterinary therapy XIV. St. Louis (MO): Saunders Elsevier [Google Scholar]
- 12.Nair S, Yadav D, Pitchumoni CS. 2000. Association of diabetic ketoacidosis and acute pancreatitis: observations in 100 consecutive episodes of DKA. Am J Gastroenterol 95:2795–2800 [DOI] [PubMed] [Google Scholar]
- 13.Nelson RW. 2003. Endocrine disorders, p 762–769 : Nelson RW, Couto CG. Small animal internal medicine, 3rd ed St Louis (MO): Mosby [Google Scholar]
- 14.Quesenberry KE, Rosenthal KL. 2004. Endocrine diseases, p79–90 : Quesenberry KE, Carpenter JW. Ferrets, rabbits, and rodents: clinical medicine and surgery, 2nd ed St Louis (MO): Saunders–Elsevier [Google Scholar]
- 15.Rand JS, Fleeman LM, Farrow HA, Appleton DJ, Lederer R. 2004. Canine and feline diabetes mellitus: nature or nurture? J Nutr 134:2072S–2080S [DOI] [PubMed] [Google Scholar]
- 16.Schoemaker NJ, Kuijten AM, Galac S. 2008. Luteinizing hormone-dependent Cushing's syndrome in a pet ferret (Mustela putorius furo). Domest Anim Endocrinol 34:278–283 [DOI] [PubMed] [Google Scholar]
- 17.Siraj ES, Samuel G, Saber S, Samuel S, Hamrahian AH, Reddy SS. 2006. Metastatic malignant insulinoma in a patient with type 2 diabetes mellitus: case presentation and literature review. Endocr Pract 12:411–416 [DOI] [PubMed] [Google Scholar]
- 18.Smith MR. 2008. Treatment-related diabetes and cardiovascular disease in prostate cancer survivors. Ann Oncol 19:vii86–vii90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner RA, Bailey EM, Schneider JF, Oliver JW. 2001. Leuprolide acetate treatment of adrenocortical disease in ferrets. J Am Vet Med Assoc 218:1272–1274 [DOI] [PubMed] [Google Scholar]
- 20.Wagner RA, Piche CA, Jochle W, Oliver JW. 2005. Clinical and endocrine responses to treatment with deslorelin acetate implants in ferrets with adrenocortical disease. Am J Vet Res 66:910–914 [DOI] [PubMed] [Google Scholar]