Abstract
The present study investigated effects of the physical form of the diet on food intake, growth, and body composition in male C57BL/6 mice. Three-week-old mice were fed isocaloric diets (AIN93G or a modification containing 25% wheat) in powdered or pelleted form. In experiment 1, mice were assigned into 4 groups offered the AIN93G or the wheat-modified diet in powdered or pelleted form. In experiment 2, mice were pair-fed the powdered diets to the ad libitum level of food intake of those fed the pelleted form of the respective diets. Body weight, food intake, and fecal excretion were recorded, and body composition was assessed on mice 1 wk before termination of the experiment. Mice fed the powdered diets showed greater increases in body weight in 2 wk of feeding than did mice fed the pelleted diets. Compared with the pelleted diets, the powdered diets supported an approximately 85% increase in the fat-mass:body-mass ratio and a 2-fold increase in the abdominal-fat-weight:carcass-weight ratio. In addition, mice fed the powdered diet showed significantly greater plasma concentrations of insulin and leptin and significantly lower plasma adiponectin, compared with their pellet-fed counterparts. Food intake of mice fed the powdered diet was 11% greater for the AIN93G and 16% greater for the wheat diet compared with that of the respective pelleted diet. These results demonstrate that C57BL/6 mice responded to the physical form of these diets in terms of food intake, which affected their growth, body composition, and plasma concentrations of insulin and adipocytokines.
Laboratory rodents are commonly used as animal models for obesity research. These models are established through various means,20 including diet-induced, spontaneous mutations (for example, ob/ob), and genetically engineered knockout methods. The diet-induced obesity models are probably the most relevant to humans, because obesity in most people is a consequence of polygenic interactions with the environment, especially dietary practice. Diet-induced obesity models require simple experimental procedures and are relatively low in cost. Strain, age, and sex of laboratory animals are known to affect growth response to high-fat diets.14 However, the effects of the physical form of the diet on animal growth have not been investigated.
We observed in our laboratory that mice gained weight differently in response to different physical forms of the same diet, and we suspected that this difference may have affected adipogenesis and the expression of plasma adipocytokines. The purpose of the present study was to investigate the effects of diet physical form (pellets compared with powder) on food intake, growth, and body composition changes in C57BL/6 mice.
Materials and Methods
This study was approved by the Animal Care and Use Committee of the USDA–ARS Grand Forks Human Nutrition Research Center. The procedures followed the NIH guidelines for the experimental use of laboratory animals.6
Animals and diets.
Male C57BL/6 mice (age, 3 wk; Harlan, Madison, WI) were housed individually in suspended stainless steel mouse cages in a room with a 12:12-h light:dark cycle. The temperature of the room was 22 ± 1 °C. The AIN93G diet18 and AIN93G diet modified by supplementation with 25% wheat were used in the present study (Table 1). The wheat (Wesley, hard-red winter wheat) was fully cooked (98 °C for 45 min), air dried, and ground to a fine powder before being added to the diet. The wheat-supplemented diet was modified so that it contained all nutrients in amounts equal to those of the AIN93G diet, based on the National Nutrient Database for hard red winter wheat.21 To make pelleted forms of each of those diets, 12% water was mixed well into the powdered diet; the resulting slurry then was pelleted (diameter, 1.27 cm) by using a laboratory pellet mill (model CL, CA Pellet Mill, San Francisco, CA). The immediate temperature of the freshly made pellets was 28 °C, measured by using an infrared temperature sensor (model Raynger ST, Raytek, Santa Cruz, CA). The pellets were air-dried at 29 °C for 48 h. All diets were stored at −20 °C, and fresh diets were provided to mice daily. Moisture content was less than 10% for all diets, measured by placing samples from frozen-stored diets in duplicates in a drying oven at 95 °C for 4 h.
Table 1.
Composition of the experimental diets
| AIN93G | Wheat-supplemented | |
| Ingredient (g/kg) | ||
| Casein | 200 | 164 |
| Corn starch | 395.8 | 216.7 |
| Cooked wheata | 0 | 250 |
| Dextrinized starch | 132 | 132 |
| Sucrose | 100 | 100 |
| Soybean oil | 70 | 66 |
| Cellulose | 50 | 19.5 |
| Mineral mixb | 35 | 35 |
| AIN-93G vitamin mix | 10 | 10 |
| L-methionine | 0.3 | 0.63 |
| L-cystine | 4.4 | 3.69 |
| Choline bitartrate | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 |
| Gross energy (kcal/g) | ||
| Calculatedc | 3.8 | 3.8 |
| Measured, powdered diet | 4.4 | 4.4 |
| Measured, pelleted diet | 4.4 | 4.4 |
Wesley, hard-red winter wheat.
Mineral mix contained calcium carbonate, anhydrous, 40.04% Ca, 555.26 g/kg; sodium chloride, 39.34% Na, 52.17 g/kg; sodium metasilicate, 9-hydrate, 9.88% Si, 1.45 g/kg; chromium potassium sulfate, 12-hydrate, 10.42% Cr, 0.275 g/kg; copper carbonate, 57.47% Cu, 0.143 g/kg; boric acid, 17.5% B, 0.082 g/kg; sodium fluoride, 45.24% F, 0.064 g/kg; nickel carbonate, 45% Ni, 0.032 g/kg; lithium chloride, 16.38% Li, 0.017 g/kg; potassium iodate, 59.3% I, 0.010 g/kg; ammonium paramolybdate, 4 hydrate, 54.34% Mo, 0.008 g/kg; ammonium vanadate, 43.55% V, 0.007 g/kg; and powdered sucrose, 390.482 g/kg.
Reference 21.
Experimental design.
In experiment 1, mice were assigned randomly into 4 groups (n = 7 or 8 each) that were fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet. Mice had free access to their assigned diet and deionized water, and they were weighed weekly. This experiment was repeated once with the same design (n = 6 or 7 per group), and data from these 2 replicates were combined for statistical analysis. Experiment 2 was a paired-feeding experiment to determine whether differences in body weight in mice fed the pelleted compared with the powdered diets was due a difference in caloric intake. Mice were assigned into 6 groups (n = 6 or 7 each), and they were fed ad libitum the pelleted or powdered diet or were pair-fed the respective powdered diet to match the level of food intake of the pellet-fed mice. The average 24-h food intake of the pellet-fed group was obtained and used as the total amount of diet that was provided to pair-fed mice on the following day in 2 allotments at a 12-h interval.13 Mice were weighed weekly, and food intake and fecal excretion were recorded daily for the paired-feeding study. The feeding period was 18 wk for all experiments. One week before termination of an experiment, body composition analysis of fat mass, lean mass, and total body water content of conscious, immobilized mice was performed by using quantitative magnetic resonance (model 100, Whole-body Composition Analyzer, Echo Medical System, Houston, TX). At experiment termination, mice were fasted overnight and then anesthetized with a mixture of ketamine and xylazine. Liver, kidneys, spleen, and abdominal adipose tissues (gonadal and perirenal) were collected and weighed, and plasma was collected and stored at −80 °C for adipocytokine analysis.
Plasma cytokine analyses.
Mouse obesity proteome profile array (R&D Systems, Minneapolis, MN) was used to assess the abundance of obesity-related cytokines in plasma from mice fed different diets. Plasma from 3 mice per group were pooled and screened for cytokine expression according to the manufacturer's protocol. Plasma concentrations of insulin (Mercodia, Uppsala, Sweden), leptin, and adiponectin (both from R&D Systems) were assessed by using sandwich ELISA assays according to manufacturers’ protocols.
Caloric intake.
Caloric content of experimental diets and feces was quantified by using bomb calorimetry (model 6200, Oxygen Bomb Calorimeter, Parr Instrument Moline, IL), and daily caloric intake was calculated by subtracting fecal caloric excretion (fecal caloric content × fecal excretion) from diet caloric intake (diet caloric content × food intake).
Gastrointestinal transit time.
Chromic oxide (a nondigestible, nonabsorbable marker; dose, 5000 mg Cr/kg diet) was used to measure the total gastrointestinal transition time16 of mice fed different diets. Mice were fasted overnight, after which they were fed 0.25 g pelleted or powdered Cr-supplemented diet followed by free access to their respective diets as normal. Feces were collected hourly for the first 10 h and then every 2 h for the next 6 h, and samples collected from each group were pooled for analysis. Fecal Cr content was quantified (model 6500; Thermo Scientific Inductively Coupled Argon Plasma, Fisher Diagnostics, Middletown, VA). Total transition time was estimated as the time from ingestion of the Cr-supplemented diet to the time of defecation of feces with the peak Cr content.16
Statistical analyses.
Experiment 1 used two-way ANOVA and Tukey contrasts to compare differences among the groups. Experiment 2 used one-way ANOVA and Tukey contrasts to analyze data from the paired-feeding experiment. All data are presented as mean ± SEM. Differences with a P value of 0.05 or less were considered significant. All statistical analyses were performed by using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
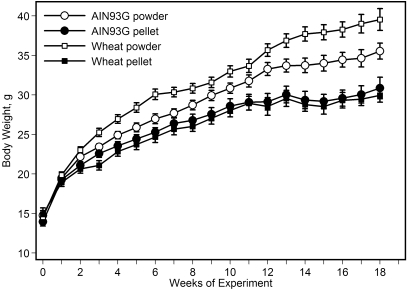
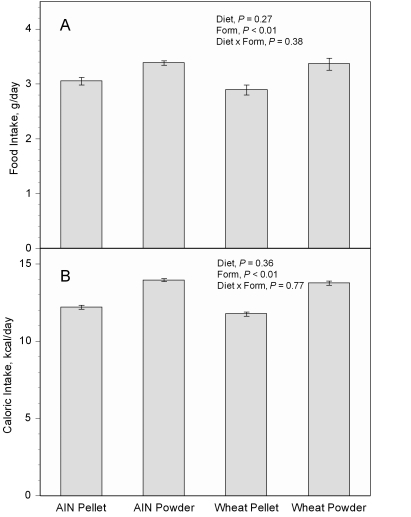
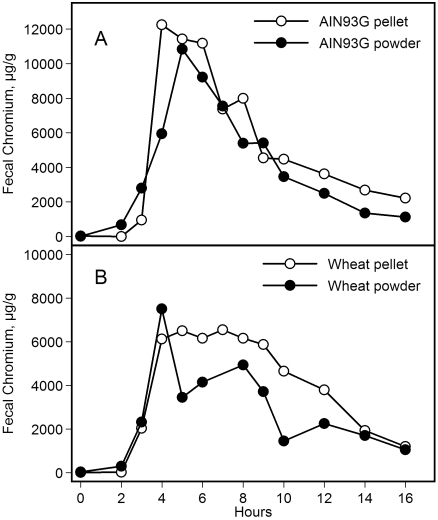
Feeding mice either version of the powdered diet (AIN93G or wheat-supplemented) increased body weight as compared with the pelleted diet (Figure 1). This difference was statistically significant (P < 0.01) 2 wk after initiation of the experiment, and the significant increase continued throughout the remainder of the experiment. Final body weights of mice at the week 18 are in Table 2. The body weight of mice pair-fed the powdered diet to the ad libitum intake level of mice that received the pelleted diet was similar to that of mice that received the pelleted diet but was significantly (P < 0.05) lower than that of mice fed the powdered diet ad libitum (Figure 2). Compared with those of mice fed the respective pelleted diet, food intake (Figure 3 A) was approximately 11% and 16% greater in mice fed the powdered AIN93G or wheat diet, respectively, and total caloric intake was approximately 14% and 17% greater, respectively (Figure 3 B); the differences in food and caloric intakes between powdered and pelleted diets were significant (P < 0.01). Total gastrointestinal transit time did not differ between mice fed powdered or pelleted diet. The time of defecation with peak Cr content was 4 to 5 h after the Cr challenge for all dietary groups (Figure 4).
Figure 1.
Body weights of mice fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet. Two-way ANOVA and Tukey contrasts were performed to compare differences among groups. The difference in body weight between groups fed the pelleted or powdered diet was significant (P < 0.01) 2 wk after initiation of the experiment, and the significant increase continued throughout the experiment. Data are presented as mean ± SEM (n = 13 to 15 per group).
Table 2.
Effects of the physical form of the diet on body weight, body composition, and organ weights of C57BL/6 mice
| AIN93G |
Wheat |
P |
|||||
| Pellet | Powder | Pellet | Powder | Diet effect | Form effect | Diet × form interaction | |
| Final body weight (g) | 30.9 ± 1.4a | 35.5 ± 1.0b | 30.0 ± 0.9a | 39.5 ± 1.4b | 0.166 | <0.001 | 0.031 |
| Fat mass:body mass (%) | 15.21 ± 1.85 | 26.67 ± 2.45 | 15.24 ± 1.40 | 29.84 ± 1.73 | 0.420 | <0.001 | 0.428 |
| Lean mass:body mass (%) | 72.36 ± 1.62 | 62.65 ± 2.14 | 72.18 ± 1.29 | 60.27 ± 1.48 | 0.462 | <0.001 | 0.527 |
| Total body water:body mass (%) | 61.36 ± 1.39 | 53.31 ± 1.68 | 61.14 ± 1.06 | 50.82 ± 1.33 | 0.346 | <0.001 | 0.426 |
| Fat mass (g) | 4.51 ± 0.56 | 8.66 ± 0.92 | 4.25 ± 0.58 | 10.95 ± 0.74 | 0.177 | <0.001 | 0.091 |
| Lean mass (g) | 21.75 ± 0.39 | 21.56 ± 0.23 | 21.39 ± 0.43 | 22.72 ± 0.45 | 0.258 | 0.116 | 0.042a |
| Body mass (g) | 30.08 ± 0.94 | 34.43 ± 1.33 | 29.22 ± 0.96 | 37.49 ± 0.87 | 0.312 | <0.001 | 0.076 |
| Abdominal fat weight: carcass weight (%) | 3.24 ± 0.45 | 6.31 ± 0.62 | 3.02 ± 0.36 | 6.75 ± 0.35 | 0.816 | <0.001 | 0.497 |
| Liver weight: carcass weight (%) | 3.89 ± 0.09 | 3.57 ± 0.09 | 3.82 ± 0.07 | 3.73 ± 0.14 | 0.654 | 0.036 | 0.258 |
| Kidney weight: carcass weight (%) | 1.50 ± 0.03 | 1.32 ± 0.06 | 1.61 ± 0.03 | 1.29 ± 0.07 | 0.424 | <0.001 | 0.195 |
| Spleen weight: carcass weight (%) | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.24 ± 0.02 | 0.24 ± 0.03 | 0.368 | 0.511 | 0.554 |
Two-way ANOVA (n = 12 to 15 per group) was performed for statistical analysis, and Tukey contrasts were performed for variables with significant (P ≤ 0.05) diet × form interaction. Within each row, means with different superscripts are significantly (P ≤ 0.05) different by Tukey contrasts. None of the pairwise comparisons are significant (P ≤ 0.05) by Tukey contrasts.
Figure 2.
Effects of paired-feeding on body weights of mice fed the (A) AIN93G or (B) wheat-supplemented diet. One-way ANOVA and Tukey contrasts were used to compare differences among groups. The difference in body weight between mice fed the powdered or pelleted diet was significant (P ≤ 0.05), but there was no difference (P > 0.05) between mice fed the pelleted diets ad libitum and those pair-fed the powdered form of the respective diet throughout the experiment. Data are presented as mean ± SEM (n = 6 to 7 per group).
Figure 3.
(A) Food intake and (B) caloric intake in mice fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet. Two-way ANOVA was used to compare differences among the groups. The interaction between diet and form was not significant (P > 0.05), so Tukey contrasts were not performed for any of the variables. Values are given as mean ± SEM (n = 6 or 7 per group).
Figure 4.
Gastrointestinal transit time of mice fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet after ingesting 0.25 g Cr-supplemented diet (n = 2 for each time point).
Powdered and pelleted forms of both diets were associated with significant differences in body composition (Table 2). The ratio of fat mass to body mass increased by approximately 85%, and the ratios of lean mass to body mass and total body water to body mass both decreased by approximately 15% in groups fed powdered diets compared with pelleted diets (P < 0.01). These changes were due to a net increase in fat mass; the lean mass was unchanged (Table 2). After the termination of the experiment, the abdominal fat (gonadal and perirenal) in mice fed powdered diet was approximately 2-fold more than that in pellet-fed mice (P < 0.01). Significant differences in the liver:carcass weight ratio and the kidney:carcass weight ratio also were present between powdered-diet- and pellet-fed mice (Table 2). In the paired-feeding experiment, the body composition of mice pair-fed the powdered diets was similar to that of pellet-fed mice but different from that of mice offered the powdered diet ad libitum (Table 3).
Table 3.
Body composition changes in C57BL/6 mice (n = 6 or 7 per group) fed the pelleted or powdered diet ad libitum or pair-fed the powdered diet to the level of intake of the pellet-fed mice
| Powdered diet |
||||
| Pelleted diet | Ad libitum feeding | Paired-feeding | ||
| AIN93G | Fat mass:body mass (%) | 28.06 ± 1.85b | 36.10 ± 0.90a | 26.78 ± 2.53b |
| Lean mass:body mass (%) | 61.51 ± 1.58a | 54.77 ± 0.81b | 62.12 ± 2.25a | |
| Total body water:body mass (%) | 52.00 ± 1.33a,b | 46.66 ± 0.79b | 53.14 ± 2.31a | |
| Wheat-supplemented | Fat mass:body mass (%) | 27.19 ± 2.08b | 34.14 ± 1.55a | 31.02 ± 1.47a,b |
| Lean mass:body mass (%) | 61.96 ± 1.59a | 56.59 ± 1.42b | 58.86 ± 1.11a,b | |
| Total body water:body mass (%) | 52.20 ± 1.47 | 48.09 ± 1.20 | 50.02 ± 1.15 | |
One-way ANOVA and Tukey contrasts were performed. Within a row, means with different superscripts are significantly (P ≤ 0.05) different.
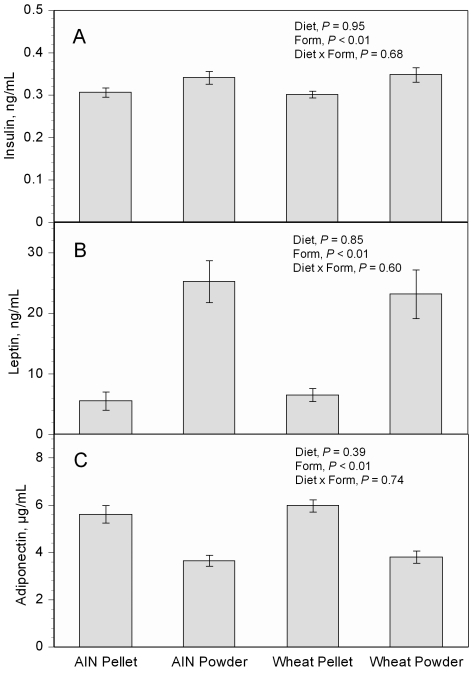
The physical forms of these diets affected plasma concentrations of insulin and adipocytokines. Mice fed powdered diets showed 11% increases in plasma insulin (P < 0.01, Figure 5 A), 4-fold increases in plasma leptin (P < 0.01, Figure 5 B), and 35% decreases in adiponectin (P < 0.01, Figure 5 C), compared with those fed the pelleted diets. Diet type (AIN93G compared with the wheat diet) did not cause significant differences in these variables in either physical form. The obesity proteome array analysis showed that different physical forms of the same diet affected the expression of plasma cytokines. The expression of hepatocyte growth factor, insulin-like growth factors 1 and 2, interleukin 11, leptin, leukemia inhibitor factor, oncostatin M, and tumor necrosis factor α were 50% or greater in mice fed powdered diets, either AIN93G or wheat-supplemented, than in mice fed pelleted diets (Table 4).
Figure 5.
Plasma concentrations of (A) insulin, (B) leptin, and (C) adiponectin in mice fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet. Two-way ANOVA was performed to compare differences among groups. Because the interaction between diet and form was nonsignificant (P > 0.05), Tukey contrasts were not performed for any of the variables. Values are mean ± SEM (n = 8 to 12 for each group).
Table 4.
Expression (arbitrary units) of obesity-related cytokines in plasma of C57BL/6 mice fed the pelleted or powdered form of the AIN93G or wheat-supplemented diet
| AIN93G |
Wheat-supplemented |
|||||
| Expression levels | Change (%)a | Expression levels | Change (%)a | |||
| Pellet | Powder | Pellet | Powder | |||
| Adiponectin | 738.3 | 567.5 | ↓ 23.1 | 331.8 | 244.9 | ↓ 26.2 |
| Agouti related protein | 3740.8 | 3754.9 | — | 1217.6 | 1264.3 | — |
| Angiopoietin-like 3 | 5231.8 | 3328.0 | ↓ 36.4 | 2657.8 | 2786.2 | — |
| C-reactive protein | 2650.9 | 1940.4 | ↓ 26.8 | 1324.8 | 1259.2 | — |
| Dipeptidyl peptidase IV | 4895.6 | 3359.5 | ↓ 31.4 | 2777.8 | 1932.0 | ↓ 30.4 |
| Endocan | 13360.6 | 12867.2 | — | 10762.8 | 11217.0 | — |
| Fetuin A | 1794.9 | 1244.4 | ↓ 30.7 | 805.3 | 567.4 | ↓ 29.5 |
| Fibroblast growth factor acidic | 458.7 | 387.3 | ↓ 15.6 | 377.0 | 270.0 | ↓ 28.4 |
| Fibroblast growth factor 21 | 10441.3 | 12618.6 | ↑ 20.9 | 9695.7 | 10249.2 | — |
| Hepatocyte growth factor | 212.7 | 333.9 | ↑ 57.0 | 243.66 | 300.9 | ↑ 23.5 |
| Intercellular adhesion molecule 1 | 4089.6 | 5992.3 | ↑ 46.5 | 3357.9 | 4680.5 | ↑ 39.4 |
| Insulin-like growth factor 1 | 2122.6 | 4272.1 | ↑ 101.3 | 1406.2 | 2070.1 | ↑ 47.2 |
| Insulin-like growth factor 2 | 558.2 | 1027.7 | ↑ 84.1 | 541.1 | 986.0 | ↑ 82.2 |
| Insulin-like growth factor binding protein 1 | 14526.0 | 13746.6 | — | 9828.8 | 9610.7 | — |
| Insulin-like growth factor binding protein 2 | 10078.6 | 10833.2 | — | 5747.4 | 6091.4 | — |
| Insulin-like growth factor binding protein 3 | 8684.5 | 8078.0 | — | 5418.4 | 6231.8 | ↑ 15.0 |
| Insulin-like growth factor binding protein 5 | 11391.1 | 11342.3 | — | 8692.3 | 8291.0 | — |
| Insulin-like growth factor binding protein 6 | 15012.2 | 14844.2 | — | 12903.4 | 12027.6 | — |
| Interleukin 6 | 308.3 | 276.3 | ↓ 10.4 | 131.1 | 183.8 | ↑ 40.2 |
| Interleukin 10 | 281.8 | 302.4 | — | 139.9 | 140.1 | — |
| Interleukin 11 | 497.0 | 943.6 | ↑ 89.9 | 288.7 | 415.1 | ↑ 43.8 |
| Leptin | 1975.3 | 6671.7 | ↑ 237.7 | 1085.6 | 3130.4 | ↑ 188.3 |
| Leukemia inhibitory factor | 599.3 | 940.5 | ↑ 56.9 | 329.6 | 430.3 | ↑ 30.5 |
| Lipocalin 2 | 15324.6 | 18757.7 | ↑ 22.4 | 14596.3 | 12440.8 | ↓ 14.8 |
| Monocyte chemotatic protein 1 | 1214.2 | 1604.5 | ↑ 32.1 | 915.3 | 1172.5 | ↑ 28.1 |
| Macrophage colony stimulating factor | 5433.1 | 5120.6 | — | 2668.5 | 2694.7 | — |
| Oncostatin M | 343.2 | 413.3 | ↑ 20.4 | 140.9 | 230.8 | ↑ 63.8 |
| Pentraxin 2 | 2979.0 | 3738.5 | ↑ 25.5 | 2099.0 | 2328.3 | ↑ 10.9 |
| Pentraxin 3 | 5133.2 | 5400.9 | — | 3568.5 | 3792.8 | — |
| Preadipocyte factor 1 | 1474.4 | 1273.9 | ↓ 13.6 | 744.1 | 776.7 | — |
| RAGE | 1728.4 | 1414.7 | ↓ 18.2 | 1344.0 | 1217.8 | — |
| RANTES | 269.9 | 224.9 | ↓ 16.7 | 244.2 | 164.7 | ↓ 32.6 |
| Retinol binding protein 4 | 783.2 | 841.1 | — | 531.6 | 398.8 | ↓ 25.0 |
| Resistin | 18832.2 | 19548.8 | — | 16779.7 | 15022.1 | ↓ 10.5 |
| Serpin E1 | 7868.7 | 10324.4 | ↑ 31.2 | 5977.7 | 6473.4 | — |
| Tissue inhibitors of metalloproteinase 1 | 3666.9 | 5355.6 | ↑ 46.1 | 1702.3 | 2192.1 | ↑ 28.8 |
| Tumor necrosis factor α | 287.1 | 497.3 | ↑ 73.2 | 194.4 | 255.3 | ↑ 31.3 |
| Vascular endothelial growth factor | 1681.3 | 1672.8 | — | 863.1 | 821.8 | — |
RAGE, receptor for advanced glycation end products; RANTES, regulated upon activation, normal T cell expressed and presumably secreted
Values within 10% are not presented.
Discussion
We investigated effects of the physical form of the diet, comparing pelleted and powdered diets, on growth and changes in body composition in C57BL/6 mice. We found significant differences in body weight, body composition, abdominal adiposity, and plasma insulin and adipocytokine concentrations between mice fed the powdered and pelleted diets, with no differences in these measurements between those given the AIN93G and wheat-supplemented diets. These responses were associated with differences in food intake, even though the diets were isocaloric and contained equal concentrations of all nutrients. The greater intake of the powdered form of each diet resulted in 14% to 17% increases in caloric intake, compared with the pelleted diet.
The most important findings concerned the increased food intake in mice fed powdered diet, which resulted in significant changes in plasma concentrations of insulin, leptin, and adiponectin and the expression of related cytokines in plasma. In obesity, adipose-derived hormones affect insulin action and energy homeostasis. Insulin and leptin are secreted into the bloodstream in proportion to the body's adipose mass,12,19 and a decrease in adiponectin is associated with insulin resistance.4 The present study showed that increases in plasma concentrations of insulin and leptin, a decrease in plasma adiponectin, and increased expression of hepatocyte growth factor, insulin-like growth factors 1 and 2, interleukin 11, leptin, leukemia inhibitor factor, oncostatin M, and tumor necrosis factor α by mice fed the powdered diets compared with pelleted diets. Insulin-like growth factors share many of the effects of insulin and similarly are involved in metabolic homeostasis.7 Hepatocyte growth factor24 and tumor necrosis factor α11 increase in diet-induced obesity, and both are angiogenic in animal models.2,10 Leukemia inhibition factor, interleukin 11, and oncostatin M belong to the interleukin 6 subfamily, and they are multifunctional cytokines, with proinflammatory effects.3 The observed changes in concentrations and expression of adipocytokines in the mice fed powdered diet are very similar to those observed in mice fed high-fat diet,15 indicating the involvement of active inflammatory reactions in powdered-diet-induced adipogenesis.
The pelleting process may have affected these diets in ways that influenced consumption by mice. One such possibility is reduction of metabolizable energy or protein digestibility by formation of Maillard products due to the heat induced by the friction of pelleting.8,23 Another is oxidative degradation during storage.17 Third is that pellet stability may have increased the rate of gastrointestinal transit, thus reducing absorptive efficiency. In the present study, the pelleting process only minimally increased the temperature of the diet: the temperature of freshly made pellets was 28 °C. All the diets were stored at −20 °C in sealed containers before the use, and the moisture content of all the diets was similar. Gastrointestinal transit time was not different for the pelleted and powdered forms of either diet, indicating that the hardness of the pelleted diets did not affect the transition time of the diet and that both forms of the diet had equal time for digestion and absorption during their transition through the gastrointestinal tract. Therefore, we concluded that the differences between mice in responses to pelleted and powdered forms of these diets were due to differences in their rates of food intake.
C57BL/6 mice are commonly used laboratory animals and are more sensitive to dietary composition changes than are other strains (for example, BALB/c and A/J mice).5 These mice become obese when they are fed a high-fat or a high-carbohydrate diet9 and develop leptin and insulin resistance,1,22 mimicking humans with obesity. In the present study, C57BL/6 mice responded differently to different physical forms of the same diet. They consumed more food when it was presented in powdered form than when it was presented as pellets. The difference was associated with corresponding differences in the intake of nutrients including calories and with differences in plasma adipocytokine concentrations. These results demonstrate the importance of considering the physical form of the diet in designing experiments to minimize confounding influences and experimental variations.
The AIN93 formulation18 is the most commonly used diet for laboratory rodents. In their publications, many investigators report the formulation of the diet but usually not its physical form. Commercially produced diets often are pelleted, whereas laboratory-produced diets typically are powdered, perhaps because many laboratories do not have pellet mills. Although neither of the diets used in the present study was adipogenic, our results clearly show differences between pelleted and powdered diets in food intake, body weight, body composition, and plasma adipocytokine concentrations and expression. Therefore, the physical form of a diet used in an experiment should be presented in scientific publications for comparison of results with those of other studies and for repeatability by other investigators.
Acknowledgments
We thank the South Dakota Wheat Commission and Mr Steve Taylor, Lyman, South Dakota, for providing us wheat sample to this study, and we gratefully acknowledge the assistance of the following staff of the Grand Forks Human Nutrition Research Center: James Lindlauf for preparing experimental diets, Kay Keehr for technical supports, Craig Lacher and William Martin for conducting Cr analysis, and vivarium staff for providing high-quality animal care.
Supported by the US Department of Agriculture, ARS, research project 5450-51000-036-00D.
The US Department of Agriculture, Agricultural Research Service, Northern Plains Area, is an equal opportunity–affirmative action employer, and all agency services are available without discrimination. Mention of trade names or commercial products in this article is solely for providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- 1.Almind K, Kulkarni RN, Lannon SM, Kahn CR. 2003. Identification of interactive loci linked to insulin and leptin in mice with genetic insulin resistance. Diabetes 52:1535–1543 [DOI] [PubMed] [Google Scholar]
- 2.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. 1992. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll G, Bell M, Wang H, Chapman H, Mills J. 1998. Antagonism of the IL6 cytokine subfamily—a potential strategy for more effective therapy in rheumatoid arthritis. Inflamm Res 47:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Haque WA, Shimomura I, Matsuzawa Y, Garg A. 2002. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab 87:2395–2398 [DOI] [PubMed] [Google Scholar]
- 5.Haramizu S, Nagasawa A, Ota N, Hase T, Tokimitsu I, Murase T. 2009. Different contribution of muscle and liver lipid metabolism to endurance capacity and obesity susceptibility of mice. J Appl Physiol 106:871–879 [DOI] [PubMed] [Google Scholar]
- 6.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 7.Jones JI, Clemmons DR. 1995. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16:3–34 [DOI] [PubMed] [Google Scholar]
- 8.Kim SW, Rogers QR, Morris JG. 1996. Maillard reaction products in purified diets induce taurine depletion in cats which is reversed by antibiotics. J Nutr 126:195–201 [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Sumiyoshi M. 2007. High-fat, high-sucrose, and high-cholesterol diets accelerate tumor growth and metastasis in tumor-bearing mice. Nutr Cancer 59:207–216 [DOI] [PubMed] [Google Scholar]
- 10.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. 1987. Macrophage-induced angiogenesis is mediated by tumour necrosis factor α. Nature 329:630–632 [DOI] [PubMed] [Google Scholar]
- 11.Liang H, Yin B, Zhang H, Zhang S, Zeng Q, Wang J, Jiang X, Yuan L, Wang CY, Li Z. 2008. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNFα signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology 149:2943–2951 [DOI] [PubMed] [Google Scholar]
- 12.Martini G, Valenti R, Giovani S, Campagna S, Franci B, Nuti R. 2001. Leptin and body composition in healthy postmenopausal women. Panminerva Med 43:149–154 [PubMed] [Google Scholar]
- 13.Moran TH. 2003. Methods for the study of the controls of food intake in mice, p 25–34 : Crawley JN. Short course II: mouse behavioral phenotyping. Washington (DC): Society for Neuroscience [Google Scholar]
- 14.Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. 2007. Involvement of sex, strain, and age factors in high-fat diet–induced obesity in C57BL/6J and BALB/cA mice. Exp Anim 56:263–272 [DOI] [PubMed] [Google Scholar]
- 15.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Batkai S, Marsicano G, Lutz B, Buettner C, Kunos G. 2008. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118:3160–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peachey SE, Dawson JM, Harper EJ. 2000. Gastrointestinal transit times in young and old cats. Comp Biochem Physiol A Mol Integr Physiol 126:85–90 [DOI] [PubMed] [Google Scholar]
- 17.Plakas SM, Lee TC, Wolke RE, Meade TL. 1985. Effect of Maillard browning reaction on protein utilization and plasma amino acid response by rainbow trout (Salmo gairdneri). J Nutr 115:1589–1599 [DOI] [PubMed] [Google Scholar]
- 18.Reeves PG, Nielsen FH, Fahey GCJ. 1993. AIN93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN76A rodent diet. J Nutr 123:1939–1951 [DOI] [PubMed] [Google Scholar]
- 19.Stylianou C, Galli-Tsinopoulou A, Farmakiotis D, Rousso I, Karamouzis M, Koliakos G, Nousia-Arvanitakis S. 2007. Ghrelin and leptin levels in obese adolescents. Relationship with body fat and insulin resistance. Hormones (Athens) 6:295–303 [DOI] [PubMed] [Google Scholar]
- 20.Tschop M, Heiman ML. 2001. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109:307–319 [DOI] [PubMed] [Google Scholar]
- 21. United States Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 20, NDB No: 20072.
- 22.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr 1997. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 99:385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela P, del Mar Arce M, Marcos A, Castrillon AM. 1997. Immunocompetence in relation to a heat-processed diet (Maillard reaction) in weanling rats. Br J Nutr 77:947–956 [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Demars LC. 2010. Effects of dietary fat on spontaneous metastasis of Lewis lung carcinoma in mice. Clin Exp Metastasis 27:581–590 [DOI] [PubMed] [Google Scholar]