Abstract
Using an isocratic high-performance liquid chromatography (HPLC) system and UV detection, a simple and precise analytical procedure was developed to quantify levels of the CB1 receptor antagonist rimonabant in the plasma of rhesus monkeys. Rimonabant was extracted from plasma samples into 5% isopropanol in hexane. After separation, the isopropanol-hexane fractions were dried to residue, redissolved in mobile phase, and then injected into the HPLC. The HPLC system included an acetonitrile–phosphate buffer (62:38, v/v) mobile phase (pH 6.7), flow rate of 1.5 mL/min, C18 column (4.6 mm i.d. × 150 mm length, 5 μm), and UV detection at 280 nm. Retention times for rimonabant and doxepin (internal standard) were 9.9 and 2.4 min, respectively. The regression of the spiked calibrator curve was linear from 60 to 4000 ng/mL (r2 = 0.996). The lower limit of quantification was 60 ng/mL, and recovery was 83.6%. Rimonabant was stable in stock solutions and monkey plasma across a range of temperatures and concentrations. To demonstrate utility, plasma rimonabant was measured in six rhesus monkeys at 60 and 240 min after intramuscular administration of 1 mg/kg rimonabant. Rimonabant levels ranged from 175 to 1290 ng/mL. The analytical assay described here provides a simple and accurate procedure for multiple within-subject measurements of the CB1 antagonist rimonabant.
Introduction
Rimonabant (SR 141716A) was the first potent and selective cannabinoid CB1 receptor antagonist to be discovered (1,2). Based on its ability to decrease obesity in both pre-clinical and clinical trials, rimonabant was approved in Europe for the management of obesity in 2006. However, concerns over increased anxiety and depressive symptoms in rimonabant-treated patients resulted in its removal from the European market, and similar concerns led to its disapproval for use in the U.S. Despite these setbacks, rimonabant continues to be evaluated for other therapeutic indications, including pharmacotherapy of drug abuse disorders (3). Moreover, rimonabant has been and continues to be an invaluable research tool for establishing CB1 receptor mechanisms involved in the effects of cannabinoids including those contained in Cannabis sativa (e.g., Δ9-tetrahydrocannabinol; Δ9-THC). For example, rimonabant blocks many of the effects of Δ9-THC in non-humans associated with its abuse liability (4–6) as well as verbal reports of marijuana intoxication in human laboratory studies (7). From studies with rimonabant, therefore, it appears that CB1 receptors mediate the abuse liability of cannabinoids.
The principal aim of this study was to develop and validate a method for quantifying rimonabant in rhesus monkey plasma. To date, there have been few published methods for the measurement of rimonabant levels in biological tissue (8–10). None of these described an assay of rimonabant in monkey plasma. Zhang et al. (8) measured rimonabant levels to study metabolism in rat liver microsomes in vitro and used a gradient elution with their high-performance liquid chromatography (HPLC)–UV system. However, many of the details of this procedure were not included in the report. Hsieh et al. (9) used a more complicated procedure, which included a column switching HPLC system and tandem mass spectrometry detection (MS–MS). McCulloch et al. (10) reported an elegant and sophisticated procedure for measurement of rimonabant in mouse plasma. This assay combined fast HPLC–MS–MS. These latter two methods are well-characterized, but the equipment required is expensive and not available in our lab. The current study was undertaken to develop an assay for rimonabant using an isocratic HPLC–UV system, without the necessity of mass spectrometry, and to validate the current procedure. Our laboratory routinely uses HPLC–UV methods to quantify a variety of CNS-acting drugs in primate plasma (11).
Experimental
Chemicals
Rimonabant (SR141716A) was obtained from the Research Technology Branch of the National Institute on Drug Abuse (Rockville, MD). HPLC-grade methanol and acetonitrile were purchased from Fisher (Fair Lawn, NJ). Doxepin and other reagents were purchased from Sigma Chemical Company (St. Louis, MO). Milli-Q water was used for preparation of all solutions (Millipore, Billerica, MA).
Equipment
The HPLC system consisted of a Waters 515 HPLC pump, Waters 717 autosampler, Waters 2487 UV detector, and PC-based Waters Empower chromatographic software (Waters, Milford, MA).
Chromatographic conditions
The HPLC analytical column was a Phenomenex Gemini C18 (4.6 × 150 mm, 5 μm) (Torrance, CA). The mobile phase was 62% (v/v) acetonitrile and 38% (v/v) of 20 mM KH2PO4 (pH 6.7). The flow rate of the mobile phase was 1.5 mL/min, and the wavelength of absorbance was 280 nm. All HPLC experiments were conducted at room temperature (23°C).
Preparation of standards and calibrators
Rimonabant powder was dissolved in methanol at a concentration of 1 mg/mL and stored in aliquots at –80°C. A working stock solution was prepared each day from the methanol stock solution at concentrations of 10 and 100 μg/mL and used to spike calibrators in blank monkey plasma. Calibrator samples were prepared daily by spiking rhesus monkey plasma to achieve final concentrations of 0, 60, 250, 500, 1000, 2000, and 4000 ng/mL. Control samples of rimonabant in monkey plasma were prepared at concentrations of 250 and 2000 ng/mL and then stored as 1 mL aliquots at –80°C.
Procedure for rimonabant extraction from monkey plasma
Calibrators, controls, and plasma samples (1 mL) were mixed with 50 μL of a solution of 10 μg/mL doxepin (internal standard, IS), 100 μL of 5 N sodium hydroxide, and 3 mL of 5% (v/v) isopropanol in hexane in borosilicate test tubes. The samples were vortexed for 30 s. Next, the samples were centrifuged at 1,500 × g for 10 min and then placed at –80°C until the aequous, lower layer was frozen solid. The organic supernatants were poured into new glass test tubes and dried to residue under a gentle stream of nitrogen. The residues were dissolved in 250 μL of mobile phase and then filtered using microfilterfuge tubes. Next, 200 μL of the final samples were injected into the HPLC system. The ratios of the peak area of rimonabant to those of the IS (response ratios) were compared against a linear regression of response ratios of the calibrator concentration curve to quantify rimonabant. Rimonabant concentrations in plasma were expressed in ng/mL.
Validation of the rimonabant HPLC–UV method
Validation of this method was performed by determination of selectivity, accuracy, precision, recovery, calibration/standard curve, lower limit of detection (LLOQ), and stability. Our method employed an IS (doxepin), so calibration curves were constructed by plotting the ratio of the peak area of rimonabant to that of the IS (response ratios) at each rimonabant concentration. Linear regression analysis of the calibration data was used to evaluate the linearity of extraction and recovery.
Selectivity was determined by analysis of blank (unspiked) extracted samples of plasma from 10 monkeys. Also, chromatographic interference by Δ9-tetrahydrocannabinol and its metabolites at the elution times for doxepin and rimonabant was tested as these were the only chemicals that would be given to the monkeys in our experiments. Precision and accuracy were measured for all calibrator and quality control (QC) samples (Tables I–II) using the mean and standard deviation (SD) of at least six tests. To determine recovery, equal amounts of rimonabant were spiked into both 1 mL monkey plasma samples and 250 μL mobile phase samples to construct extracted and unextracted concentration curves, then the slopes of the linear regressions of those curves were compared. The LLOQ was the lowest calibrator (60 ng/mL). Stability of rimonabant was tested in methanol stock solutions (1 mg/mL) for six months, in working stock solutions used to spike calibrators for 6 h, in processed (extracted) samples for 24 h, and in control samples (250 and 2000 ng/mL) during three freeze-thaw cycles for 24 h at room temperature and up to one month stored at –80°C.
Table I.
Validation of Rimonabant Calibrator Curve (Monkey Plasma)
| Spike Concentration | Back-calculated concentration (± SD)* | Precision (CV%)† | Accuracy (%) |
|---|---|---|---|
| 60 ng/mL | 59.1 ± 6.55 | 11.1% | 98.5% |
| 250 ng/mL | 248 ± 32.7 | 13.2% | 99.2% |
| 500 ng/mL | 455 ± 63.5 | 14.0% | 91.0% |
| 1000 ng/mL | 1028 ± 112 | 10.8% | 103% |
| 2000 ng/mL | 1998 ± 187 | 9.38% | 99.9% |
| 4000 ng/mL | 3999 ± 111 | 2.77% | 99.9% |
SD = standard deviation.
CV% = Percent coefficient of variation (100 × SD/mean).
Table II.
Precision and Accuracy of Quality Control Samples
| Spike Concentration | Empirical Concentration (± SD) | Precision (CV%) | Accuracy (%) |
|---|---|---|---|
| 250 ng/mL | 261 ± 35.1 (n = 14) | 13.5% | 104.0% |
| 2000 ng/mL | 1982 ± 277 (n = 15) | 14.0% | 99.1% |
Dosing of rimonabant to rhesus monkeys
Six adult rhesus monkeys (three female and three male, weighing between 6.2 and 9.1 kg) were treated with a 1 mg/kg intramuscular dose of rimonabant. Heparinized blood was collected from intravenous catheters at 10 min before intramuscular injection and then at 60 and 240 min post-injection. The blood was centrifuged at 1500 × g for 10 min. The upper plasma layer was removed, placed in polypropylene tubes, and stored at –80°C for analysis at a later time.
Results
Chromatography of rimonabant
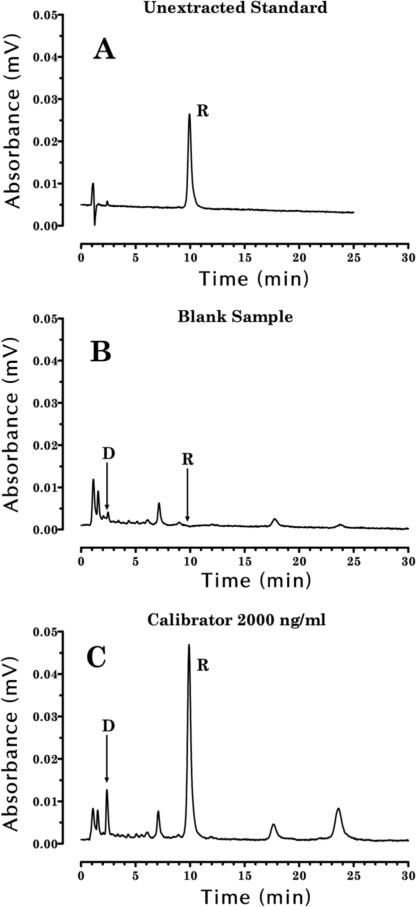
A chromatogram of rimonabant and doxepin standards shows that these drugs eluted at 9.9 and 2.4 min, respectively (Figure 2A). Figure 2C shows a chromatogram of an extracted monkey plasma sample spiked with 2000 ng/mL of rimonabant and 500 ng/mL of doxepin.
Figure 2.
Chromatograms of (A) 50 ng of rimonabant standard injected into the HPLC system, (B) a blank monkey sample after the extraction procedure, (C) a monkey sample spiked with 2000 ng/mL of rimonabant and 500 ng/mL of doxepin (IS). Arrows indicate the location of the elution of doxepin (D) and rimonabant (R) on the chromatograms.
Experiments were performed using wavelengths of detection of 214 and 280 nm. Although the extinction coefficients of rimonabant and doxepin were higher at 214 nm than 280 nm, results with 280 nm provided adequate sensitivity for our experiments and significantly less interference from coeluting peaks in chromatograms of extracted monkey plasma samples. Also, experiments were performed to establish a maximum run-time for extracted samples of less than 30 min by modifying the organic content of the mobile phase. Using the procedure described in the experimental section, only two peaks of significance elute after the rimonabant peak in extracted monkey plasma samples (Figure 2C).
Selectivity, IS, and calibrator curve
Experiments to test selectivity suggested that there was no matrix, drug, or reagent interference. A chromatogram of an extracted blank monkey plasma sample indicated that neither extraction reagent nor endogenous plasma peaks interfered with the elution of doxepin or rimonabant (Figure 2B). Although a small but visible peak occurred near the elution time of doxepin (Figure 2B), its peak area represented less than 0.5% of the IS peak in extracted monkey plasma samples. Blank plasma samples from 10 different monkeys were processed to verify that endogenous compounds did not produce significant peaks that eluted coincidentally with either doxepin or rimonabant. Also, Δ9-tetrahydrocannabinol and its metabolites, all compounds that might be given to monkeys during behavioral experiments, did not elute coincidentally with either doxepin or rimonabant.
Doxepin was selected as the internal standard because it absorbed light at 280 nm, eluted at 2.4 min on our chromatographic system, was recovered through the extraction process at 84.6%, and resulted in no significant interfering peaks at its elution time. Doxepin was used as the IS at a concentration of 500 ng/mL.
Plasma samples from untreated rhesus monkeys were spiked with rimonabant at concentrations of 0, 60, 250, 500, 1000, 2000, and 4000 ng/mL and analyzed on each of three separate days. The drugs were extracted from monkey plasma samples and analyzed with HPLC–UV as described in the experimental section. The range of concentrations from 60 to 4000 ng/mL was selected because it covers the range of plasma concentrations expected from the rimonabant doses used in our behavioral experiments with monkeys. The linear regression of combined calibrator curves from three experiments had an r2 value of 0.996 (y = 0.004533x – 0.0984). The precision and accuracy of the back-calculated values of rimonabant for all calibrator concentrations are shown in Table I. The precision of the back-calculated values ranged between coefficient of variation (CV%) values of 2.77 and 14.0. Accuracy ranged from 91% to 103%.
LLOQ and recovery
The LLOQ for rimonabant in spiked monkey plasma was 60 ng/mL. Precision and accuracy for this concentration for six replicates were 11.1% and 98.5%, respectively (Table I). Recovery of rimonabant through the extraction procedure was 83.6%, which was determined by comparing the slope of the extracted plasma calibrator curve to that of unextracted rimonabant standards.
Precision and accuracy of quality control samples
Control samples containing either 250 or 2000 ng/mL of rimonabant were prepared and stored at –80°C until the day of analysis. These samples were analyzed in sets of five replicates on each of three days. The combined inter-day results show that the mean and SD of the samples spiked with 250 and 2000 ng/mL of rimonabant were 261 ± 35 and 1982 ± 277 ng/mL, respectively (Table II). Precision was 13.5% and 14.0% for 250 and 2000 ng/mL samples, respectively. Accuracy was found to be 104% and 99.1% for 250 and 2000 ng/mL samples, respectively. Our results show that 79% and 73% of the samples tested at 250 and 2000 ng/mL, respectively, were within 15% of their relative nominal value.
Stability of rimonabant in monkey plasma and stock solutions
Rimonabant was found to be stable during a variety of experiments. Rimonabant was stable when stored in stock solutions of methanol at a concentration of 1 mg/mL at –80°C for six months with an accuracy of 99.3%. Working stock solutions of rimonabant in methanol at concentrations of 10 and 100 μg/mL were stable for 6 h at room temperature with accuracies of 98.7% and 99.4%, respectively. The accuracy values of low (250 ng/mL) and high (2000 ng/mL) concentrations of rimonabant in monkey plasma were 96.0% and 99.8%, respectively, after three freeze-thaw cycles and 100% and 95.0%, respectively, after storage for 24 h at room temperature. Also, rimonabant was stable in monkey plasma control samples spiked with 250 and 2000 ng/mL of rimonabant and stored for one month at –80°C. Finally, rimonabant was found to be stable in processed (extracted) analytical samples when stored at room temperature for 24 h with an accuracy of 97.7%.
Plasma Levels of rimonabant in rhesus monkeys
Six adult rhesus monkeys (three female and three male) weighing between 6.2 and 9.1 kg were treated with a 1 mg/kg intramuscular dose of rimonabant. Plasma levels of rimonabant ranged from 416 to 1290 ng/mL (807 ± 367 SD ng/mL) at 60 min and from 175 to 676 ng/mL (353 ± 171 SD ng/mL) at 240 min post-injection (Table III).
Table III.
Plasma Rimonabant Levels in Six Rhesus Monkeys*
| Time after injection |
||
|---|---|---|
| Monkey | 60 min | 240 min |
| 1 | 1115 | 375 |
| 2 | 959 | 676 |
| 3 | 1290 | 175 |
| 4 | 438 | 305 |
| 5 | 625 | 304 |
| 6 | 416 | 282 |
| Mean ± SD | 807 ± 367 | 353 ± 171 |
All values are plasma rimonabant concentrations in ng/mL. Blood samples were collected at 60 and 240 min after intramuscular injection of 1 mg/kg rimonabant.
Discussion and Conclusions
This study has resulted in the development and validation of a simple and accurate assay for the measurement of rimonabant in monkey plasma samples. This procedure involves a liquid–liquid extraction of rimonabant into an isopropanolhexane mixture and analysis using an isocratic HPLC method and UV detection at 280. The extraction is straightforward with a recovery of 83.6%, and the assay is suitable for quantification of rimonabant in monkey plasma samples at concentrations between 60–4000 ng/mL. These levels are measurable in monkeys treated with rimonabant doses between 0.5–3.2 mg/kg (data not shown). Few previous reports describe an HPLC procedure for quantification of rimonabant. Zhang et al. (8) described a gradient HPLC–UV technique for rimonabant and its metabolites in rat liver microsomes but did not include a complete characterization or validation of the procedure. Hsieh et al. (9) developed a procedure using HPLC that included a fused-core silica column and detection with MS–MS. McCulloch et al. (10) described a procedure for measurement of rimonabant in mouse plasma. This assay combined fast HPLC with MS–MS detection. While these procedures were elegant and accurate, MS–MS equipment is expensive and not available in most laboratories.
To demonstrate the utility of this assay, plasma levels of rimonabant were measured before and after treatment of rhesus monkeys with an intramuscular dose of 1 mg/kg. The results indicate that the plasma levels of rimonabant at 60 and 240 min vary with time and fall in the middle of the calibrator curve. Future experiments will involve intramuscular and intravenous doses of rimonabant from 0.1 to 3.2 mg/kg, so it appears that this assay is well-suited for the quantification of plasma levels of rimonabant in these studies. These future studies will address the relationship between the pharmacokinetics and pharmacodynamics of rimonabant.
Our principle conclusion is that rimonabant can be conveniently, accurately, and sensitively quantified in rhesus monkey plasma using HPLC with UV detection. The analytical assay described here provides a suitable procedure for multiple, within subject measurements of the CB1 receptor antagonist rimonabant as an adjunct to behavioral experiments using rhesus monkeys.
Figure 1.
Structures of rimonabant HCl and doxepin HCl.
Acknowledgments
This work was supported by USPHS grant DA19222.
References
- 1.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, et al. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 2.Rinaldi-Carmona M, Barth F, Heaulme M, Alonso R, Shire D, Congy C, Soubrie P, Breliere JC, Le Fur G. Biochemical and pharmacological characterization of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- 3.Beardsley PM, Thomas BF. Current evidence supporting a role of cannabinoid CB1 receptor antagonists as potential pharmacotherapies for drug abuse disorders. Behavior. Pharmacol. 2005;16:275–296. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 5.Järbe TUC, Lamb RJ, Lin S, Makriyannis A. (R)-methanandamide and Δ9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacol. 2001;156:369–380. doi: 10.1007/s002130100730. [DOI] [PubMed] [Google Scholar]
- 6.McMahon LR. Discriminative stimulus effects of the cannabinoid CB1 antagonist SR141716A in rhesus monkeys pretreated with Δ9-tetrahydrocannabinol. Psychopharmacol. 2006;188:306–314. doi: 10.1007/s00213-006-0500-6. [DOI] [PubMed] [Google Scholar]
- 7.Huestis MA, Forelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716A. Arch. Gen. Psych. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Ma P, Wang W, Cole RB, Wang G. In vitro metabolism of diarylpyrazoles, a novel group of cannabinoid receptor ligands. Drug Metab. Dispos. 2005;33:508–517. doi: 10.1124/dmd.104.001974. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh Y, Duncan CJG, Brisson J-M. Fused-core silica column high-performance liquid chromatography/tandem mass spectrometric determination of rimonabant in mouse plasma. Anal. Chem. 2007;79:5668–5673. doi: 10.1021/ac070343g. [DOI] [PubMed] [Google Scholar]
- 10.McCulloch M, Zhou X, Xu Y, Brunell S, Spear L. Determination of endocannabinoid receptor antagonist SR141716 (rimonabant) in plasma by liquid chromatograph tandem mass spectrometry. J. Chromatogr. B. 2008;863:258–265. doi: 10.1016/j.jchromb.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon LR, Javors MA, France CP. Changes in relative potency among positive GABAA receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacol. 2007;192:135–145. doi: 10.1007/s00213-006-0692-9. [DOI] [PubMed] [Google Scholar]