Abstract
Transient intraluminal infusion of porcine pancreatic elastase into the infrarenal segment of the abdominal aorta is the most widely used animal model of abdominal aortic aneurysm (AAA) ever since it was first described in rats by Anidjar and colleagues.1 The rationale for its development was based on the disrupted nature of elastin observed in AAAs. This rat model has been modified to produce AAAs in the infrarenal aortic region of mice.2 The model has the ability to add broad insight into the pathobiology of AAA due to the emergence of numerous transgenic and gene knockout mice. Moreover, it is a viable platform to test potential therapeutic agents for AAA. In this video, we demonstrate the elastase infusion AAA procedure used in our laboratory.
Mice are anesthetized using 2.5% isoflurane, and a laparotomy is performed under sterile conditions. The abdominal aortais isolated with the assistance of an operating stereomicroscope (Leica). After placing temporary ligatures around the proximal and distal aorta, an aortotomy is created at the bifurcation with the tip of a 30-gauge needle. A heat-tapered segment of PE-10 polyethylene tubing is introduced through the aortotomy and secured. The aortic lumen is subsequently perfused for 5-15 minutes at 100 mm Hg with saline containing type I porcine pancreatic elastase (4.5 U/mL; Sigma Chemical Co.). After removing the perfusion catheter, the aortotomy is repaired without constriction of the lumen.
Protocol
Mice are anesthetized with inhaled isoflurane 2.0-3.0ml/L.
The abdomen is shaved and scrubbed with alcohol and betadine.
The mouse is placed supine on the operative field.
Sterile gloves are worn by the surgeon, and the instruments used have been sterilized with hot bead sterilizer.
A long midline abdominal incision is made. Abdominal contents are retracted outside the abdomen with sterile gauze to expose the abdominal aorta.
The abdominal aorta is isolated from the level of the left renal vein to the bifurcation.
The branches of the abdominal aorta are exposed and ligated with 10-0 sutures. All aortic branches within 1.0cm of the bifurcation are ligated.
Temporary 6.0 silk ligatures are placed around the proximal and distal portions of the aorta
An aortotomy is created at the bifurcation with a 30-gauge needle.
Heat-tapered polyethylene tubing (PE-10) is introduced through the aortotomy and secured with a tie.
Using a saline bag hung at the height of 136 cm to calibrate 100 mmHg, the aorta is filled with the saline containing 4.5 U/mL Type I porcine pancreatic elastase. The aorta typically dilates to 150-170% of its original diameter during the 5-15 minute elastase perfusion.
The perfusion catheter is then removed and the aortotomy closed with a 10-0 suture to avoid constriction.
After the aortotomy is closed, the distal ligature is removed.
If there is little or no bleeding from the repaired aortotomy, the proximal ligature is removed. The expanded aorta fills with blood immediately.
The intestines are returned to the abdomen, and the wound is closed in two layers with 6-0 nylon / polypropylene suture.
0.25% Bupivicaime drops (appx one drop for each centimeter of the incision size) are placed on the wound and one dose of Caprofen (5mg/kg) is given subcutaneously.
The mouse is placed on a warm area and usually recovers within 15 mins.
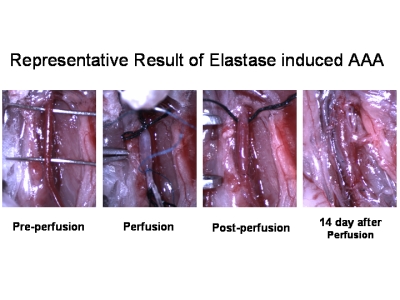 Figure 1.Representative Result of Elastase induced AAA.
Figure 1.Representative Result of Elastase induced AAA.
Discussion
Previous studies have noted significant lot-to-lot variation in AAA development from different commercial elastase preparations3. Thus, it is critical to monitor production lots as well as to have appropriate control groups in order to document the ability of the preparation to induce AAAs.
Interestingly, susceptibility to AAA development in this model is dependent on the mouse strain used3. The mouse used in the current video is of the C57Bl/6 strain which has consistent development of AAA using the outlined protocol. Elastase concentration and/or incubation time may be adjusted to accommodate other strains.
Although the technique of creation of AAA with elastase in mice is technically challenging, it is an important technique for studying the underlying molecular mechanisms of AAA formation and development. Practice and technical modifications have resulted in a success rate of over 90% in our laboratory. We believe that the video and the accompanying text will help reduce the learning curve for laboratories that wish to use this technique.
Troubleshooting
The branches of the abdominal aorta should be ligated with 10-0 sutures, preventing bleeding during the operation and insufficient expansion during elastase infusion because of leaking.
It is important to have a clear and expansive operating field. Therefore, use 6-0 suture instead of clamps to temporarily occlude the aorta
The lumen of the abdominal aorta should be cleared with saline solution to prevent thrombosis after the operation.
It is important that the tip of the heat tapered PE-10 tube should be smooth in order to avoid scratching the endothelial layer when inserted.
Air bubbles inside the vessels should be avoided when infusing elastase as well as when the aortotomy is closed.
Acknowledgments
This work was supported the SCCOR grant “AAA Disease: Mechanisms, Stratification, and Treatment” (P50 HL083800-03) from the National Institutes of Health
References
- Anidjar S, Salzmann JL, Gentric D. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82(3):973–973. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- Pyo R, Lee JK, Shipley JM. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105(11):1641–1641. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsten CG, 3rd, Calton WC, Johanning JM. Elastase is not sufficient to induce experimental abdominal aortic aneurysms. J Vasc Surg. 2001;33(6):1255–1255. doi: 10.1067/mva.2001.112706. [DOI] [PubMed] [Google Scholar]


