Abstract
BACKGROUND
Patient-reported outcomes (PROs) have shown independent prognostic value for patients with non-small cell lung cancer (NSCLC). However, translating PROs into useful prognostic information for individual patients has been problematic.
METHODS
Ninety-four patients with advanced NSCLC and an Eastern Cooperative Oncology Group performance status (PS) of 0–2 who qualified for chemotherapy rated symptom severity using the M. D. Anderson Symptom Inventory before and after their first chemotherapy cycle. Prognostic values of baseline symptoms and changes in symptom severity were examined by Cox proportional hazards models.
RESULTS
In multivariate analysis, controlled for demographic and other factors, baseline coughing rated ≥4 independently predicted significantly higher risk for shorter survival (hazard ratio [HR], 8.69; P < .0001). Patients with coughing ≥4 and PS = 2 were more likely to have shorter survival (HR, 20.6; P < .0001) than patients with coughing <4 and PS = 0–1. A 1-point or greater increase in severity of fatigue (P < .05), shortness of breath, or poor appetite (P < .01) from baseline to end of the first chemotherapy cycle also was independently associated with higher risk for poor survival.
CONCLUSION
An increased risk for shorter survival was indicated by moderate to severe coughing at baseline or by increased fatigue or shortness of breath during the first chemotherapy cycle in patients with advanced NSCLC. Although cross-validation is needed, these data suggest that an individual patient’s symptom severity scores, quickly obtainable in the clinic, might contribute clinically useful information for treatment planning for that patient.
Keywords: NSCLC, symptom, MDASI, patient-reported outcome (PRO), performance status, survival analysis
INTRODUCTION
Patients with advanced non-small cell lung cancer (NSCLC) generally survive only 8–10 months after diagnosis, yet during this relatively short time they suffer from significant symptom burden driven by rapid disease progression.1,2 High levels of physical symptoms (eg, pain, fatigue, pain, coughing) and affective symptoms (eg, distress and sadness) greatly affect functioning and quality of life.3 For patients with advanced NSCLC, it is widely accepted that those with the poorest performance status (Eastern Cooperative Oncology Group performance status [PS] = 3–4)4 will have poor survival rates; those who have better performance status (PS = 0–2) and thus qualify for first-line chemotherapy will have improved but quite variable overall survival rates. To know indicators for potentially shorter survival could help clinicians plan treatment and select candidates for drug intervention clinical trials, and would provide evidence-based knowledge for better communication with patients.
Because of the limited survival associated with advanced NSCLC, patient ratings of symptom burden and quality of life (patient-reported outcomes, or PROs)3 are believed to be more clinically relevant than response to treatment, the typical oncology clinical-trial endpoint.5 PROs have been shown to be reliable, easily measured endpoints that provide independent prognostic information and crucial parameters for the treatment of patients with lung cancer.3,6–14 Much of this research has suggested that pretreatment symptom severity and changes in global PRO scores during chemotherapy are predictors of survival.
Most of the literature cited above reported that pretreatment symptoms and/or changes in global symptom scores during chemotherapy were predictors of survival. While these findings apply to groups of patients, there is limited knowledge about how an individual patient’s symptom report might be used to predict that patient’s near-term survival. For example, no report has described what specific type of baseline symptoms (if any) or which degree of measurable change in symptom severity over time would be most critical for establishing clinically useful criteria to predict overall survival. The lack of such quantitative information is a major factor preventing the meaningful use of PROs to guide patient management in routine practice.5
We conducted a prospective longitudinal study to quantitatively define the association between symptom severity ratings and survival outcomes in individual patients with advanced NSCLC who qualified for chemotherapy. The M. D. Anderson Symptom Inventory (MDASI),15 a symptom-assessment tool designed and validated for administration to cancer patients, was used to evaluate multiple symptoms. We hypothesized that moderate to severe symptom levels at baseline and worsening symptom burden during the first chemotherapy cycle would be independent predictors of overall survival in patients with advanced NSCLC who were eligible for chemotherapy (PS = 0–2).
MATERIALS AND METHODS
Patient Sample
We recruited patients from the thoracic medical oncology clinic at The University of Texas M. D. Anderson Cancer Center. Eligible patients had stage IIIB or IV NSCLC, were at least 18 years old, spoke English, had PS = 0, 1, or 2, and were scheduled for first-line chemotherapy. Patients provided informed consent to participate in the study. The M. D. Anderson Cancer Center Institutional Review Board approved the study.
Assessment Methods
Symptom assessment
Patients completed the MDASI before and after the first cycle of chemotherapy. The MDASI15 assesses the severity of 13 symptoms (pain, fatigue, nausea, vomiting, dry mouth, shortness of breath, lack of appetite, difficulty remembering, drowsiness, disturbed sleep, sadness, distress, numbness) over the previous 24 hours on a 0–10 numeric scale, where 0 = “not present” and 10 = “as bad as you can imagine.” We additionally assessed 2 other symptom items common to patients with NSCLC (coughing, constipation). In paper-and-pencil format, the MDASI takes less than 5 minutes to complete.
Other measures
Demographic information, clinician-estimated PS, and previous and current cancer therapy were obtained from medical records. Comorbidities were documented using the Charlson Comorbidity Index score.16 Patient survival was tracked through searches of M. D. Anderson databases containing date-of-death information or, if necessary, searches of outside sources of death information.
Statistical Analysis
On the basis of previous research on symptom-severity cut points17 and symptom-management practice guidelines,18,19 we dichotomized baseline MDASI symptom scores as “mild” (rated 0–3 on the MDASI’s 0–10 scale) or “moderate to severe” (rated 4 or higher). Changes in symptom scores between baseline and the end of the first chemotherapy cycle were also dichotomized according to whether the symptom increased ≥1 point on the 0–10 scale (a “symptom increase”), or did not increase (either no change or a decrease in symptom score). The selection of one point as the indicator of symptom increase from baseline to the end of one chemotherapy cycle was based on the distribution of severity scores, where 1 point represented approximately 0.5 standard deviation of the sample, a value that has been suggested as a clinically meaningful change.20,21
Univariate Cox proportional hazards models were used to screen for relationships between candidate predictor variables (15 dichotomized baseline symptom variables and 15 dichotomized symptom-increase variables) and overall survival. Component scores of mean symptom severity were defined from symptoms that showed a significant relationship to overall survival in the univariate analysis (P < .05). These component scores were used in the multivariate analyses.
Four multivariate Cox proportional hazards models were fitted to examine the prognostic values of baseline symptoms and symptom change, adjusted for age, gender, race, previous chemotherapy, comorbidities, and PS. The baseline models used data from all patients who completed baseline symptom assessments and who contributed covariate data to examine the prognostic value of both baseline component symptom scores (Model 1a) and individual symptoms (Model 1b). The symptom-increase models used data from only those patients who reported symptoms by the end of the first chemotherapy cycle and for whom covariate data were available to examine the prognostic value of both symptom-increase component scores (Model 2a) and individual symptoms (Model 2b). For each of these models, final predictors were selected by stepwise regression with entry level 0.1 and stay level 0.05. Cox survival curves were plotted to model the relationship between survival time and either baseline symptom severity or symptom increase during the first cycle. Model fitting for multivariate analysis were examined by Akaike information criterion.22
RESULTS
Table 1 presents demographic and disease-related characteristics for the patient sample. The median time span between diagnosis of lung cancer and enrollment into the study was 55 days. Of the 94 patients recruited, 41 (44%) were newly diagnosed. During the course of our study, 86 patients (91%) received conventional platinum-based and/or paclitaxel-based chemotherapy and 8 (9%) received single-agent chemotherapy (pemetrexed or gemcitabine). Poor performance status (PS = 2) was noted in 6 of the 8 black or Hispanic patients (75%), compared with 22 of 86 (25%) of white non-Hispanic patients.
Table 1.
Patient Demographic Information (N = 94)
| n (%) | ||
|---|---|---|
| Gender | Male | 63 (67.0) |
| Female | 31 (33.0) | |
|
| ||
| Age, years | Mean (SD) | 61.2 (9.5) |
|
| ||
| Range | 31.7 to 85.0 | |
|
| ||
| Race | Black non-Hispanic | 7 (7.4) |
| Hispanic | 1 (1.1) | |
| White non-Hispanic | 86 (91.5) | |
|
| ||
| Marital status | Married | 78 (83.0) |
|
| ||
| Divorced or widowed | 9 (9.6) | |
|
| ||
| Separated or single | 7 (7.4) | |
|
| ||
| Job status | Employed or homemaker | 31 (33.7) |
|
| ||
| Retired | 39 (42.4) | |
|
| ||
| On medical leave or disabled | 20 (21.7) | |
|
| ||
| Education | Greater than high school degree | 58 (62.4) |
|
| ||
| Baseline PS | 0 | 16 (17.2) |
| 1 | 49 (52.7) | |
| 2 | 28 (30.1) | |
|
| ||
| Previous cancer therapy | Yes | 53 (56.4) |
|
| ||
| Type of previous treatment | Chemotherapy | 24 (25.5) |
| Surgery | 17 (18.1) | |
| Radiation | 32 (34.0) | |
|
| ||
| Charlson Comorbidity Index score (0–37) | 0 | 39 (42.4) |
|
| ||
| 1 | 23 (25.0) | |
|
| ||
| 2 | 14 (15.2) | |
|
| ||
| 3 or more | 18 (17.4) | |
|
| ||
| Baseline cancer stage | IIIB | 7 (7.4) |
|
| ||
| IV | 87 (92.6) | |
SD indicates standard deviation; PS, Eastern Cooperative Oncology Group performance status.
Fifty-nine patients (63%) who completed baseline assessments also contributed symptom data at week 3, the end of the first chemotherapy cycle. The remaining 35 patients (37%) provided only baseline data: 10 patients (11%) did not undergo chemotherapy because of newly found brain metastases; 1 (1%) died and 3 (3%) withdrew from chemotherapy before completing the first chemotherapy cycle due to disease progression; and 21 (22%) underwent chemotherapy but did not contribute a symptom assessment at the end of the first cycle. Of the 94 patients included in our analysis, 75 (80%) died within 120 weeks of beginning chemotherapy. The median overall survival time for the entire sample was 37 weeks (95% confidence limit [CL], 27.3, 43.9).
Baseline Symptoms as Predictors of Overall Survival
Of the 15 symptoms measured, fatigue, shortness of breath, disturbed sleep, and pain had the highest mean severity ratings at baseline (N = 94) (Table 2). No significant differences in baseline symptom levels were noted between newly diagnosed patients and previously treated patients, nor were there significant differences according to race or gender.
Table 2.
MDASI Symptom Severity at Baseline and Univariate Analysis of Symptoms as Predictors of Overall Survival (N = 94)
| Baseline Symptom Severity (MDASI 0–10 Scale) | Cox Proportional Hazard Regression Model for Baseline Symptoms (4–10 vs 0–3)* | |||||
|---|---|---|---|---|---|---|
| Variable | Mean* (SD) | % ≥4† | Participants/Events | HR | 95% CL | |
| Coughing | 1.46 (1.81) | 8.5 | 94/75 | 5.60‡ | 2.58 | 12.19 |
| Nausea | 0.29 (0.98) | 3.2 | 93/74 | 3.88§ | 1.18 | 12.82 |
| Lack of appetite | 1.70 (2.19) | 18.1 | 94/75 | 2.43|| | 1.35 | 4.40 |
| Distress | 1.72 (2.34) | 16.0 | 94/75 | 2.18§ | 1.15 | 4.13 |
| Fatigue | 2.89 (2.45) | 34.0 | 94/75 | 2.09|| | 1.27 | 3.42 |
| Shortness of breath | 2.22 (2.14) | 21.3 | 94/75 | 1.61 | 0.93 | 2.79 |
| Vomiting | 0.10 (0.53) | 1.1 | 94/75 | 2.81 | 0.38 | 20.72 |
| Difficulty remembering | 1.28 (1.83) | 9.6 | 94/75 | 1.60 | 0.79 | 3.24 |
| Numbness | 0.83 (1.97) | 6.5 | 93/74 | 1.53 | 0.65 | 3.60 |
| Disturbed sleep | 2.11 (2.47) | 21.3 | 94/75 | 1.52 | 0.85 | 2.72 |
| Pain | 1.98 (2.44) | 24.5 | 94/75 | 1.49 | 0.83 | 2.68 |
| Dry mouth | 1.68 (2.26) | 18.1 | 94/75 | 1.27 | 0.68 | 2.39 |
| Drowsiness | 1.88 (2.05) | 19.2 | 94/75 | 1.04 | 0.58 | 1.88 |
| Constipation | 1.47 (2.52) | 11.8 | 93/74 | 0.89 | 0.44 | 1.79 |
| Sadness | 1.82 (2.55) | 17.0 | 94/75 | 0.84 | 0.43 | 1.65 |
MDASI indicates M. D. Anderson Symptom Inventory; SD, standard deviation; HR, hazard ratio; CL, confidence limit.
Mean symptom severity score, MDASI 0–10 scale.
A symptom rated 4 or higher on the MDASI’s 0–10 scale was considered to be moderate to severe.
Significant at P < .0001.
Significant at P < .05.
Significant at P < .01.
Univariate analysis
Results of univariate Cox proportional hazards model analysis suggested that overall survival was predicted by the 5 moderate to severe baseline symptoms: coughing (associated with the highest risk for death), nausea, lack of appetite, distress, and fatigue (Table 2). Among patient characteristic covariates, race (hazard ratio [HR], 0.24; P < .05) and PS = 2 (HR, 2.17; P < .05) was significantly associated with overall survival.
Multivariate analyses
Multivariate Cox proportional hazards models examined the prognostic value of the 5 baseline symptoms that had significant prognostic value in the univariate analysis (n = 91). Patient characteristic covariates (age, gender, race, previous chemotherapy, and level of comorbidities) were adjusted in the model.
First, we fitted a component score of the five baseline symptoms with significant prognostic value (Table 4, Model 1a). Significant predictors of shorter survival were a component score that included a patient’s report of at least 1 moderate or severe symptom, PS = 2, and being minority. Second, we examined all 5 individual symptom variables in the model (Table 4, Model 1b). After stepwise variable selection, moderate to severe coughing was the only highly significant symptom predictor of survival (HR, 8.69; P < .0001). Patients who rated their baseline coughing as moderate to severe had a median survival time of 10.4 weeks, compared with 37.1 weeks for patients with no or mild baseline coughing (P < .001). Of the patient characteristic covariates, PS = 2 and being minority were independent predictors of shorter survival.
Table 4.
Multivariate Analysis: Stepwise Cox Regression of Moderate to Severe Baseline Symptoms,* Symptom Increase,† and Patient Characteristicsas Predictors of Overall Survival
| Participants/Events | HR | 95% CL | P | ||
|---|---|---|---|---|---|
| Model 1a. Baseline symptom component score and patient characteristics‡ as predictors | |||||
| Entire sample with all ethnicities | 91/73 | ||||
| 5 baseline symptoms§ (moderate to severe*) | 2.66 | 1.48 | 4.80 | .001 | |
| PS = 2 | 2.08 | 1.22 | 3.55 | .007 | |
| Race = white non-Hispanic | 0.36 | 0.15 | 0.87 | .023 | |
| White non-Hispanic sample | 84/66 | ||||
| 5 baseline symptoms§ (moderate to severe*) | 2.92 | 1.59 | 5.36 | .0006 | |
| Model 1b. Individual baseline symptoms and patient characteristics|| as predictors | |||||
| Entire sample with all ethnicities | 90/72 | ||||
| Coughing (moderate to severe*) | 8.69 | 3.53 | 21.38 | < .0001 | |
| PS = 2 | 2.01 | 1.15 | 3.01 | .014 | |
| Race = white non-Hispanic | 0.16 | 0.01 | 0.39 | < .0001 | |
| White non-Hispanic sample | 83/65 | ||||
| Coughing (moderate to severe*) | 7.83 | 3.05 | 20.12 | <.0001 | |
| Model 2a. Symptom increase† component score and patient characteristics‡ as predictors | |||||
| Entire sample with all ethnicities | 56/47 | ||||
| Symptom increase† in any 1 of 6 symptoms¶ | 4.16 | 1.71 | 10.16 | .002 | |
| 5 baseline symptoms§ | 2.47 | 1.19 | 5.10 | .015 | |
| PS = 2 | 2.34 | 1.08 | 5.08 | .032 | |
| White non-Hispanic sample | 52/43 | ||||
| 5 baseline symptoms§ | 2.36 | 1.15 | 4.85 | .019 | |
| Symptom increase† in any 1 of 6 symptoms¶ | 4.32 | 1.76 | 10.64 | .001 | |
| Model 2b. Symptom increase† in individual symptoms and patient characteristics|| as predictors | |||||
| Entire sample with all ethnicities | 53/44 | ||||
| Fatigue | 3.54 | 1.21 | 10.36 | 0.021 | |
| Shortness of breath | 4.07 | 1.28 | 12.95 | 0.018 | |
| Race = white non-Hispanic | 0.05 | 0.01 | 0.48 | 0.009 | |
| White non-Hispanic sample | 49/40 | ||||
| Fatigue | 4.17 | 1.28 | 13.55 | 0.018 | |
| Lack of appetite | 7.36 | 2.07 | 26.14 | 0.002 | |
HR indicates hazard ratio; CL, confidence limit; PS, Eastern Cooperative Oncology Group performance status.
A symptom rated 4 or higher on the MDASI’s 0–10 scale was considered to be moderate to severe.
“Symptom increase” was defined as a symptom-score increase of 1 or more points on the MDASI’s 0–10 scale between baseline and the end of the first chemotherapy cycle (21 days, on average).
Age, gender, race, PS, previous chemotherapy, and level of comorbidities were included as covariates in the Cox model.
Coughing, nausea, lack of appetite, distress, and fatigue.
All baseline symptoms, age, gender, race, PS, previous chemotherapy, and level of comorbidities were included as covariates in the multivariate Cox model.
Fatigue, shortness of breath, vomiting, difficulty remembering, numbness, and lack of appetite.
Because of potential confounding of race with PS (6 of 8 minority patients had PS = 2), we refitted both of the baseline models after excluding data collected from 10 minority patients (n = 84). Both a baseline symptom component score that included at least 1 moderate or severe symptom (Table 4, Model 1a), and moderate to severe coughing continued to be highly significant predictors of survival in the new models (Table 4, Model 1b).
Synergistic effects of baseline coughing and performance status as predictors of overall survival
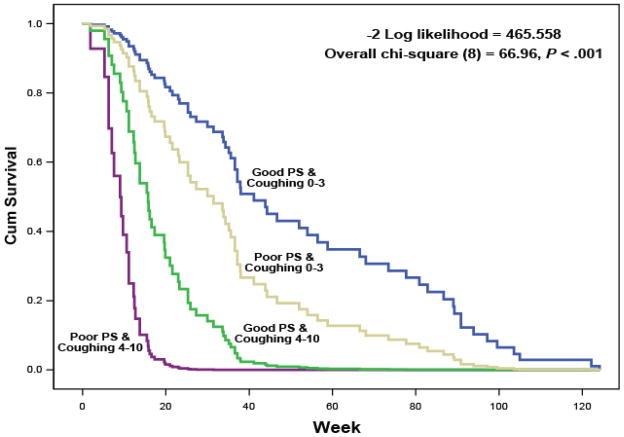
To explore the synergistic effects of baseline coughing and PS on patients’ survival, we created a 4-level interaction variable between baseline coughing and PS and fitted a Cox model that included this variable and other patient covariates (age, gender, race, previous chemotherapy, and level of comorbidities). Cox survival curves are presented in Fig. 1. Compared with HRs for patients with no or mild coughing (rated 0–3 on the 0–10 scale) and good performance status (PS = 0–1), HR = 2.0 (95% CL, 1.1, 3.6; P = .029) for subjects with coughing = 0–3 and PS = 2; HR = 5.5 (95% CL, 1.2, 26.4; P = .032) for patients with coughing ≥4 and PS = 0–1; and HR = 20.6 (95% CL, 7.0, 60.3; P < .0001) for patients with coughing ≥4 and PS = 2.
Figure 1.
Cox proportional hazard regression curves for survival duration based on moderate to severe baseline coughing (≥4 on the MDASI’s 0–10 scale) versus mild baseline coughing (≤3 on the 0–10 scale), by good performance status (PS = 0 or 1) versus poor performance status (PS = 2).
Symptom Increase as a Predictor of Overall Survival
The 59 patients who completed their first cycle of chemotherapy and contributed symptom data reported fatigue, shortness of breath, and pain as the most severe symptoms (Table 3). From baseline to the end of the first chemotherapy cycle, 3 symptoms (fatigue, disturbed sleep, and pain) had the highest prevalence of increase of 1 or more points on the MDASI’s 0–10 scale.
Table 3.
MDASI Symptom Severity at End of First Cycle of Chemotherapy and Univariate Analysis of Symptom Increase* as Predictors of Overall Survival (n = 59)
| Symptom Severity at End of First Cycle of Chemotherapy | Cox Proportional Hazard Regression Model for Symptom Increase* | |||||
|---|---|---|---|---|---|---|
| Variable | Mean† (SD) | % with Symptom Increase* | Participants/Events | HR | 95% CL | |
| Fatigue | 3.32 (2.45) | 49.2 | 59/49 | 2.405‡ | 1.324 | 4.368 |
| Shortness of breath | 2.57 (2.64) | 34.5 | 58/48 | 2.295§ | 1.190 | 4.425 |
| Vomiting | 0.36 (1.00) | 19.0 | 58/48 | 2.213§ | 1.036 | 4.727 |
| Difficulty remembering | 1.60 (2.13) | 34.5 | 58/48 | 2.167§ | 1.182 | 3.970 |
| Numbness | 1.38 (2.26) | 33.3 | 57/47 | 2.080§ | 1.115 | 3.880 |
| Lack of appetite | 1.76 (2.37) | 25.9 | 58/48 | 2.067§ | 1.057 | 4.044 |
| Nausea | 0.90 (1.93) | 31.0 | 58/48 | 1.886 | 0.982 | 3.621 |
| Constipation | 1.41 (2.32) | 24.5 | 53/43 | 1.884 | 0.904 | 3.927 |
| Disturbed sleep | 2.17 (2.20) | 42.4 | 59/49 | 1.408 | 0.773 | 2.564 |
| Drowsiness | 2.17 (2.13) | 37.9 | 58/48 | 1.395 | 0.764 | 2.548 |
| Coughing | 1.66 (1.77) | 34.5 | 58/48 | 1.393 | 0.755 | 2.570 |
| Sadness | 1.69 (2.49) | 34.5 | 58/48 | 1.367 | 0.740 | 2.523 |
| Dry mouth | 1.95 (2.36) | 34.5 | 58/48 | 1.299 | 0.705 | 2.395 |
| Distress | 2.05 (2.53) | 34.5 | 58/48 | 1.231 | 0.661 | 2.293 |
| Pain | 2.49 (2.54) | 39.0 | 59/49 | 1.030 | 0.570 | 1.863 |
MDASI indicates M. D. Anderson Symptom Inventory; SD, standard deviation; HR, hazard ratio; CL, confidence limit
“Symptom increase” was defined as a symptom-score increase of 1 or more points on the MDASI’s 0–10 scale between baseline and the end of the first chemotherapy cycle (21 days, on average).
Mean symptom severity score, MDASI 0–10 scale.
Significant at P <.01.
Significant at P < .05.
Univariate analysis
In univariate Cox regression analyses (n = 59) of symptom increase during the first chemotherapy cycle, fatigue (P < .01), shortness of breath, vomiting, difficulty remembering, numbness, and lack of appetite (all P < .05) were each significantly associated with decreased survival (Table 3).
Multivariate analyses
We examined the prognostic effect of symptom increase in the 6 symptoms identified in the univariate analysis, both as a component score (Table 4, Model 2a) and as individual symptoms (Table 4, Model 2b). Age, gender, race, PS, previous chemotherapy, and level of comorbidities were included as covariates in these analyses (n = 56). First, we found that a component score that included a symptom increase in any 1 of the 6 symptoms significantly predicted shorter survival (P = .0017) (Table 4, Model 2a). Other significant predictors of survival in this model were the baseline symptom severity component score (P = .015) and poor baseline performance status (PS = 2) (P = .032).
Second, a stepwise variable selection of candidate predictors was performed to determine which individual variables retained significance in the multivariate Cox model (Table 4, Model 2b). We found that symptom increase in fatigue (P = .021) and shortness of breath (P = .018) were significant predictors of survival. Being white non-Hispanic (P = .009) also was predictive of better survival. Elimination of minority patients from the analysis (n = 52) had a noticeable effect in Model 2b: fatigue and lack of appetite became significant predictors.
Model Fitting for Multivariate Analyses
We compared the Akaike information criterion (AIC)22 for the best model fit between models with a component symptom score (Models 1a, 2a) and models with single symptom items (Models 1b, 2b). We found that the single-symptom models fitted the data better than did the models with component scores. For baseline models of both the all-patient sample and the white non-Hispanic patient sample, we consistently found that the model with baseline coughing and PS (Model 1b) showed the smallest AIC, which indicates the best model fit (Table 5).
Table 5.
Akaike Information Criteria (AIC) for Model Comparison*
| Baseline Models | AIC | |
|---|---|---|
| All Patients | White Non-Hispanic Patients | |
| PS + baseline coughing | 480.030 | 432.681 |
| PS + baseline component score | 485.474 | 434.095 |
| Baseline coughing only | 493.048 | 443.175 |
| PS only | 494.370 | 444.028 |
| Baseline component score only | 498.193 | 443.543 |
PS, Eastern Cooperative Oncology Group performance status.
Age, gender, race, previous chemotherapy, and comorbidity were included as covariates in all models.
DISCUSSION
Whereas previous studies have shown that both symptom ratings and responses to quality-of-life questionnaires are associated with survival, especially for patients who have cancer with a shorter prognosis, this study is among the first to examine whether measurement of baseline symptoms or symptom change in an individual patient with advanced cancer might assist in estimating the prognosis for that patient.
Results of this study support our hypothesis that moderate to severe baseline symptoms and worsening symptom burden during the first cycle of chemotherapy are strong independent predictors of overall survival in patients with advanced NSCLC who were eligible to receive chemotherapy. Of the multiple symptoms rated by patients, moderate to severe coughing (rated as 4 or greater on the MDASI’s 11-point scale) at baseline had the most significant independent predictive value for poor survival in this patient population, especially for patients with poor performance status (PS = 2). Additionally, we found that fatigue, shortness of breath, or poor appetite that had increased in severity by 1 or more points on the MDASI from baseline to the end of the first chemotherapy cycle were independent predictors of poorer overall survival in these patients.
Translational and clinical research has set the stage for personalizing chemotherapy in the management of NSCLC to improve response to treatment and survival of patients with NSCLC.23 Some molecular biomarkers are promising as to their prognostic value for tumor response and survival in patients with advanced NSCLC.23–25 Ultimately, however, the clinical application of these biomarkers may rely on the feasibility and ease of testing them in clinical practice. In contrast, symptom assessment via patient report can provide a simple, readily available, yet robust prediction of the patient’s near-term survival, especially when more elaborate tests are not available in daily oncology practice. Further investigation into the additional prognostic value of symptom measures along with biomarkers for advanced NSCLC is merited.
Previous research on patient-reported outcomes has examined both health-related quality-of-life and symptom information as predictors of survival. For example, Eton and colleagues6 reported that changes in quality-of-life component scores over 2 cycles of chemotherapy were predictive of clinical outcomes in a group of patients with advanced lung cancer. Our study adds to these findings by suggesting that an individual patient’s symptom scores—particularly when scores exceed specified cut points—have utility in predicting outcomes, at least for patients with advanced disease. Such cut points are often used in the clinic to control symptoms. On the basis of widely accepted symptom-control guidelines,18,19 we provisionally set 4 or greater on a 0–10 scale to describe a moderate to severe symptom and its association with survival outcomes. The potential utility of such a categorization on severity scales has been well investigated for pain management in individual patients,26 and it has been explored for categorizing the severity of other symptoms as well.17,27,28
Our results support for measuring a few highly relevant symptoms as a simple, robust tool for predicting outcomes in a busy clinic setting. Repeated symptom ratings can be collected reasonably quickly: patients typically complete the MDASI in 5 minutes or less. In the current study, we took advantage of the MDASI’s multisymptom approach and its simple 0–10 rating scale to compare a large number of symptoms. Fatigue, shortness of breath, and pain were the most severe symptoms by the end of the first chemotherapy cycle (Table 3), identical to findings from a retrospective study of the prevalence and intensity of lung cancer symptoms near the time of death.7 This study not only confirmed the prognostic value of the component scores, but also identified the specific symptoms that were most relevant to overall survival status. In fact, the examination of model fitting (Table 5) demonstrated the better prognostic value of specific symptoms compared with component scores. Our results have added evidence that a single-symptom score is responsive to changes over time and is unambiguous as to which specific symptom is changing, and to what degree.29
Investigating the interaction between symptom severity and baseline performance status as a predictor of survival is justified by the considerable number of patients (approximately 30%) with advanced NSCLC who had poor performance status (PS = 2) in this study. Although these patients typically are qualified for chemotherapy, they can expect only a small survival benefit.30 Even with a small sample size, the study clearly demonstrated that the risk for death in patients with moderate to severe coughing and poor performance status at baseline was 20.6 times higher than for patients with only mild coughing and good performance status (PS = 0–1) (P < .0001), a strong indicator of which patients would be most likely to die before the end of a chemotherapy clinical trial. This model could potentially provide a practical tool for clinical use, as it may more precisely predict overall survival than either symptom severity alone or PS alone.
This study was limited in that it was conducted in a single institution and with a mostly white non-Hispanic patient sample; the impact of race or ethnicity on survival is thus inconclusive. The multivariate analyses that excluded the minority patients showed a diluted impact from PS on overall survival and demonstrated an increased role for symptom report as an independent predictor. This result warrants further study in a sample containing sufficient minority patients to confirm the role of race/ethnicity in PRO-predicted survival. Also, although previous reports indicate that pain is an important prognostic factor in lung cancer, it was not a significant predictor of overall survival in the current study.12 Improvements in the standard care for pain management in oncology practice in many cancer treatment centers in recent years may have diluted the potential prognostic impact of pain in this study.
As with any other potential marker for survival or progression, recommendations about patient care cannot be made without repeated cross-validation studies of the prognostic value of symptom-report models in other cohorts of patients with advanced NSCLC being treated in medical oncology clinics. Cross validation in a much larger multi-institutional study would greatly enhance any recommendations about the use of symptom reports as predictors of outcome. Nonetheless, the results obtained from this relatively small patient sample were statistically significant, with at least 90% power to detect a hazard ratio of 2.5 or higher for baseline moderate to severe symptoms at an alpha level of .05. This level of power strongly supports the effect and clinical relevance of the results and thus the use of symptom reports in predicting outcomes in advanced NSCLC.
The stability of symptom report based on a single baseline time point needs to be established. Even so, the potential predictive power of symptom report in individual patients, as found in this study, is noteworthy and can easily be examined in other databases via the methods we report here. The increasing use of symptom measures at baseline and longitudinally in observational studies and clinical trails could provide data to further evaluate the observations reported here. Symptom assessment by MDASI takes less than five minutes, and MDASI symptom data can be obtained remotely via electronic (computer or telephone-computer systems) that cause little patient burden. The use of symptom report as a predictor is also supported by the evident clinical and biologic significance of increasing symptom severity as a marker of disease severity.
In conclusion, this study highlights the importance of validated symptom-burden assessment tools, such as the MDASI, in gaining patient-report information that, beyond facilitating better symptom control in oncology care, is useful for predicting overall survival in patients with advanced cancer. Such symptom-based prognostic information, taken together with physician-rated PS, may help clinicians gain a sense of the expected near-term survival for individual patients with advanced NSCLC who qualify for chemotherapy.
Acknowledgments
The authors thank Linda McCrory, RN and Beth Johnson, RN for data collection and Marilyn Morrissey, MPH, for protocol management. The authors acknowledge the editorial assistance of Jeanie F. Woodruff, ELS.
Footnotes
Financial Disclosures:
Sources of Support: This study was funded by a grant (R01 CA026582) from the National Cancer Institute of the National Institutes of Health. Neither the NCI nor the NIH had any role in the study design, data collection, analysis, interpretation, or preparation of the report.
Conflicts of Interest: The authors declare no conflicts of interest. The corresponding author states that she had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Statement of Informed Consent: All patients provided informed consent to participate in the study. The M. D. Anderson Cancer Center Institutional Review Board approved the study.
References
- 1.Cooley ME. Symptoms in adults with lung cancer. A systematic research review. J Pain Symptom Manage. 2000;19:137–153. doi: 10.1016/s0885-3924(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 2.Temel JS, Pirl WF, Lynch TJ. Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer. 2006;7:241–249. doi: 10.3816/CLC.2006.n.001. [DOI] [PubMed] [Google Scholar]
- 3.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monographs. 2007;37:16–21. doi: 10.1093/jncimonographs/lgm005. [DOI] [PubMed] [Google Scholar]
- 4.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 5.Gralla RJ, Griesinger F. Interpreting clinical trials in lung cancer: impact of methodology and endpoints. J Thorac Oncol. 2007;2:S51–S58. doi: 10.1097/01.JTO.0000269734.27047.3e. [DOI] [PubMed] [Google Scholar]
- 6.Eton DT, Fairclough DL, Cella D, Yount SE, Bonomi P, Johnson DH. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol. 2003;21:1536–1543. doi: 10.1200/JCO.2003.07.128. [DOI] [PubMed] [Google Scholar]
- 7.Tishelman C, Petersson LM, Degner LF, Sprangers MAG. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 8.Ganz PA, Lee JJ, Siau J. Quality of life assessment. an independent prognostic variable for survival in lung cancer. Cancer. 1991;67:3131–3135. doi: 10.1002/1097-0142(19910615)67:12<3131::aid-cncr2820671232>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Gralla RJ, Thatcher N. Quality-of-life assessment in advanced lung cancer: considerations for evaluation in patients receiving chemotherapy. Lung Cancer. 2004;46:S41–S47. doi: 10.1016/s0169-5002(04)80040-0. [DOI] [PubMed] [Google Scholar]
- 10.Sloan JA, Loprinzi CL, Kuross SA, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998;16:3662–3673. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 11.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 12.Efficace F, Bottomley A, Smit EF, et al. Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann Oncol. 2006;17:1698–1704. doi: 10.1093/annonc/mdl183. [DOI] [PubMed] [Google Scholar]
- 13.Belani CP, Pereira JR, von PJ, et al. Effect of chemotherapy for advanced non-small cell lung cancer on patients’ quality of life. A randomized controlled trial. Lung Cancer. 2006;53:231–239. doi: 10.1016/j.lungcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26:1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 15.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 17.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35:126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedetti C, Brock C, Cleeland C, et al. NCCN practice guidelines for cancer pain. Oncology (Huntington) 2000;14:135–150. [PubMed] [Google Scholar]
- 19.Mock V, Atkinson A, Barsevick A, et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Huntington) 2000;14:151–161. [PubMed] [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 22.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 23.Simon GR, Begum M, Bepler G. Setting the stage for tailored chemotherapy in the management of non-small cell lung cancer. Future Oncol. 2008;4:51–59. doi: 10.2217/14796694.4.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERCC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2:902–906. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 25.Niedernhofer LJ, Bhagwat N, Wood RD. ERCC1 and non-small-cell lung cancer. N Engl J Med. 2007;356:2538–2540. doi: 10.1056/NEJMc070742. [DOI] [PubMed] [Google Scholar]
- 26.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 27.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998;82:1904–1908. doi: 10.1002/(sici)1097-0142(19980515)82:10<1904::aid-cncr13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Sloan JA, Aaronson N, Cappelleri JC, Fairclough DL, Varricchio C. Assessing the clinical significance of single items relative to summated scores. Mayo Clin Proc. 2002;77:479–487. [PubMed] [Google Scholar]
- 30.Socinski MA. The role of chemotherapy in the treatment of unresectable stage III and IV nonsmall cell lung cancer. Respir Care Clin N Am. 2003;9:207–236. doi: 10.1016/s1078-5337(02)00089-8. [DOI] [PubMed] [Google Scholar]