Abstract
Interstitial fibrosis and hypoxia accelerate the progression of CKD, but clinical tools to quantitate these factors in patients are lacking. Here, we evaluated the use of two magnetic resonance imaging (MRI) techniques, diffusion-weighted (DW)-MRI and blood oxygen level-dependent (BOLD)-MRI, to assess kidney fibrosis and hypoxia of the cortex in 142 patients with either diabetic nephropathy (n = 43), CKD without diabetes (n = 76), or acute kidney injury (AKI) (n = 23). Apparent diffusion coefficient (ADC) values of DW-MRI correlated with estimated glomerular filtration rates (eGFR) in the diabetic nephropathy and CKD groups (r2 = 0.56 and r2 = 0.46, respectively). Although the T2* values of BOLD-MRI and eGFR displayed good correlation in the CKD group (r2 = 0.38), we did not observe a significant correlation between these values in the diabetic nephropathy group, suggesting that factors other than tubulointerstitial alteration determine the degree of hypoxia in the renal cortex. In the AKI group, neither the T2* nor ADC values correlated with eGFR. Renal biopsies from patients with CKD demonstrated that the T2* and ADC MRI values correlated with renal pathology. Taken together, ADC and T2* values appear to serve as accurate indices for evaluating renal tubulointerstitial alterations and parenchymal hypoxia, respectively, in the cortex. Functional MRI can thus contribute to multilateral, noninvasive, in vivo assessment of kidney function.
Chronic kidney disease (CKD) is characterized by progressive loss of kidney function resulting from chronic tubulointerstitial injury, which encompasses tubular atrophy and interstitial fibrosis. Such alterations decrease renal oxygenation, which in turn initiates and promotes fibrotic responses via various cytokine signaling pathways and cell-signaling events.1 Because fibrosis and hypoxia are regarded as major factors leading to the progression of CKD, the ability to accurately and noninvasively evaluate these factors directly would aid in the treatment of CKD. Although determining the degree of renal parenchymal fibrosis and hypoxia in patients remains challenging, recent advances in magnetic resonance imaging (MRI) may allow these processes to be evaluated in vivo.
Two promising functional MRI techniques for assessing kidney function are diffusion-weighted (DW)-MRI and blood oxygen level-dependent (BOLD)-MRI. DW-MRI measures apparent diffusion coefficient (ADC) values and quantifies the combined effects of blood microcirculation and Brownian motion of water molecules within tissues.2 The experimental application of DW-MRI for evaluating liver cirrhosis demonstrated that ADC values are significantly lower in cirrhotic livers as compared with normal livers.3 The applicability of DW-MRI in kidney disease was also evaluated in a preliminary trial, which revealed that patients with renal failure have significantly lower ADC values in the cortex and medulla than normal patients.4 More recently, this technique was successfully used to demonstrate decreases in ADC values in ligated kidneys in a mouse unilateral urethral obstruction model of interstitial fibrosis.5 The second promising MRI technique, BOLD-MRI, noninvasively assesses tissue oxygen bioavailability by measuring relative changes in deoxyhemoglobin, an endogenous contrast agent.6,7 BOLD-MRI, which was developed primarily for visualizing active regions in the brain, has been used experimentally to demonstrate acute and transient changes in oxygenation levels in the renal cortex8 and medulla.9–11 Additionally, the capability of BOLD-MRI to evaluate chronic, progressive, parenchymal hypoxia in renal allografts has been reported.12 Despite the potential of BOLD-MRI and DW-MRI for assessing renal function, the applicability of these two techniques for evaluating the progression of CKD in vivo has not been conclusively demonstrated.
In this study, we applied DW- and BOLD-MRI to examine their potential for detecting renal fibrosis and hypoxia in vivo in 142 patients with varying degrees of renal impairment. Initially, DW- and BOLD-MRI was first performed to define appropriate scanning parameters in a subset of patients and ten healthy volunteers. After the scanning parameters were defined, the kidneys of patients with either CKD without diabetes (n = 76), diabetic nephropathy (n = 43), or acute kidney injury (AKI) without diabetes (n = 23) were examined by DW- and BOLD-MRI, followed by conventional abdominal CT, to obtain ADC and T2* values, respectively. The MRI values were then compared with estimated glomerular filtration rates (eGFR). If the patient displayed asymmetry in kidney size, estimated renal plasma flow values were determined by renal dynamic scintigraphy to estimate split renal function. Moreover, kidney biopsies were also performed in a subset of CKD patients, and the degree of fibrosis was morphologically evaluated. Finally, the renal plasma flow values and biopsy fibrosis data were used to confirm that the obtained MRI values correlated with the physiologies and pathologies observed by the functional MRI.
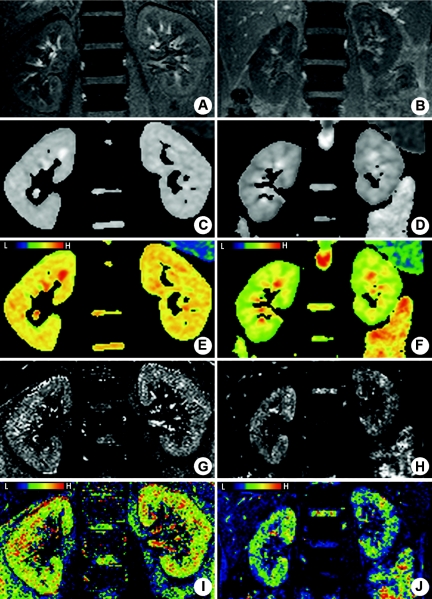
Demographics and clinical characteristics of the three patient groups are presented in Table 1. Ten healthy volunteers were also included as controls. Representative MRI images of kidneys from a healthy 37-year-old male volunteer and a 40-year-old female patient in stage 5 CKD with chronic glomerulonephritis are shown in Figure 1. The coronal proton density-weighted images (PDWI) (Figure 1, A and B) were used as anatomical references. Notably, although the cortex and medulla were easily distinguishable in the kidney of the healthy volunteer (Figure 1A), the cortico-medullary junction was ill-defined in the CKD patient (Figure 1B).
Table 1.
Comparison of clinical and laboratory data between the three patient groups
| CKD without Diabetes | Diabetic Nephropathy | AKI without Diabetes | |
|---|---|---|---|
| Number of cases | 76 | 43 | 23 |
| Male | 44 | 31 | 13 |
| Female | 32 | 12 | 10 |
| Age (years) | 51.5 ± 18.1 | 59.0 ± 11.1 | 61.9 ± 21.3 |
| Laboratory findings | |||
| eGFR (ml/min per 1.73 m2) | 45.8 ± 30.1 | 43.8 ± 27.7 | 16.0 ± 10.2a |
| Serum albumin (mg/dl) | 3.39 ± 0.89 | 3.57 ± 0.72 | 3.00 ± 0.72a |
| HbAlc (%) | 7.0 ± 1.2 | ||
| Hb (g/dl) | 12.3 ± 2.5 | 12.6 ± 2.6 | 9.6 ± 1.6a |
| Urinary protein (mg/g uCr) | 1549.2 ± 2415.8 | 980.0 ± 2229.1 | 1238.7 ± 1233.8 |
| Primary diseases (n) | Hypertensive nephrosclerosis (20) | Diabetic nephropathy (43) | Prerenal AKI (12) |
| Vasculitic syndrome (8) | Acute interstitial nephritis (8) | ||
| Chronic glomerulonephritis (37) | Acute glomerulonephritis (3) | ||
| Chronic interstitial nephritis (7) | |||
| Asymptomatic hematuria (4) |
The study participants were diagnosed with either CKD without diabetes, diabetic nephropathy, or AKI without diabetes. No significant differences were observed between the CKD without diabetes and diabetic nephropathy groups. Anti-neutrophil cytoplasmic antibody-associated renal limited vasculitis is described as vasculitic syndrome. Note that in the case of AKI, eGFR was calculated using the serum creatinine concentration determined at the nearest point of the magnetic resonance imaging scan, even if the levels were at steady state. CKD, chronic kidney disease; AKI, acute kidney injury; eGFR, estimated GFR; HbA1c, hemoglobin A1c; Hb, hemoglobin; uCr, urinary creatinine; and (n), number of cases.
aP < 0.01, compared with CKD without diabetes group.
Figure 1.
BOLD- and DW-MRI of kidneys. Representative magnetic resonance images of a 37-year-old healthy male volunteer (A, C, E, G, and I) and a 40-year-old female chronic kidney disease patient with chronic glomerulonephritis (B, D, F, H, and J) without diabetes. (A and B) Coronal proton density-weighted half-Fourier single-shot fast spin echo images. (C through F) Apparent diffusion coefficient (ADC) maps. (G through J) T2* maps. In E, F, I, and J, pseudo-color has been applied to accurately discriminate the difference between the ADC/T2* values of C, D, G, and H, respectively. For example, blue represents the areas of lowest T2* values and oxyhemoglobin levels, whereas green, yellow, and red, in that order, represent increasing T2* values and higher oxyhemoglobin levels on the colored T2* map.
We observed significantly different ADC and BOLD-MRI T2* maps between the normal functioning kidneys of a representative healthy volunteer (Figure 1, C, E, G, and I) and those of a stage 5 CKD patient (Figure 1, D, F, H, and J). Notably, a higher number of green areas were observed in the maps of the CKD patient kidney (Figure 1, F and J), corresponding to lower ADC and T2* values, when compared with those of the normal kidneys (Figure 1, E and I). We also measured these values in the cortex to avoid transient effects that are often observed in the medulla, and then evaluated their basal levels. As expected, the T2* value of BOLD-MRI in the medulla varied depending on the body fluid volume and administration of diuretic agents, a result consistent with previous reports.9–11
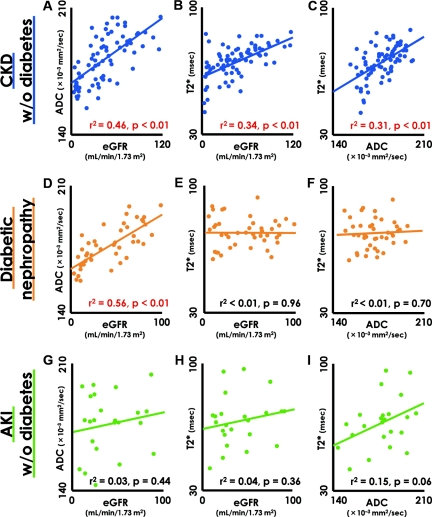
On the basis of the BOLD- and DW-MRI analysis that appeared to detect the degree of hypoxia and fibrosis of the kidney, we next examined the correlation between T2* and ADC values and residual renal function, as indicated by eGFR. In nondiabetic CKD patients, decreased eGFR were accompanied by reduced ADC values (Figure 2A). Similarly, T2* values and eGFR also displayed a significant positive correlation (Figure 2B). In patients with diabetic nephropathy, the ADC values significantly correlated with eGFR in a manner identical to that of nondiabetic CKD (Figure 2D). In contrast, there was no correlation between T2* values and eGFR, although several subjects showed lower T2* values than the healthy volunteers (Figure 2E). Overall, the average T2* value in diabetic nephropathy was significantly different from that in CKD without diabetes (diabetic nephropathy, 73.98 ± 7.99; CKD without diabetes, 70.54 ± 9.41; P = 0.046). In patients with AKI, it was not surprising that neither T2* nor ADC correlated with eGFR (Figure 2, G and H), because constantly fluctuating serum creatinine (SCr) levels do not reflect accurate eGFR. More importantly, interstitial fibrosis and/or edema and the degree of parenchymal hypoxia are presumably defined by the primary causes of AKI rather than by residual renal function. There was no statistically significant correlation between ADC and T2* values; however, we noted a tendency for lower ADC values in individuals with more severe hypoxia (Figure 2I). We also examined other factors that can affect the T2* value and the prognosis of diabetic nephropathy. The values for proteinuria, glycosylated hemoglobin (HbA1c), hemoglobin, and serum albumin levels; systolic BP; and usage of angiotensin receptor blocker and angiotensin-converting enzyme inhibitors were all independent of the T2* values (data not shown). Moreover, we evaluated the correlation between kidney size (longest length) and MRI values and found that renal size positively correlated with eGFR, ADC, and T2* values in nondiabetic CKD patients. In contrast, although renal size displayed a weak correlation with ADC values in patients with diabetic nephropathy, T2* values were completely independent of renal size (Supplemental Figure 1). According to our data, it appears that renal size reflects the degree of interstitial fibrosis, which can be evaluated by measuring ADC values using DW-MRI. Smaller kidneys have more severe interstitial fibrosis, lower ADC values, and decreased eGFR.
Figure 2.
Relationship among ADC values, T2* values, and residual kidney function. (A through C) Decreased eGFR is accompanied by reduced apparent diffusion coefficient (ADC) and T2* values in CKD patients without diabetes. (D and E) In the case of diabetic nephropathy patients, the ADC value and eGFR showed a significant positive correlation (D), but no correlation was noted between the T2* value and eGFR (E). (F) ADC was not a determinant of the T2* value. (G–I) In the AKI patients, ADC and T2* values were independent of eGFR. In the correlation graphs, closed circles indicate individual subjects, whereas continuous lines in the graph indicate a regression line. The red-colored data indicate statistical significance of correlation. CKD, chronic kidney disease; AKI, acute kidney injury; eGFR, estimated GFR.
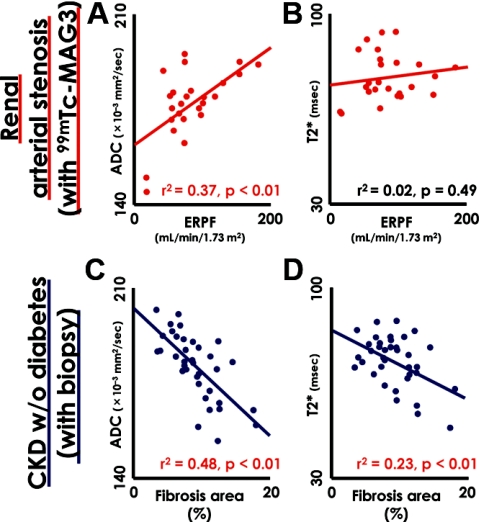
To validate whether the observed functional MRI values correlated with actual pathology and renal function, we examined renal biopsies in CKD patients who required biopsy for differential diagnosis. We also compared the ADC and T2* values with effective renal plasma flow (ERPF) estimated by 99mTc-mercaptoacetyltriglycine (99mTc-MAG3) scintigraphy in 13 subjects that showed bilateral differences in kidney size >1.5 cm resulting from renal artery stenosis. These subjects were eliminated from the BOLD- and DW-MRI analysis, because SCr does not accurately reflect net renal function in such cases. The demographics and clinical characteristics of the evaluated patients are summarized in Supplemental Table 1. The 99mTc-MAG3-estimated ERPF and ADC values showed a significant positive correlation (Figure 3A). Moreover, we evaluated the degree of interstitial fibrosis in the renal biopsy specimens of 37 CKD patients without diabetes by Masson's trichrome staining. The percentage of fibrotic area relative to the cross-sectional area in the renal biopsy were significantly correlated with not only ADC, but also T2* values, obtained from the MRI analysis (Figure 3, C and D). Thus, we concluded that the functional MRI values correlated well with the pathologic and physiologic aspects of kidney pathology.
Figure 3.
Correlation between kidney biopsies and 99mTc-MAG3 scintigraphy and functional magnetic resonance imaging (MRI) results. (A) The ADC values and the ERPF determined by diffusion-weighted (DW)-MRI and 99mTc-MAG3 scintigraphy, respectively, showed a significant positive correlation. (B) However, no correlation was observed between the BOLD-MR T2* values and ERPF. (C and D) The percentage of fibrotic area relative to the cross-sectional area (fibrosis area [%]) in each renal biopsy of 37 CKD patients, as determined by Masson's trichrome staining, is plotted against ADC (C) and T2* (D) values determined by functional MRI. Increased fibrosis was significantly correlated with reduced ADC and T2* values in CKD patients without diabetes. The continuous lines in the graph indicate a regression line. The red-colored data indicate statistical significance of correlation. CKD, chronic kidney disease; 99mTc-MAG3, 99mTc-mercaptoacetyltriglycine; ERPF, effective renal plasma flow.
Residual renal function can be defined by the degree of chronic tubulointerstitial alterations,13 which decrease renal water content and also restrict the fibrosis-mediated diffusion of water molecules. The ADC values observed in our study likely reflect the degree of chronic tubulointerstitial alterations caused by decreased renal oxygenation, which in turn initiates and promotes the fibrotic response.1 Although chronic tubulointerstitial alterations may not be the primary factor determining parenchymal hypoxia in diabetic nephropathy, reduction in the density of peritubular capillaries has been demonstrated in both diabetic14 and nondiabetic nephropathy.15 Streptozotocin-induced diabetic rats, which serve as a model for early-stage human diabetic nephropathy, exhibit hypoxic areas as large as those in nondiabetic rats with glomerulonephritis, although peritubular capillaries are not affected.16 These results suggest the existence of other factors, such as impairment of oxygen diffusion in the interstitium, inability of tubule-epithelial cells to utilize oxygen, or abnormal oxygen affinity in diabetic red blood cells, which contribute to cellular hypoxia under conditions of hyperglycemia.17 Moreover, amelioration of oxidative stress improved renal oxygenation in an animal model of diabetic nephropathy.18,19 Oxidative stress can stimulate tissue hypoxia via depletion of nitric oxide without inducing any apparent histologic changes. Thus, oxygenation of the kidney is determined by the balance between supply and demand, which is also strongly affected by factors that do not accompany architectural changes of the kidney.18,19
To our knowledge, this is the first study to noninvasively detect hypoxia in the renal parenchyma of CKD patients in vivo on the basis of a decrease of BOLD-MRI T2* values. Although we did not compare T2* values and partial pressures of oxygen directly measured by electrodes or other methods, we consider that this finding is important because convincing evidence exists from animal models20 and human biopsies15 that chronic hypoxia promotes tubulointerstitial fibrosis and the progression of CKD.20 Moreover, we are not aware of any studies that have compared the levels of hypoxia in diabetic or other nephropathies. As shown here, BOLD-MRI can detect parenchymal hypoxia in both CKD and diabetic nephropathy patients; thus, the evaluation of hypoxia by BOLD-MRI could also be used as a prognostic factor for predicting the response of kidney disease to other factors, such as new medications.
In conclusion, we have demonstrated the clinical potential of combining the functional MRI techniques, DW-MRI and BOLD-MRI, as a relatively simple, noninvasive clinical tool that obviates the need for potentially toxic radiocontrast agents and/or ionizing radiation. Although considerable research is required to establish functional MRI of kidneys as a routine clinical examination, this approach may allow the identification of the underlying pathophysiology of various renal diseases, which is expected to be valuable in making clinical decisions. In a future study, we plan to investigate the relationship between MRI-measured values and renal-function prognosis.
CONCISE METHODS
Subject Characteristics
A total of 142 patients (88 men and 54 women) admitted to the Departments of Nephrology and Endocrinology & Diabetes of our hospital were recruited for participation in this study. Ten healthy volunteers (five men and five women; average age, 36 years). All of the subjects were subjected to both functional and standard MRI, and laboratory tests were performed within 1 month of the MRI scans. The eGFR was calculated using the Modification of Diet in Renal Disease formula, which is recommended by the Japanese Society of Nephrology. Diagnosis of CKD was on the basis of persistent proteinuria or chronic decline in eGFR (<60 ml/min per 1.73 m2) and were classified into stages as follows: eGFR ≥90, stage 1; 60 to 89, stage 2; 30 to 59, stage 3; 15 to 29, stage 4; <15, stage 5. Clinical history, laboratory findings, and renal biopsy results were all used to make the primary diagnosis. AKI was diagnosed using AKIN criteria,21 which is on the basis of increases in SCr over a 48-hour period, rather than 7 days. Stage 1 AKI was defined as an increase in SCr of 26.5 μmol/L (0.3 mg/dl) or 150 to 200% from baseline levels, stage 2 as an increase in SCr of 200 to 300%, and stage 3 as an increase in SCr of either >354 μmol/L (>4 mg/dl) or >300% or the commencement of acute renal replacement therapy. Among the 23 patients diagnosed with AKI, eight, ten, and five were stages 1, 2, and 3, respectively. MRI scans were performed less than 10 days after hospital admission. A diagnosis of diabetic nephropathy meant the presence of diabetes mellitus with proteinuria, which was defined as 30 mg/dl of protein in the urine or greater, as measured by a test-tape method in outpatient clinics. Subjects with obstructive nephropathy and primary nephrotic syndrome were excluded. The study protocol was approved by the Institutional Review Board of Saitama Medical University and conformed to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all of the patients before their participation in this study.
Magnetic Resonance Imaging Techniques
Magnetic resonance imaging was performed with a 1.5-T Imager (Sonata; Siemens, Erlangen, Germany) and a six-channel body coil. For morphologic evaluation, the images were acquired with a coronal proton density-weighted half-Fourier single-shot fast spin echo (PDWI), a coronal T2-weighted half-Fourier single-shot fast spin-echo sequence (HASTE), and a coronal T1-weighted fast low-angle shot gradient-echo sequence (T1WI).
BOLD-MRI was performed with a multiple gradient-recalled-echo sequence. Three coronal sections of kidneys were acquired with a 5-mm section thickness and a 1-mm intersection gap. The field of view and the matrix were 400 × 400 mm and 256 × 256, respectively; one signal was acquired. The repetition time was 65 milliseconds, and the flip angle was 30°. The following 12 in-phase echo times were used 4.76, 9.53, 14.3, 19.1, 23.8, 28.6, 33.3, 38.1, 42.9, 47.6, 52.4, and 57.2 s/mm to remove the out-of-phase subtraction effect caused by the fat component. Twelve T2*-weighted images corresponding to the 12 different gradient echoes were acquired for each section with 26-second breath holds.
Coronal multisection echo-planar DW-MRI was performed with the following parameters: 21 sections (section thickness, 5 mm; intersection gap, 1 mm); field of view, 400 × 400 mm; matrix, 128 × 128; six signals acquired; bandwidth, 1500 Hz/pixel; and partial Fourier factor, 6/8. The following eight diffusion gradient b values were used: 0, 50, 100, 150, 300, 500, 750, and 1000 s/mm2. The gradients were applied in three orthogonal directions and subsequently averaged to minimize the effects of diffusion anisotropy. A parallel imaging technique with a reduction factor of 2 was applied. Respiratory triggering was used with a minimum repetition time of 5900 milliseconds and an echo time of 74 milliseconds. Section positioning was used with the axial HASTE. The minimum acquisition time was 8 minutes 57 seconds.
The BOLD- and DW-MRI findings of all participating subjects were independently analyzed by two observers (I.T. and E.K.), who were blinded to the SCr levels to eliminate bias. T2* and ADC maps were calculated on a pixel-by-pixel basis by fitting a linear regression method through the logarithms of the signal intensities versus their 12 echo times of in-phase and eight b values, respectively. Signal intensities in the region of interest (ROI) were obtained using the different in-phase echo times and b values of DW-MRI, as described previously.4,12 T2* and ADC maps of the kidney were generated using the software on the MRI scanner. T2* and ADC values were determined by measuring the signal intensity on the map using OsiriX, which is image-processing software for digital imaging and communications in medicine. The ROI was manually defined in the cortex (ROI, 2.65 to 12.61 cm2; mean, 5.56 cm2). PDWI was used as an anatomical reference because of the good contrast between the medulla and cortex. HASTE and T1WI were referred at the identical level to avoid areas of cysts, mass lesions, and stones. We also examined the variation and reproducibility of MRI values using healthy volunteers; the degree of variability for both T2* and ADC values was approximately 4% for the identical subject. Moreover, we observed a significant difference in T2* values between the cortex and medulla in healthy controls (cortex: 74.61 ± 5.30; medulla: 59.35 ± 4.98; P < 0.001, t test).
Morphologic Examination of Kidney Tissue
Biopsy was performed when patients were admitted into our hospital to establish an accurate diagnosis. Because hematoma would exert unexpected influences on MRI results, MRI was performed before biopsy. Four-micrometer sections were cut from paraffin blocks and processed for Masson's trichrome staining. Interstitial fibrosis was assessed quantitatively using a Mac SCOPE (Version 2.5; Mitani Corp., Fukui, Japan). In this analysis, all glomeruli and vessels were subtracted from a given field, yielding a target area of tubulointerstitium. Collagenous, fibrotic areas in blue were then quantified and expressed as a mean percentage area per field.22 Before performing renal scintigraphy, the subjects were well hydrated. 99mTc-MAG3 was injected intravenously, and 99mTc-MAG3 clearance was measured using a collimator. Split renal function was calculated from the percentage contributed from each kidney.
Statistical Analyses
All of the values are expressed as the means ± SD. One-way ANOVA with post hoc Tukey's honestly significantly different test was used to compare the mean values of the three groups. The Pearson's product-moment correlation coefficient was used to evaluate associations between variables. The values of P < 0.05 were considered to be statistically significant. All of the statistical analyses were performed using JMP version 8 statistical software (JMP Japan, Tokyo, Japan).
DISCLOSURES
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Le Bihan D, Breton E, Lallemand D, Aubin M, Vignaud J, Laval-Jeantet M: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497–505, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Boulanger Y, Amara M, Lepanto L, Beaudoin G, Nguyen B, Allaire G, Poliquin M, Nicolet V: Diffusion-weighted MR imaging of the liver of hepatitis C patients. NMR Biomed 16: 132–136, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Thoeny HC, De Keyzer F, Oyen RH, Peeters RR: Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: Initial experience. Radiology 235: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M: Assessment of renal fibrosis with diffusion-weighted MR imaging: Study with murine model of unilateral ureteral obstruction. Radiology 255: 772–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennan RP, Zhong J, Gore JC: Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med 31: 9–21, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR: Microscopic susceptibility variation and transverse relaxation: Theory and experiment. Magn Reson Med 31: 601–610, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Schachinger H, Klarhofer M, Linder L, Drewe J, Scheffler K: Angiotensin II decreases the renal MRI blood oxygenation level-dependent signal. Hypertension 47: 1062–1066, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Epstein FH, Veves A, Prasad PV: Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care 25: 575–578, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC: The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 19: 780–788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thoeny HC, Zumstein D, Simon-Zoula S, Eisenberger U, De Keyzer F, Hofmann L, Vock P, Boesch C, Frey FJ, Vermathen P: Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: Initial experience. Radiology 241: 812–821, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Risdon RA, Sloper JC, De Wardener HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 14. Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Eardley K, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage C, Howie A, Kaur K, Cooper M, Adu D, Cockwell P: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Shibata R, Ueda S, Yamagishi S, Kaida Y, Matsumoto Y, Fukami K, Hayashida A, Matsuoka H, Kato S, Kimoto M, Okuda S: Involvement of asymmetric dimethylarginine (ADMA) in tubulointerstitial ischaemia in the early phase of diabetic nephropathy. Nephrol Dial Transplant 24: 1162–1169, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Kowluru R, Bitensky MW, Kowluru A, Dembo M, Keaton PA, Buican T: Reversible sodium pump defect and swelling in the diabetic rat erythrocyte: Effects on filterability and implications for microangiopathy. Proc Natl Acad Sci U S A 86: 3327–3331, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mimura I, Nangaku M: The suffocating kidney: Tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol 6: 667–678, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Palm F, Teerlink T, Hansell P: Nitric oxide and kidney oxygenation. Curr Opin Nephrol Hypertens 18: 68–73, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Matsumoto M, Tanaka T, Yamamoto T, Noiri E, Miyata T, Inagi R, Fujita T, Nangaku M: Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol 15: 1574–1581, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury N: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H: Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol 16: 133–143, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.