Abstract
TGF-β/Smad3 signaling promotes fibrosis, but the development of therapeutic interventions involving this pathway will require the identification and ultimate targeting of downstream fibrosis-specific genes. In this study, using a microRNA microarray and real-time PCR, wild-type mice had reduced expression of miR-29 along with the development of progressive renal fibrosis in obstructive nephropathy. In contrast, Smad3 knockout mice had increased expression of miR-29 along with the absence of renal fibrosis in the same model of obstruction. In cultured fibroblasts and tubular epithelial cells, Smad3 mediated TGF-β1-induced downregulation of miR-29 by binding to the promoter of miR-29. Furthermore, miR-29 acted as a downstream inhibitor and therapeutic microRNA for TGF-β/Smad3-mediated fibrosis. In vitro, overexpression of miR-29b inhibited, but knockdown of miR-29 enhanced, TGF-β1-induced expression of collagens I and III by renal tubular cells. Ultrasound-mediated gene delivery of miR-29b either before or after established obstructive nephropathy blocked progressive renal fibrosis. In conclusion, miR-29 is a downstream inhibitor of TGF-β/Smad3-mediated fibrosis and may have therapeutic potential for diseases involving fibrosis.
Tissue scarring or fibrosis is a hallmark and final pathway leading to end-stage kidney disease. It is now clear that TGF-β1 is a key mediator and mediates renal fibrosis by activating its downstream mediators, Smad2 and Smad3, which are negatively regulated by the inhibitor Smad7.1,2 Recent studies revealed that Smad3, but not Smad2, is a key signaling pathway of fibrogenesis in response to many fibrogenic mediators such as TGF-β1, angiotensin II, and advanced glycation end products.2–6 This is supported by the findings that mice lacking Smad3 are protected against fibrosis in many disease models, including irradiative skin disorder, ischemic and hypertensive cardiac remodeling, bleomycin-induced pulmonary fibrosis, dimethylnitrosamine-induced hepatic fibrosis, and unilateral ureteral obstructive (UUO) nephropathy.7–12 In contrast, conditional deletion of Smad2 from the kidney enhances renal fibrosis.6 The critical role for TGF-β/Smad signaling in fibrogenesis is further demonstrated by the finding that forced expression of Smad7 within the tissues inhibits, but loss of Smad7 promotes, fibrosis in a variety of disease models.13–17 However, mice lacking either TGF-β1 or Smad3 have impaired immunity, which results in deathly inflammation in multiple organs or the development of autoimmune disease.18–21 Lessons learned from the knockout mice strongly indicate that strategies aimed to prevent or treat fibrosis need to precisely target the downstream specific gene(s) of TGF-β/Smad signaling that directly regulates fibrosis rather than block the general effects of the TGF-β signaling pathway.
A recent discovery of microRNAs (miRNAs) as key regulators in the pathophysiological process of many diseases including cancers, metabolic diseases, neurologic disorders, infectious diseases, and other illness led us to hypothesize that TGF-β1 may mediate fibrosis by regulating Smad3-dependent miRNAs that are specifically involved in fibrosis. Increasing evidence shows that the miR-29 family is the best characterized miRNA associated with TGF-β1-mediated fibrosis.22–25 Indeed, miR-29 is downregulated by TGF-β1 in vitro and in the fibrotic tissues after cardiac infarction,22 bleomycin-induced systemic sclerosis and pulmonary fibrosis,23,24 and carbon tetrachloride-induced hepatic fibrogenesis.25 However, expression of miR-29 and the functional importance of miR-29 in renal fibrosis remain unexplored. Furthermore, the signaling mechanisms through which TGF-β1 regulates miR-29 expression are unknown. On the basis of the known role of Smad3 in fibrosis,2–12 we therefore hypothesized that miR-29 may be negatively regulated by TGF-β/Smad3 signaling and may function as a downstream inhibitor of TGF-β/Smad3-mediated fibrosis. This study examined this hypothesis in cells lacking Smad3 and in a well characterized progressive renal-fibrosis mouse model of UUO nephropathy induced in Smad3 knockout (KO) mice. Furthermore, the functional role and therapeutic potential for miR-29 in the fibrogenesis were determined in vitro by overexpression or knockdown of miR-29 and in vivo in a mouse model of UUO nephropathy using the ultrasound-microbubble-mediated inducible miR-29b gene transfer.
RESULTS
miR-29 Is Lost with Progressive Renal Fibrosis in Smad3 WT Mice, but Increased with Protection against Renal Fibrosis in Smad3 KO Mice in UUO Nephropathy
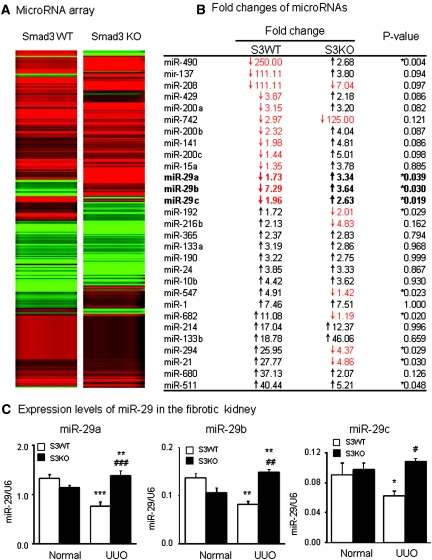
To determine whether miR-29 is regulated by Smad3 during fibrogenesis, we first examined the miRNA expression profile in a well characterized renal fibrosis mouse model of UUO nephropathy induced in Smad3 wild-type (WT) and KO mice by miRNA microarray and real-time PCR. As shown in Figure 1 (A and B), compared with normal controls, miRNA microarray detected that a number of miRNAs such as miR-192, miR-216a, miR-547, miR-682, miR-294, and miR21 were significantly increased in Smad3 WT mice with severe renal fibrosis but lost in Smad3 KO mice in which renal fibrosis was protected as described previously.12 In contrast, many miRNAs were markedly downregulated in the UUO kidney of Smad3 WT mice but significantly increased in Smad3 KO mice. Of them, the miR-29 family including miR-29a, miR-29b, and miR-29c was of particular interest, proving to be consistently decreased in the fibrotic kidney of Smad3 WT mice and significantly increased in Smad3 KO mice in the absence of severe renal fibrosis as previously reported.12 Changes of miR-29 expression profiles were further confirmed by quantitative real-time PCR (Figure 1C). Consistent with the finding by miRNA microarray, miR-29a and miR-29b were lost in the UUO kidney of Smad3 WT mice but significantly upregulated in Smad3 KO mice, although the change of miR-29c was marginally significant. Nevertheless, in the fibrotic kidney, deletion of Smad3 prevented a loss of miR-29 a,b,c family in the UUO kidney. These results suggest that TGF-β may regulate miR-29 expression during renal fibrosis via the Smad3-dependent mechanism.
Figure 1.
miRNA array and real-time PCR detect that the miR-29 family is lost in the UUO kidney of Smad3 WT, but increases in Smad3 KO mice. (A) miRNA expression profile in the UUO kidney of Smad3 WT and KO mice. (B) List of fold changes of miRNAs in the UUO kidney in Smad3 WT and KO mice. The data are normalized to normal Smad3 WT or KO mice. (C) Real-time PCR results of miR-29 family members expression in Smad3 WT and KO mice. Each bar represents the mean ± SEM for at least five mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus normal mice; #P < 0.05, ##P < 0.01, ###P < 0.001 versus Smad3 WT UUO.
miR-29 Expression Is Negatively Regulated by TGF-β/Smad3, Not Smad2, Signaling
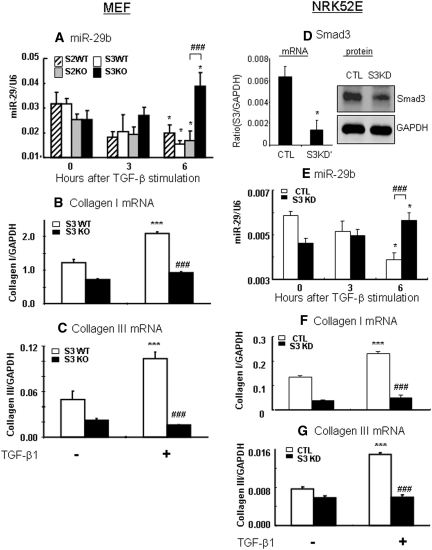
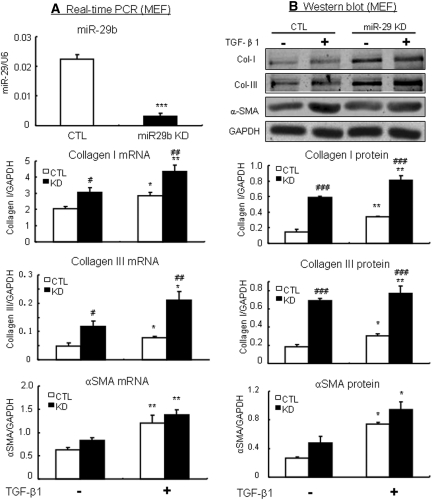
We next investigated the signaling mechanisms by which TGF-β1 regulates miR-29 expression. Because miR-29b-1 is coexpressed with miR-29a, whereas miR-29b (miR-29b-2) is coexpressed with miR-29c,22 miR-29b was used as a representative miRNA of the miR-29 family member for the entire study in vitro and in vivo. As shown in Figure 2A, TGF-β-induced loss of miR-29b expression was negatively regulated by Smad3, but not Smad2, because the addition of TGF-β1 caused remarkably lower levels of miR-29b in Smad2 WT, Smad2KO, and Smad3 WT mouse embryonic fibroblast (MEF). In contrast, MEF lacking Smad3 enhanced miR-29b expression in response to TGF-β1. Further studies revealed that TGF-β1-induced downregulation of miR-29b was associated with a significant upregulation of collagen I and III in Smad3 WT MEF but absent in Smad3 KO MEF (Figure 2, B and C). Because we have previously shown that MEF cells lacking Smad2 largely enhance TGF-β1-induced collagen I and III expression,6 the effect of deleting Smad2 on collagen expression was not shown in this study.
Figure 2.
TGF-β1 downregulates miR-29b and upregulates collagen I and III expression via the Smad3-dependent, not Smad2-dependent, mechanism. (A) Real-time PCR shows that TGF-β1 downregulates miR-29b expression in Smad3 WT, which is enhanced in Smad3 KO MEF. In contrast, MEF lacking Smad2 shows no protective effect on TGF-β1-downregulated miR-29b expression. (B and C) Collagen I and III mRNA expression in Smad3 WT and KO MEF in response to TGF-β1 (2 ng/ml). (D) Real-time PCR and Western blot analysis detect that Smad3 is significantly deleted from NRK52E cells that are stably expressed Smad3 siRNA. (E) Real-time PCR shows that TGF-β1 downregulates miR-29b expression in NRK52E cells, which is significantly enhanced in Smad3 knockdown NRK52E cells when compared with empty vector control NRK52E cells. (F and G) Collagen I and III mRNA expression in Smad3 KD and control NRK52E cells. Each bar represents the mean ± SEM for at least four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus baseline levels; #P < 0.05, ##P < 0.01, ###P < 0.001 versus Smad3 KO or KD cells or as indicated. CTL, control.
The regulating role of Smad3 in miR-29b expression during fibrosis in response to TGF-β1 was also examined in a stable Smad3 knockdown (KD) kidney tubular epithelial cell (TEC) line (NRK52E), another key cell type in tubulointerstitial fibrosis. The results shown in Figure 2 (D through F) clearly revealed that TGF-β1 downregulated miR-29b and upregulated collagen I and collagen III by NRK52E cells, which was prevented by knocking down Smad3.
Smad3 Interacts with miR-29b Promoter
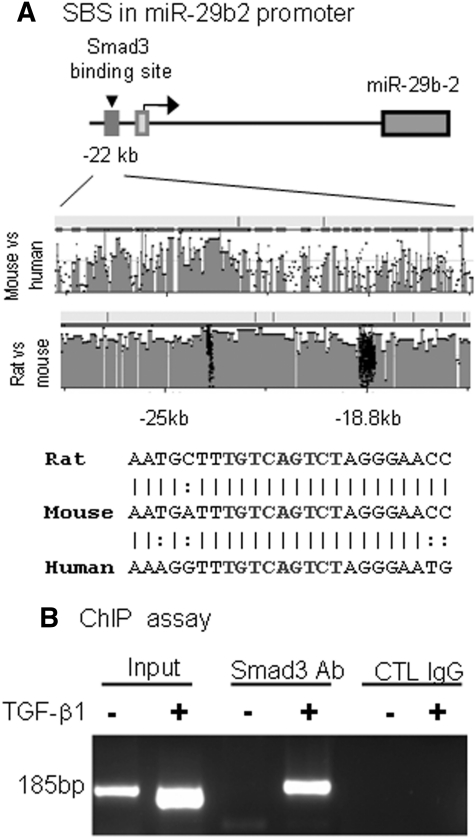
We next examined whether miR-29 is a target gene of Smad3 by examining the potential Smad-binding site in miR-29b promoter using rVista 2.0 (http://rvista.dcode.org/). As shown in Figure 3A, there was a potential binding site in a highly conserved region ∼22 kb upstream of miR-29b2. Sequence analysis revealed a Smad response element (TGTCAGTCT) located in this region, which is conserved in rat, mouse, and human miR-29b2 promoters (Figure 3A).
Figure 3.
Smad3 interacts with miR-29b2 promoter. (A) Sequence analysis shows that a Smad3-binding site (SBS) locates at the promoter region 22 kb upstream of miR-29b2. DNA sequence alignments indicate a highly conserved binding site between species. (B) ChIP assay shows that Smad3 physically binds miR-29b2 promoter in response to TGF-β1 (2 ng/ml for 24 hours).
By using a chromatin immunoprecipitation (ChIP) assay, we also detected that Smad3 interacted with the miR-29b2 promoter, and this interaction was markedly increased after TGF-β1 treatment (Figure 3B), indicating that miR-29b may be a transcriptional target of TGF-β/Smad 3 signaling.
Overexpression of miR-29b Inhibits, but Knockdown of miR-29b Enhances, TGF-β-induced Collagen I and III, but Not Alpha-Smooth Muscle Actin (α-SMA) Expression in Vitro
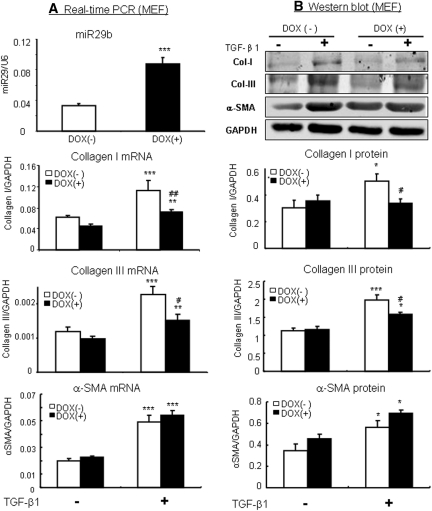
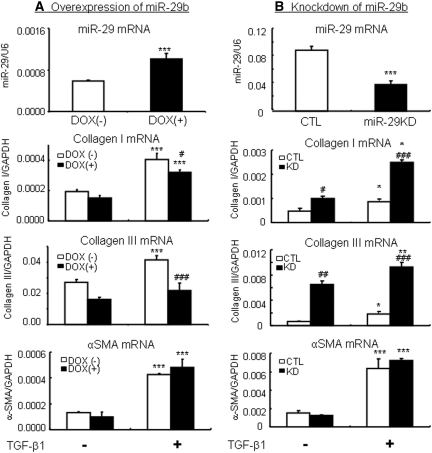
To study the functional role of miR-29 in fibrosis, overexpression or knockdown of miR-29b was carried out in vitro in MEF and NRK52E cells in which miR-29b expression was induced by the addition of doxycycline (Dox) but knocked down with pSuper-sh-miR-29b plasmids. Real-time PCR and Western blot analysis demonstrated that Dox-induced miR-29b overexpression in MEF virtually blocked TGF-β1-induced collagen I and III mRNA and protein expression (Figure 4). In contrast, knockdown of miR-29b in MEF largely enhanced collagen I and III expression (Figure 5). Similar results were also found in NRK52E TEC cells as shown in Figure 6.
Figure 4.
Overexpression of miR-29b in MEF cells inhibits TGF-β1-induced collagen I and III, but not α-SMA expression. (A) Real-time PCR. (B) Western blotting. The results show that the addition of doxycycline (DOX, 2 μg/ml for 24 hours) induces miR-29b expression in a Dox-inducible miR-29b expressing MEF cells (Smad3WT), thereby blocking TGF-b1 (2 ng/ml)-induced collagen I and III expression. Note that overexpression of miR-29b has no effect on α-SMA expression. Each bar represents the mean ± SEM for at least four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus baseline levels; #P < 0.05, ##P < 0.01, ###P < 0.001 versus nondoxycycline-treated cells.
Figure 5.
Knockdown of miR-29b in MEF cells enhances TGF-β1-induced collagen I and III expression but not α-SMA expression. (A) Real-time PCR. (B) Western blotting. The results show that stable knockdown of miR-29b in MEF cells (Smad3WT) significantly enhances TGF-β1 (2 ng/ml)-induced collagen I and III expression but not α-SMA expression. Note that knockdown of miR-29b also significantly enhances constitutive levels of collagen I and III mRNA and protein expression without TGF-β1 stimulation. Each bar represents the mean ± SEM for at least four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus baseline levels; #P < 0.05, ##P < 0.01, ###P < 0.001 versus control cells. CTL, control.
Figure 6.
Overexpression of miR-29b inhibits, but knockdown of miR-29b promotes, TGF-β1-induced collagen I and III expression by NRK52E cells. (A) Real-time PCR shows that addition of doxycycline (DOX, 2 μg/ml for 24 hours) induces miR-29b expression in a Dox-inducible miR-29b expressing NRK52E tubular epithelial cell line, thereby blocking TGF-β1 (2 ng/ml)-induced collagen I and III mRNA expression. (B) In contrast, stable knockdown of miR-29β in NRK52E cells significantly enhances TGF-β1 (2 ng/ml)-induced collagen I and III mRNA expression. Note that the change in miR-29b expression has no effect on α-SMA expression. Each bar represents the mean ± SEM for at least four independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus baseline levels; #P < 0.05, ##P < 0.01, ###P < 0.001 versus nondoxycycline-treated cells or vector control cells (CTL).
Interestingly, knockdown of miR-29b also resulted in a significant upregulation of basal levels of collagen I and III expression by both MEF and NRK52E cells (Figures 5 and 6B). However, overexpression or knockdown of miR-29b in both MEF and NRK52E cells did not alter the expression patterns of α-SMA under normal or high TGF-β1 conditions (Figures 4 through 6).
Gene Transfer of miR-29 Prevents Renal Fibrosis in a Mouse Model of Obstructive Nephropathy
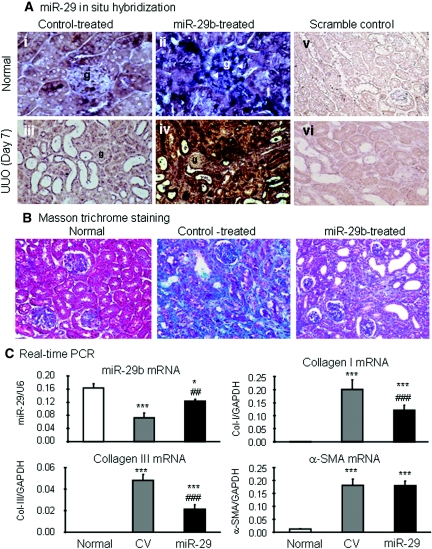
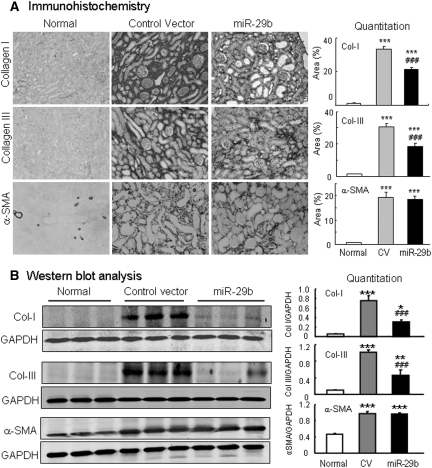
We next determined the functional role of miR-29 in TGF-β/Smad3-mediated fibrosis in a mouse model of obstructive nephropathy in which progressive renal fibrosis is Smad3-dependent.12 The Dox-inducible miR-29b-expressing plasmid system (pTet-on and pTRE2-miR-29b) was transfected into the normal left kidney of mouse using an established ultrasound-microbubble-mediated gene transfer technique, followed immediately by ligating the left ureter to induce UUO nephropathy as described previously.14 The results in Figures 7 and 8 clearly illustrate that progressive renal fibrosis in UUO nephropathy was associated with a loss of miR-29b. In contrast, miR-29b gene transfer resulted in higher levels of miR-29b transgene expression within the kidney and therefore prevented progressive renal fibrosis at day 7 after UUO. By in situ hybridization, moderate miR-29b was constitutively expressed by all glomerular and tubulointerstitial cells in normal mouse kidneys (Figure 7Ai) but was largely reduced in the fibrotic kidney at day 7 after UUO (Figure 7Aiii). Ultrasound-microbubble-mediated miR-29b gene transfer resulted in higher levels of miR-29b expression by all kidney cell types, particularly in mesangial cells, arterial wall, and TEC (Figure 7Aii), thereby preventing a loss of miR-29 from the UUO kidney at day 7 (Figure 7Aiv), and therefore attenuated progressive renal fibrosis as determined at the histologic level by Masson trichrome staining (Figure 7B), at mRNA levels by real-time PCR (Figure 7C), and at protein levels by immunohistochemistry and Western blot analysis (Figure 8). Interestingly, ultrasound-mediated miR-29 gene transfer did not alter high levels of α-SMA expression and α-SMA+ myofibroblast accumulation in the UUO kidney (Figures 7C and 8). The results obtained from this study indicated that overexpression of miR-29 at the time of disease induction was able to prevent renal fibrosis in the UUO nephropathy.
Figure 7.
Ultrasound-microbubble-mediated miR-29b gene transfer prevents renal fibrosis in a mouse model of UUO nephropathy at day 7. (A) In situ hybridization shows that moderate expression of miR-29b is found in normal glomerular and tubulointerstitial cells (i) and is largely enhanced by ultrasound-mediated miR-29 transfer, resulting in higher levels of miR-29b expression, particularly by mesangial cells (arrowheads), arterial wall, and TEC (ii). In the UUO kidney treated with control plasmid, miR-29 expression is largely reduced (iii) but is remarkably increased with ultrasound-mediated miR-29 gene therapy (iv). (v and vi) Scramble control. (B) Masson trichrome stain detects that severe renal fibrosis (green materials) is developed in the control-treated UUO kidney but largely reduced in the UUO kidney treated with miR-29. (C) Real-time PCR shows that miR-29 gene therapy prevents reduction of miR-29, thereby inhibiting collagen I and III but not α-SMA mRNA expression. Each bar represents the mean ± SEM for at least six mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus normal mice; #P < 0.05, ##P < 0.01, ###P < 0.001 versus control vector (CV)-treated mice. Magnifications, ×900 (A, i and ii); ×200 (A, iii through vi and B).
Figure 8.
Ultrasound-microbubble-mediated miR-29b gene transfer prevents renal fibrosis in a mouse model of UUO nephropathy at day 7. (A) Immunohistochemistry. (B) Western blotting. The results show that ultrasound-mediated miR-29 gene therapy inhibits collagen I and III expression and deposition, but not α-SMA expression and myofibroblast accumulation in the UUO kidney. Each micrograph or bar represents the mean ± SEM for at least six mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus normal mice; ###P < 0.001 versus control vector (CV)-treated mice. Magnification, ×100.
miR-29 Has Therapeutic Effect on Renal Fibrosis in the Established UUO Nephropathy
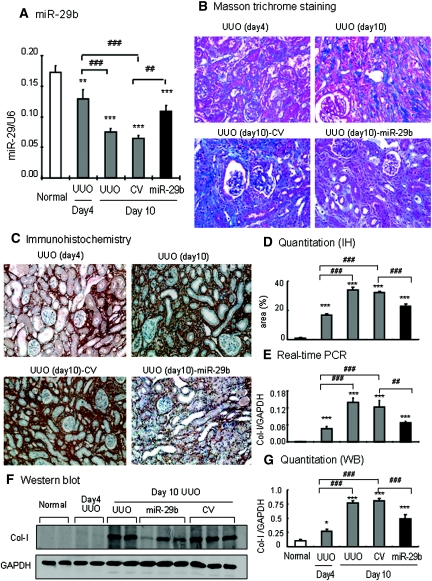
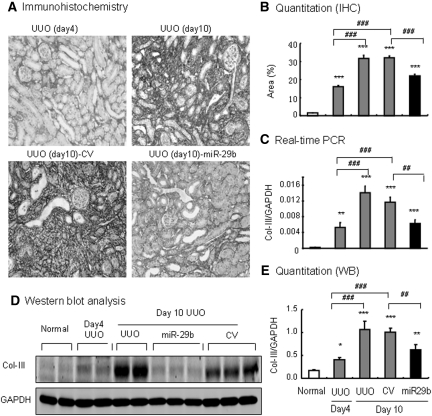
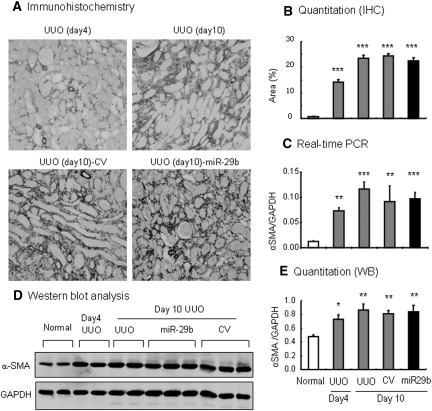
We further determined whether miR-29 has therapeutic potential for renal fibrosis. This was examined in the established UUO nephropathy in which miR-29b mimic was transfected into the UUO kidney at day 4 after the UUO nephropathy. In both UUO and control-treated animals, a rapid progression of renal fibrosis was associated with a progressive loss of miR-29 over the 10-day disease course (Figure 9A). Excitingly, miR-29b gene therapy at day 4 after UUO prevented a progressive loss of miR-29b (Figure 9A), thereby inhibiting progressive tubulointerstitial fibrosis from day 4 onwards as demonstrated by blocking tubulointerstitial fibrosis (Figure 9B), collagen I and collagen III mRNA expression, and protein accumulation (Figures 9, C through G, and 10). However, gene therapy with miR-29b from day 4 after UUO did not reverse the severity of renal fibrosis, because all of the fibrosis parameters were comparable between day 4 before treatment and day 10 after treatment (Figures 9 and 10). Again, miR-29 treatment did not alter α-SMA mRNA expression and α-SMA+ myofibroblast accumulation in the established UUO nephropathy (Figure 11).
Figure 9.
Ultrasound-microbubble-mediated miR-29b gene transfer holds progressive renal fibrosis in an established mouse model of UUO nephropathy at day 10. (A) Real-time PCR for miR-29b expression. (B) Masson trichrome stain. (C) Immunohistochemistry for collagen I deposition. (D) Quantitative analysis of collagen I deposition within the UUO kidney. (E) Real-time PCR for collagen I mRNA expression. (F) Western blotting (WB). (G) Quantitative analysis of collagen I expression by Western blotting. The results show that ultrasound-mediated miR-29b gene therapy from day 4 of UUO restores the loss of miR-29b and holds progressive renal fibrosis such as collagen I expression and disposition as determined by histology, immunohistochemistry, real-time PCR, and Western blotting. Each micrograph or bar represents the mean ± SEM for at least six mice. *P < 0.05, ***P < 0.001 versus normal mice; ##P < 0.01, ###P < 0.001 versus control vector (CV)-treated mice or as indicated. Magnification, ×200. IHC, immunohistochemistry.
Figure 10.
Ultrasound-microbubble-mediated miR-29b gene transfer blocks collagen III expression in an established mouse model of UUO nephropathy at day 10. (A) Immunohistochemistry for collagen III deposition. (B) Quantitative analysis of collagen III deposition within the UUO kidney. (C) Real-time PCR for collagen III mRNA expression. (D) Western blotting (WB) for collagen III expression. (E) Quantitative analysis of collagen III expression detected by Western blotting. The results show that ultrasound-mediated miR-29b gene therapy from day 4 of UUO holds progressive renal fibrosis such as collagen III expression and disposition as determined by immunohistochemistry, real-time PCR, and Western blotting. Each micrograph or bar represents the mean ± SEM for at least six mice. *P < 0.05, **P < 0.01, ***P < 0.001 versus normal mice; ##P < 0.01, ###P < 0.001 versus control vector (CV)-treated mice. Magnification, ×200. IHC, immunohistochemistry.
Figure 11.
Ultrasound-microbubble-mediated miR-29b gene transfer has no effect on expression of α-SMA in an established mouse model of UUO nephropathy at day 10. (A) Immunohistochemistry. (B) Quantitative analysis of immunostaining. (C) Real-time PCR. (D) Western blotting (WB). (E) Quantitative analysis of Western blots. The results show that ultrasound-mediated miR-29b gene therapy from day 4 of UUO does not influence α-SMA expression and α-SMA+ myofibroblast accumulation. Each micrograph or bar represents the mean ± SEM for at least six mice. Magnification, ×200. IHC, immunohistochemistry; CV, control vector.
DISCUSSION
Fibrosis is a hallmark of end-stage renal disease and is a major cause of therapeutic failure. Although TGF-β/Smad3 has been considered as a major pathway for fibrogenesis,1,2 the diverse roles of this pathway in fibrosis and immunity have hampered the development of anti-TGF-β treatment in general. Thus, treatment for fibrosis should aim to specifically target the TGF-β/Smad3-dependent genes that are directly involved in fibrogenesis rather than block the general effect of the TGF-β/Smad signaling pathway. In this study, we identified that miR-29 was a downstream target gene of TGF-β/Smad3 in fibrosis and was negatively regulated by TGF-β/Smad3, not Smad2. More importantly, we also found that miR-29 acted as a downstream inhibitor of TGF-β/Smad3-mediated fibrosis and had the therapeutic potential for disease associated with fibrosis. Results from this study demonstrated that, in the context of fibrosis, targeting the Smad3-miR-29 axis may represent a novel and specific therapy for a wide range of diseases associated with fibrosis.
Although miR-29 is reduced in response to TGF-β1 and in scar tissues associated with cardiac infarction,22 bleomycin-induced systemic sclerosis and pulmonary fibrosis,23,24 and carbon tetrachloride-induced hepatic fibrosis,25 the signaling mechanism through which TGF-β1 regulates miR-29 expression remains unclear. In this study, we found that TGF-β1 negatively regulated miR-29 expression during fibrogenesis via the signaling mechanism of Smad3, not Smad2. By using miRNA microarray and real-time PCR, we found that miR-29 a,b,c family members were substantially reduced in the fibrotic kidney of UUO nephropathy in Smad3 WT mice but were significantly increased in Smad3 KO mice in which renal fibrosis was absent as a result of a lack Smad3.12 These findings strongly indicate that renal fibrosis is associated with a loss of miR-29 that is mediated by a TGF-β/Smad3-dependent mechanism. This was further confirmed in vitro in both MEF and TEC lacking Smad3. Similar to previous studies in fibroblasts from heart, lung, skin, and hepatic stellate cells,22–25 we also detected that the addition of TGF-β1 largely reduced miR-29b expression in Smad3 WT MEF and TEC, which was associated with a marked upregulation of collagen I and III. In contrast, deletion of Smad3 enhanced miR-29b expression, thereby inhibiting collagen matrix expression by MEF and TEC. Interestingly, disruption of Smad2 from MEF cells did not alter the lower level of TGF-β1-induced miR-29b expression, indicating that TGF-β1 downregulates miR-29 expression via the mechanism of Smad3, not Smad2. The finding that Smad3, not Smad2, negatively regulates miR-29 expression may be associated with the ability of Smad3, not Smad2, to bind DNA sequence directly through its MH1 domain.26 Further evidence supporting the interaction of Smad3 with miR-29 came from the findings that at least two conserved Smad3-binding sites were found in the promoter region of miR-29 and that Smad3 was able to interact with the miR-29 promoter region as detected by the ChIP assay. The transcriptional start site and the promoter region of miR-29 have been clearly identified with ∼20 kb upstream from the miR-29b/c cluster on chromosome 1 and have been shown to be able to interact with the NF-κB-YY1 pathway to regulate differentiation of skeletal muscle progenitor cells.27 In this study, we also identified miR-29 as a target of Smad3, and TGF-β/Smad3 is an essential signaling mechanism negatively regulating miR-29 expression during fibrogenesis. The specific regulatory role of Smad3, not Smad2, in downregulation of miR-29 may explain our recent finding that Smad3, not Smad2, mediates angiotensin II-induced cardiovascular remodeling and renal fibrosis,2–4 in advanced glycation end product-mediated diabetic renal fibrosis,5 and in a mouse model of UUO nephropathy.6
An interesting observation from this study is that cells lacking Smad3 were promoted expression of miR-29 in response to TGF-β1, suggesting a Smad3-independent TGF-β signaling mechanism in positively regulating miR-29 expression when Smad3 is absent. Indeed, the regulating network of microRNAs is complicated. Different genes can be regulated by a single microRNA, and a single microRNA can regulate many different genes. Thus, Smad3 may act as a suppressive gene to negatively regulate miR-29 expression during TGF-β-mediated fibrosis, but deletion of Smad3 may also promote some other genes/factors that can positively regulate miR-29 expression via either direct or indirect mechanisms, which is complicated and requires further investigation.
This study also demonstrated that miR-29 negatively regulated fibrosis by targeting the process of collagen matrix synthesis rather than by inhibiting myofibroblast accumulation. This was evidenced by the fact that overexpression of miR-29b suppressed, but knockdown of miR-29b enhanced, TGF-β1-induced collagen I and collagen III expression without altering α-SMA expression by MEF and TEC. Similarly, overexpression of miR-29b in the UUO kidney also blocked collagen I and III matrix deposition without the inhibitory effect on α-SMA expression and myofibroblast accumulation. The selective effect of miR-29 on controlling collagen matrix expression without influencing α-SMA expression and myofibroblast accumulation may be associated with the ability of miR-29 to specifically target extracellular matrix including collagen I, collagen III, fibrilin, and elastin,22–25 but not α-SMA, because no miR-29-binding sites were detected on α-SMA 3y′-untranslated region by the programs with Targetscan (http://targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), and PicTar (http://pictar.mdc-berlin.de). Thus, a loss of miR-29 impairs the suppressive effect on collagen matrix expression. This may explain why TGF-β-induced further collagen I and III expression in cells when miR-29 was disrupted. Moreover, TGF-β1 is also capable of inducing collagen synthesis via a miR-29-independent mechanism. Recent studies in TGF-β-induced miR-192 to mediate renal fibrosis support this notion.28,29
The most significant finding in this study was the successful development of ultrasound- microbubble-mediated miR-29 therapy for renal fibrosis in both the induction phase and established UUO nephropathy in mice. By using antisense inhibitor oligonucleotides (AMO), miRNA-based therapy has produced favorable therapeutic results in cardiovascular diseases.30–32 However, the lower level of tissue uptake of AMO is a key obstacle in developing the AMO strategy for clinical use. We have previously shown that the use of ultrasound-microbubble-mediated gene transfer is able to highly transfect the Dox-inducible Smad7 gene into the kidney to block activation of TGF-β/Smad signaling, thereby inhibiting progressive renal fibrosis in rat models of UUO nephropathy and remnant kidney disease without notable side effects.14,15 In this study, we developed this technology to effectively deliver Dox-inducible miR-29 plasmids into both normal and diseased kidney in which higher levels of miR-29 transgene were expressed by all kidney cell types, particularly by mesangial cells, arterial wall, and TEC as detected by in situ hybridization as well as by real-time PCR. Another advantage using this inducible technique is that the expression level of miR-29 within the kidney could be tightly controlled by the addition of doses of Dox in the drinking water. To avoid the potential side effect caused by overexpression of miR-29, we controlled miR-29 expression within the normal level with the addition of Dox at a dose of 200 μg/ml in the daily drinking water as determined by real-time PCR. Thus, by using the ultrasound-based miRNA therapy system, we not only expressed miR-29 in the normal kidney to prevent renal fibrosis after the UUO, we were also able to restore miR-29 to the normal level within the fibrotic kidney, thereby inhibiting progressive renal fibrosis in the established UUO nephropathy. Outcomes from this study demonstrated that miR-29 is a therapeutic agent for fibrosis and that the ultrasound-microbubble-mediated system is a safe and effective method for the miRNA-based therapy in general.
In conclusion, this study reveals that miR-29 was negatively regulated by TGF-β via the Smad3, not Smad2, signaling pathway, which is a novel signaling mechanism in the complex process of fibrosis in vivo and in vitro. Furthermore, we also find that miR-29 was a downstream inhibitor of TGF-β/Smad3-mediated fibrosis by specifically targeting the collagen type of extracellular matrix synthesis. Thus, in the context of fibrosis, the Smad3-miR-29 axis may represent a therapeutic target related to fibrosis. This was proven by the ability of restored miR-29 in the fibrotic kidney to block progressive renal fibrosis in the established UUO nephropathy in mice, providing the first evidence for miR-29 as a therapeutic agent for fibrosis. The success in the development of miR-29 as an anti-fibrosis therapy strategy is largely dependent upon the use of the ultrasound-microbubble technique to effectively deliver the Dox-inducible miR-29 into the diseased kidney. Therefore, ultrasound-microbubble-mediated inducible miR-29 may represent a novel and effective therapy for a wide range of diseases associated with fibrosis.
CONCISE METHODS
MicroRNA Microarray and Quantitative Reverse Transcription PCR (qRT-PCR)
MicroRNA profiles in fibrotic kidneys were examined by microarray assays in groups of six normal and UUO kidneys in Smad3 WT or KO mice using a TaqMan MicroRNA RT kit with Multiplex RT rodent primer pool (Applied Biosystems) as described previously.28 miRNA TaqMan assays were performed using a 7900 HT Fast real-time PCR system (Applied Biosystems). Relative quantification was performed using the ΔΔCt method. The data were normalized with U6 and RNU48 (Applied Biosystems) as endogenous control and then analyzed with real-time StatMiner (Integromics, Madrid, Spain).
Cell Culture
To dissect the specific role and mechanisms through which TGF-β1 signals to Smad2 and or Smad3 to regulate miR-29 expression, characterized MEF lacking Smad2 or Smad3 were used for this study.33 This is because renal fibroblasts lacking Smad2 are not available because of the embryonic lethality of Smad2 KO mice, although Smad3 KO mice survive.6,7 The stable cell lines with Smad2 or Smad3 gene knockdown in NRK52E were generated and characterized as described previously.4–6 Recombinant human TGF-β1 at a dose of 2 ng/ml (R&D Systems, Minneapolis, MN) was added into the cell culture to induce fibrosis responses. At least four independent experiments were performed throughout the study.
ChIP Analysis
ChIP assay was performed by transcription factor ChIP kit (Diagenode, Liège, Belgium) as described previously.28 In brief, Smad3 WT and KO MEFs pretreated with TGF-β1 were firstly cross-linked with 1% formaldehyde for 10 minutes at 37°C, then quenched with glycine, and sonicated using a Bioruptor (Diagenode) to generate 300- to 600-bp DNA fragments. Immunoprecipitation was performed with Smad3 antibody (Upstate), and normal isotype IgG was used as a control. Precipitated DNAs were detected by PCR using specific primers: 5′-TGCTTGGGAAGGTAAGGATG-3′ and 5′-TTCCCCCAGTACAAGACAGC-3′ as described previously.28
Establishment of Doxycycline-inducible miR-29 Stable Cell Lines
A Dox-regulated miR-29b expressing plasmid was prepared using Tet-on system plasmids. Briefly, a murine miR-29b1 cDNA was amplified by PCR with the forward primer 5′-gac acG GAT CCa aTG TAA GCC TCG TGC TCA CTG-3′ and the reverse primer 5′-gac ttc tcg aGT CGA Caa GGT CAT GTG CAC TGG GAA G-3′ (underlined: BamHI and SalI sites, respectively). The PCR-amplified product was subsequently cloned into a tetracycline-inducible vector, pTRE2-hygro (Clontech) to obtain pTRE2-miR-29b. To achieve Dox-induced miR-29b transgene expression, pTRE2-miR-29b and pEFpuro-p-Tet-on were cotransfected into MEF and NRK52E cells using Lipofectamine LTX (Invitrogen). Dox-induced miR-29b overexpression cell lines were selected by coculture with puromycin and hygromycin. The cells were then treated with TGF-β1 to induce fibrosis responses. At least four independent experiments were performed throughout the study.
Establishment of Smad3 or miR-29b Knockdown Stable Cell Lines
To delineate the regulating role of Smad3 in TGF-β1-induced miR-29 expression, NRK52E cells that stably expressed Smad3 siRNA were used as described previously.4,5 To establish miR-29b knockdown stable cell line, the gene-specific insert sequences for miR-29b1 (sense: TAG TGA TTG TCT AGC ACC ATT and antisense: AAT GGT GCT AGA CAA TCA CTA) and miR-29b2 (sense: ATC TTT GTA TCT AGC ACC ATT and antisense: AAT GGT GCT AGA TAC AAA GAT) were synthesized and subcloned into pSuper-puro and pSuper-neo vectors (OligoEngine, Seattle, WA) to generate pSuper-miR-29b RNA interference constructs according to the manufacturer's instructions. The vector allows direct synthesis of small hairpin RNA transcripts, which targets stem loop region of premiRNA. A siRNA with no predicted target site in the rat genome was inserted into pSUPER-puro and served as a negative control. The pSuper plasmids were then transfected into MEF and NRK52E using Lipofactamine LTX (Invitrogen) and then subjected to G418 and puromycin selection. The expression of miR-29b was determined by real-time PCR. Stable cell lines were treated with TGF-β1 to induce fibrosis responses. At least four independent experiments were performed throughout the study.
Unilateral Urethral Obstruction Model and Ultrasound-mediated Gene Transfer of Inducible miR-29b
A well characterized mouse UUO model was induced in male C57BL/6J mice (age, 6 to 8 weeks; 20 to 25 g) by ligation of the left ureter as previously reported.6,14,17 Immediately after ligation, 200 μl of the mixture of pTRE2-miR-29b (200 μg)-Tet-on plasmids/Sonovue (Bracco Diagnostics, Princeton, NJ) in a 1:1 ratio (volume:volume) or the control empty vectors (pTRE2-Tet-on/Sonovue) were injected into tail vein, followed by 4 minutes of ultrasound treatment (2 W/cm2) by placing the ultrasound probe on the skin opposite to the kidney as described previously.5 For interventional study, ultrasound-mediated gene transfer was carried out at the 4th day after UUO. To induce miR-29b transgene expression, a dose of Dox (200 μl) with a concentration of 200 μg/ml was administered into the peritoneal cavity immediately after ultrasound-mediated gene transfer and followed by additional Dox in drinking water (200 μg/ml) during the entire study period. Groups of eight mice were euthanized 7 days after gene transfer. In addition, groups of eight UUO mice without ultrasound treatment were euthanized at 4th and 10th days as disease control, and eight normal age-matched mice were used as normal control. The experimental procedure was approved by the Animal Experimentation Ethics Committee at The Chinese University of Hong Kong.
RNA Extraction and Real-Time PCR Examination
Total RNA was isolated from the cultured cells and kidney tissues using Tri-Reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions. Real-time PCR for miR-29a,b,c was performed using TaqMan microRNA assay kit (Applied Biosystems) with U6 as endogenous control and Bio-Rad iQ SYBR Green supermix with Opticon2 (Bio-Rad, Hercules, CA) as described previously.4,6,28 The primers used in this study, including mouse and rat collagen I, collagen III, α-SMA, Smad3, and GAPDH, were described previously.4–6 The ratio for the mRNA interested was normalized with internal controls (U6 or GAPDH) and expressed as the mean ± SEM.
Immunohistochemistry
Immunohistochemistry was performed in paraffin sections using a microwave-based antigen retrieval technique.4–6 The antibodies used in this study included collagen I, collagen III (Southern Tech, Birmingham, AL), and α-SMA (Sigma). After immunostaining, the sections were counterstained with hematoxylin. Percentages of positive staining area were quantified using the Image-Pro plus software (Media Cybernetics, Bethesda, MD) as described previously.5,6
Western Blot Analysis
Protein from kidney tissues and cultured cells were extracted with radioimmune precipitation assay lysis buffer, and Western blot analysis was performed as described previously.4–6 After blocking nonspecific binding with 5% BSA, the membranes were then incubated overnight at 4°C with the primary antibody against collagen I, III, α-SMA, Smad3, and GAPDH (Chemicon, Temecula, CA), followed by IRDyeTM 800-conjugated secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). The signals were detected using the LiCor/Odyssey infrared image system (LI-COR Biosciences, Lincoln, NE). Signal intensities of each Western blot were quantified by using the LiCor/Odyssey followed by analysis with Image J software (National Institutes of Health).
In Situ Hybridization
To define the expression pattern of miR-29 by renal cells and determine the miR-29 transfection efficiency and transgene expression, in situ hybridization of miR-29 was performed in normal and UUO kidneys following the established protocol.34 Briefly, formalin-fixed, paraffin-embedded kidney tissues were deparaffinized and treated with protease (10 μg/ml) for 8 minutes, followed by prehybridization with the buffer and then hybridization with digoxigenin anti-sense miR-29b probe (5′-AACACTGATTTCAAATGGTGCTA-3′) at 45°C overnight. After stringency washing, the sections were incubated with anti-digoxigenin antibody (Roche Diagnostics) overnight at 4°C and developed with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate to produce positive signals. A scramble probe (5′-GTGTAACACGTCTATACGCCCA-3′) was used as a negative control for all studies.
Statistical Analyses
Data obtained from this study are expressed as the mean ± SEM and analyzed using one-way ANOVA, followed by t test using SPSS software (Chicago, IL).
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from the Research Grant Council of Hong Kong (RGC CUHK5/CRF/09, GRF 768207, and 767508 to H.Y.L. and 763908 and 764109 to A.C.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Lan HY: Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci 13: 4984–4992, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC: Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 17: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Wang W, Huang XR, Canlas E, Oka K, Truong LD, Deng C, Bhowmick NA, Ju W, Bottinger EP, Lan HY: Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circ Res 98: 1032–1039, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang F, Huang XR, Chung AC, Hou CC, Lai KN, Lan HY: Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol 221: 390–401, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Chung AC, Zhang H, Kong YZ, Tan JJ, Huang XR, Kopp JB, Lan HY: Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc Nephrol 21: 249–260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY: Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB: Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 160: 1057–1068, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG: Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116: 2127–2138, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Huang XR, Chung AC, Yang F, Yue W, Deng C, Lau CP, Tse HF, Lan HY: Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension 55: 1165–1171, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Zhao J, Shi W, Wang YL, Chen H, Bringas P, Jr, Datto MB, Frederick JP, Wang XF, Warburton D: Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 282: L585–L593, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Latella G, Vetuschi A, Sferra R, Catitti V, D'Angelo A, Zanninelli G, Flanders KC, Gaudio E: Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int 29: 997–1009, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I: Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest 104: 5–11, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ: Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hou CC, Wang W, Huang XR, Fu P, Chen TH, Sheikh-Hamad D, Lan HY: Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 166: 761–771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, Ueberham E, Gebhardt R, Kanzler S, Geier A, Breitkopf K, Weng H, Mertens PR: Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 135: 642–659, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Chung AC, Huang XR, Zhou L, Heuchel R, Lai KN, Lan HY: Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant 24: 1443–1454, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T: Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S: Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90: 770–774, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C: Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 18: 1280–1291, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB: Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1: 260–266, 1999 [DOI] [PubMed] [Google Scholar]
- 22. van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN: Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 105: 13027–13032, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O: MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62: 1733–1743, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J: MIR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T: Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology, 53: 209–218, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Yagi K, Goto D, Hamamoto T, Takenoshita S, Kato M, Miyazono K: Alternatively spliced variant of Smad2 lacking exon 3. Comparison with wild-type Smad2 and Smad3. J Biol Chem 274: 703–709, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC: NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14: 369–381, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chung AC, Huang XR, Meng X, Lan HY: miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J Am Soc Nephrol 21: 1317–1325, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A 104: 3432–3437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G: MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z: The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13: 486–491, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Luo X, Lin H, Pan Z, Xiao J, Zhang Y, Lu Y, Yang B, Wang Z: Down-regulation of miR-1/miR-133 contributes to re-expression of pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol Chem 283: 20045–20052, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB: Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem 276: 19945–19953, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Obernosterer G, Martinez J, Alenius M: Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat Protoc 2: 1508–1514, 2007 [DOI] [PubMed] [Google Scholar]