Abstract
Identifying potential modifiable risk factors to reduce the incidence of vascular access thrombosis in hemodialysis could reduce considerable morbidity and health care costs. We analyzed data from a subset of 1426 HEMO study subjects to determine whether more frequent intradialytic hypotension and/or lower predialysis systolic BP were associated with higher rates of vascular access thrombosis. Our primary outcome measure was episodes of vascular access thrombosis occurring within a given 6-month period during HEMO study follow-up. There were 2005 total episodes of vascular access thrombosis during a median 3.1 years of follow-up. The relative rate of thrombosis of native arteriovenous fistulas for the highest quartile of intradialytic hypotension was approximately twice that of the lowest quartile, independent of predialysis systolic BP and other covariates. There was no significant association of intradialytic hypotension with prosthetic arteriovenous graft thrombosis after multivariable adjustment. Higher predialysis systolic BP was associated with a lower rate of fistula and graft thrombosis, independent of intradialytic hypotension and other covariates. In conclusion, more frequent episodes of intradialytic hypotension and lower predialysis systolic BP associate with increased rates of vascular access thrombosis. These results underscore the importance of including vascular access patency in future studies of BP management in hemodialysis.
Vascular access is often referred to as the “Achilles' heel” of patients on maintenance hemodialysis, given the complications with its creation and maintenance. Problems associated with vascular access can cause considerable morbidity, including inadequate dialysis and exposure to additional invasive procedures such as temporary catheter placement and angioplasty.1 In 2007, Medicare spending on ESRD neared $24 billion,2 with an estimated $1.8 billion spent annually on vascular access care alone.3 Finding potential modifiable risk factors to target to reduce the incidence of vascular access thrombosis is therefore imperative.
Native arteriovenous fistulas and prosthetic arteriovenous grafts are the two main types of permanent vascular accesses. Thrombosis is the most common cause of secondary vascular access failure (i.e., failure of functioning vascular access) and is associated with luminal stenosis in 60% to 80% of cases.1,4,5 However, because 20% to 40% of cases of access thrombosis occur in the absence of stenosis, and because not all stenotic accesses thrombose, other factors must contribute to access thrombosis. Among other factors, low-flow states secondary to low BP and hemoconcentration secondary to large-volume ultrafiltration have been proposed to precipitate access thrombosis.1,6 These putative causes of access thrombosis make intuitive sense, but few studies have actually examined these factors in a systematic manner.
The HEMO study was a multicenter randomized clinical trial of dialysis dose and membrane flux in prevalent patients on hemodialysis in the United States, with detailed information on comorbid conditions and vascular access complications collected for the duration of the study.7,8 We conducted a secondary analysis of data from the HEMO study to test the hypothesis that subjects with more frequent episodes of intradialytic hypotension or lower predialysis BP would have higher rates of vascular access thrombosis compared with subjects with fewer episodes of intradialytic hypotension or higher predialysis BP. We also hypothesized that more frequent episodes of large-volume ultrafiltration, perhaps by causing hemoconcentration, would be associated with higher rates of vascular access thrombosis.
RESULTS
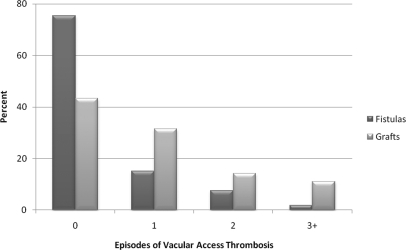
In our cohort of 1426 subjects followed for a median of 3.1 years (interquartile range [IQR] 1.8 to 4.7 years), there were a total of 2005 episodes of vascular access thrombosis. There was a median of 12.5% (IQR 0% to 28.6%) of dialysis sessions with episodes of intradialytic hypotension and 22% (IQR 0% to 44.4%) of dialysis sessions with episodes of large-volume ultrafiltration during the entire follow-up period. Overall, 55.0% of the cohort had zero episodes, 25.5% had one episode, 11.4% had two episodes, and 8.1% had three or more episodes of vascular access thrombosis. A significantly smaller proportion of subjects with a baseline fistula had episodes of vascular access thrombosis than subjects with a baseline graft (Figure 1).
Figure 1.
Vascular access thrombosis is more frequent with grafts than with fistulas.
Baseline demographic and other characteristics are shown in Table 1. Subjects in higher quartiles of intradialytic hypotension during the period from baseline to 4 months were older; more often female; used grafts more often than fistulas; and had a higher prevalence of diabetes mellitus, ischemic heart disease, congestive heart disease, and peripheral vascular disease. Subjects in higher quartiles of intradialytic hypotension also tended to have lower baseline predialysis diastolic BP (DBP), lower postdialysis systolic BP (SBP) and DBP, and lower minimum recorded SBP and DBP.
Table 1.
Baseline demographic and other characteristics of the HEMO study cohort in the study presented here
| Baseline Characteristic | Quartile of Percent of Kinetic Modeling Sessions with Intradialytic Hypotension during the Period from Baseline to 4 Months |
||||
|---|---|---|---|---|---|
| Q1 n = 529 | Q2 n = 205 | Q3 n = 319 | Q4 n = 365 | Pa | |
| Age, years, mean (SD) | 56.0 (14.6) | 55.4 (14.8) | 59.2 (12.7) | 59.0 (13.0) | <0.0001 |
| Female sex, % | 44.6 | 56.1 | 58.9 | 66.6 | <0.0001 |
| Black race, % | 61.4 | 68.1 | 64.6 | 68.2 | 0.06 |
| Arteriovenous access type, % | 43.7 | 36.1 | 34.2 | 28.0 | <0.001 |
| fistula | 56.3 | 63.9 | 65.8 | 72.0 | |
| graft | |||||
| Current smoker, % | 20.8 | 13.2 | 17.6 | 13.4 | 0.01 |
| Past medical history, % | |||||
| diabetes mellitus | 32.7 | 38.1 | 48.6 | 55.1 | <0.0001 |
| ischemic heart disease | 30.1 | 37.6 | 39.2 | 46.6 | <0.0001 |
| congestive heart failure | 32.7 | 38.5 | 38.6 | 44.1 | 0.0007 |
| peripheral vascular disease | 18.5 | 24.4 | 27.3 | 27.7 | 0.0005 |
| Medication use, % | |||||
| aspirin | 22.3 | 31.2 | 29.8 | 29.9 | 0.009 |
| warfarin | 7.9 | 11.2 | 6.9 | 9.9 | 0.6 |
| angiotensin converting enzyme inhibitor | 22.7 | 28.3 | 24.1 | 24.4 | 0.7 |
| calcium channel blocker | 50.5 | 53.2 | 47.3 | 49.6 | 0.6 |
| erythropoietin | 89.4 | 89.3 | 90.6 | 91.5 | 0.3 |
| Years on dialysis, median (IQR) | 2.4 (1.0 to 4.7) | 2.2 (1.1 to 5.4) | 1.8 (0.8 to 4.3) | 2.2 (1.0 to 4.9) | 0.8 |
| Serum albumin, g/dl, mean (SD) | 3.7 (0.3) | 3.7 (0.4) | 3.6 (0.3) | 3.6 (0.3) | <0.0001 |
| Hematocrit (%) | 33.7 (4.5) | 33.3 (4.6) | 33.7 (4.3) | 33.8 (4.6) | 0.7 |
| Predialysis (mmHg) | |||||
| SBP (SD) | 152 (21) | 152 (22) | 154 (22) | 152 (23) | 0.7 |
| DBP (SD) | 83 (13) | 84 (13) | 82 (12) | 80 (13) | 0.001 |
| Postdialysis (mmHg) | |||||
| SBP (SD) | 141 (22) | 138 (21) | 138 (22) | 132 (19) | <0.0001 |
| DBP (SD) | 77 (13) | 76 (12) | 74 (12) | 71 (10) | <0.0001 |
| Dialysis session minimum (mmHg) | |||||
| SBP (SD) | 126 (18) | 122 (18) | 119 (17) | 108 (16) | <0.0001 |
| DBP (SD) | 72 (11) | 71 (11) | 67 (10) | 61 (11) | <0.0001 |
aCalculated using two-sided Cochran–Armitage trend test or linear regression as appropriate.
Intradialytic Hypotension and Vascular Access Thrombosis
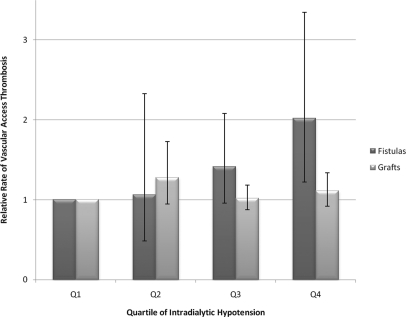
The association of vascular access thrombosis and intradialytic hypotension varied significantly by baseline vascular access type (P value for interaction = 0.04). We therefore present results stratified by baseline access type (fistulas versus grafts). In unadjusted analyses, intradialytic hypotension was strongly associated with fistula thrombosis (P = 0.0092); subjects with fistulas at baseline in the highest quartile of intradialytic hypotension had a risk of fistula thrombosis that was 2.45 times that of subjects with fistulas in the lowest quartile of intradialytic hypotension (95% confidence interval [CI] 1.55 to 3.87). Similarly, intradialytic hypotension was associated with graft thrombosis (P = 0.043); subjects with grafts at baseline in the highest quartile of intradialytic hypotension had a rate of graft thrombosis that was increased by 26% (95% CI 6% to 50%) relative to subjects in the lowest quartile of intradialytic hypotension. After multivariable adjustment, the association between intradialytic hypotension and access thrombosis was attenuated for fistulas and no longer statistically significant for grafts (Table 2, Figure 2).
Table 2.
Multivariable-adjusted negative binomial regression showing the association of each parameter with the relative rate of vascular access thrombosis stratified by baseline access type
| Parameter | Fistulas |
Grafts |
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| Quartile of intradialytic hypotensiona | ||||||
| Q1 | 1.00 | Reference | – | 1.00 | Reference | – |
| Q2 | 1.06 | 0.48 to 2.33 | 0.88 | 1.28 | 0.95 to 1.73 | 0.11 |
| Q3 | 1.41 | 0.96 to 2.08 | 0.08 | 1.02 | 0.87 to 1.18 | 0.82 |
| Q4 | 2.02 | 1.22 to 3.35 | 0.0063 | 1.11 | 0.92 to 1.34 | 0.27 |
| Age (per 10-year increase) | 0.85 | 0.73 to 0.99 | 0.032 | 0.99 | 0.92 to 1.06 | 0.71 |
| Female gender (versus male) | 0.74 | 0.49 to 1.12 | 0.15 | 0.93 | 0.77 to 1.12 | 0.43 |
| Black race (versus nonblack) | 1.29 | 0.82 to 2.03 | 0.27 | 1.17 | 0.96 to 1.44 | 0.12 |
| Current smoker (versus no) | 1.07 | 0.65 to 1.74 | 0.80 | 1.03 | 0.78 to 1.35 | 0.86 |
| Past medical history (versus none) | ||||||
| diabetes mellitus | 0.98 | 0.61 to 1.56 | 0.93 | 1.13 | 0.94 to 1.35 | 0.20 |
| ischemic heart disease | 0.75 | 0.49 to 1.14 | 0.18 | 0.92 | 0.76 to 1.11 | 0.37 |
| congestive heart failure | 1.22 | 0.82 to 1.82 | 0.32 | 0.92 | 0.75 to 1.12 | 0.40 |
| peripheral vascular disease | 1.51 | 0.93 to 2.46 | 0.094 | 1.14 | 0.93 to 1.39 | 0.20 |
| Hospitalized during the assessment period (versus no) | 1.26 | 0.91 to 1.75 | 0.17 | 1.12 | 0.98 to 1.29 | 0.61 |
| Predialysis SBP (per 1-SD increase) | 0.92 | 0.74 to 1.15 | 0.45 | 0.88 | 0.80 to 0.97 | 0.0099 |
| Serum albumin (per 0.1-g/dl increase) | 0.92 | 0.87 to 0.97 | 0.0037 | 0.96 | 0.93 to 0.98 | 0.0014 |
| Hematocrit (per 1% increase) | 0.98 | 0.98 to 1.03 | 0.48 | 1.00 | 0.98 to 1.01 | 0.51 |
| Randomization group | ||||||
| high versus conventional dose | 0.96 | 0.66 to 1.39 | 0.83 | 1.09 | 0.91 to 1.30 | 0.36 |
| high versus low flux | 1.45 | 1.01 to 2.10 | 0.046 | 1.03 | 0.86 to 1.22 | 0.78 |
Analyses adjusted for all variables listed in the table, as well as clinical center. RR, relative rate.
aGlobal P value for fistulas = 0.069; grafts = 0.41.
Figure 2.
Subjects with more frequent episodes of intradialytic hypotension are more likely to experience vascular access thrombosis. Analyses adjusted for age, sex, black race, current smoking, diabetes mellitus, ischemic heart disease, congestive heart failure, peripheral vascular disease, hospitalization, serum albumin, hematocrit, mean predialysis SBP, intervention group, and clinical center. Error bars indicate 95% CIs. Ref, reference group. *P = 0.0063 versus quartile 1 (Q1).
BP Parameters and Vascular Access Thrombosis
In separate models, lower pre- and postdialysis SBP were associated with higher relative rates of vascular access thrombosis (Table 3) after adjustment for intradialytic hypotension. There was no evidence that associations of pre- or postdialysis SBP and vascular access thrombosis varied by baseline access type (P values for interaction >0.10). Similar associations were observed for pre- and postdialysis DBP and mean arterial pressure (MAP), although these were not always statistically significant.
Table 3.
Multivariable-adjusted relative rates of vascular access thrombosis by blood pressure parameter
| Per SD increase | Predialysis |
Postdialysis |
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| SBP | 0.89 | 0.81 to 0.97 | 0.0089 | 0.91 | 0.83 to 1.00 | 0.028 |
| DBP | 0.92 | 0.83 to 1.01 | 0.066 | 0.92 | 0.84 to 1.02 | 0.087 |
| MAP | 0.90 | 0.83 to 0.98 | 0.017 | 0.92 | 0.85 to 1.00 | 0.034 |
Analyses adjusted for age, sex, black race, current smoking, diabetes mellitus, ischemic heart disease, congestive heart failure, peripheral vascular disease, serum albumin concentration, hematocrit, hospitalization, baseline access type, quartile of intradialytic hypotension, baseline access × intradialytic hypotension interaction, intervention group, and clinical center.
Large-Volume Ultrafiltration and Vascular Access Thrombosis
The frequency of episodes of large-volume ultrafiltration was directly correlated with the frequency of episodes of intradialytic hypotension, but the magnitude of the association was relatively low (Spearman ρ = 0.10, P = 0.0003). However, large-volume ultrafiltration was not significantly associated with vascular access thrombosis in unadjusted or fully adjusted models (P = 0.79 and 0.60, respectively). For example, in multivariable-adjusted models, subjects in the highest quartile of large-volume ultrafiltration had a 1.01 (95% CI 0.80 to 1.27) higher relative rate of access thrombosis compared with patients in the lowest quartile. There was no evidence that the association of large-volume ultrafiltration and vascular access thrombosis varied by baseline access type (P value for interaction = 0.20).
Other Factors Associated with Vascular Access Thrombosis
Table 2 shows the results of multivariable adjusted models after stratifying by baseline access type. Older age was associated with a lower rate of fistula thrombosis. Female sex and black race were not associated with fistula or graft thrombosis. Subjects randomized to high-flux dialyzer membranes had a higher rate of fistula thrombosis compared with subjects randomized to low-flux dialyzer membranes. These associations were not observed for subjects with grafts. For all subjects regardless of baseline vascular access type, lower baseline serum albumin concentration was associated with higher rates of vascular access thrombosis. Mean predialysis hematocrit was not associated with vascular access thrombosis.
Companion Analyses
Of the 1426 subjects in our analysis, 94 (6.6%) had a change in access type from baseline. In a companion analysis that adjusted for vascular access type as a time-varying covariate, there was some evidence of an interaction between vascular access type and intradialytic hypotension on access thrombosis, although the association was not statistically significant (P value for interaction = 0.088). Subjects with fistulas or grafts in the highest quartile of intradialytic hypotension had a rate of access thrombosis that was 1.25 times higher than that of subjects in the lowest quartile of intradialytic hypotension (95% CI 1.04 to 1.51). Additional companion analyses that adjusted for baseline use of aspirin, warfarin, angiotensin converting enzyme inhibitors, and calcium channel blockers did not materially change our results. For example, with additional adjustment for baseline medication use, the relative rate of fistula thrombosis for subjects in the highest quartile of intradialytic hypotension was 2.02 times higher than subjects in the lowest quartile (95% CI 1.21 to 3.38).
DISCUSSION
Our analysis shows for the first time that intradialytic hypotension is significantly associated with arteriovenous fistula (but not prosthetic graft) thrombosis in patients on hemodialysis. We anticipated that our results would differ by access type because the anatomical configurations and hence hemodynamics of fistulas and grafts differ greatly. Fistulas require only one surgical anastomosis, are almost entirely endothelialized, and once mature do not often thrombose. In our analysis, over 75% of patients dialyzing with fistulas at baseline had no thrombosis events for the duration of follow-up. Why then did we demonstrate a more robust association of intradialytic hypotension with fistula rather than graft thrombosis? We hypothesize that fistulas, being relatively thrombosis resistant, may require more extreme hemodynamic derangements such as those caused by repeated episodes of intradialytic hypotension to thrombose. In contrast, grafts are composed of unendothelialized foreign material, attracting macrophages that release various cytokines, such as vascular endothelial growth factor, fibroblast growth factor, and PDGF.9 Therefore, grafts are inherently thrombogenic even in stable hemodynamic settings, and intradialytic hypotension would have minimal contribution to the graft's intrinsically high risk of thrombosis.
Our analysis also shows that lower pre- and postdialysis SBP are associated with a higher rate of access thrombosis, regardless of baseline access type, which is consistent with previous studies.10,11 Lower BP may lead to decreased access blood flow, which has been shown to independently predict subsequent access thrombosis.5 Moreover, achievement of the current recommended target BP is associated with higher rates of intradialytic hypotension.12 Importantly, lower BP and intradialytic hypotension are two potentially modifiable risk factors for access thrombosis and may account for at least some of the 20% to 40% of access thromboses that occur in the absence of obvious structural abnormalities. Relatively higher BP targets may be preferred in patients with known stenosis in their vascular accesses, recent intervention, or other characteristics of increased access thrombosis risk. However, our observational analysis cannot establish causation, and an alternative explanation may be that intradialytic hypotension and lower BP, predictors of mortality in hemodialysis,13–16 may be markers of other comorbid conditions that predispose to access thrombosis. Accordingly, lower baseline serum albumin concentration, a marker of inflammation, malnutrition, and higher mortality risk in hemodialysis,17 was also significantly associated with higher rates of access thrombosis in our analysis.
We hypothesized that large-volume ultrafiltration could cause postdialysis hemoconcentration and increase the risk of access thrombosis. However, our analyses did not show an association between more frequent large-volume ultrafiltration and vascular access thrombosis. The HEMO study did not have information on postdialysis hematocrit, so whether subjects with large-volume ultrafiltration had actual elevations in their postdialysis hematocrit levels was not known. Large-volume ultrafiltration was only weakly associated with intradialytic hypotension in our analysis, which is consistent with a previous study that demonstrated no correlation between the reduction in relative plasma volume and intradialytic hypotension.18 The development of intradialytic hypotension is a complex process that depends on more than just the volume of ultrafiltration because susceptible patients may have an individualized threshold ultrafiltration volume19 in addition to other factors such as impaired arteriolar tone, left ventricular dysfunction, and autonomic instability.20–22
We did not show a significant association of sex, current smoking, or diabetes mellitus on the relative rates of access thrombosis, consistent with several previous studies.11,23–26 Interestingly, for patients with fistulas, older age was associated with lower rates of fistula thrombosis (15% lower rate per 10-year increment in age); we observed no association of age with graft thrombosis. These results contrast with those reported by Saran et al., who showed that older age was associated with a decreased risk of graft, but not fistula thrombosis.25 Overall, the relation of age with vascular access failure has been mixed, with some studies showing a detrimental association of older age24,27 and several others showing no significant relation of age11,23,28,29 with access failure. One reason for the differences may be that most of the other studies were performed in incident hemodialysis patients and examined only time to the first access failure, whereas our analysis compared rates of access thrombosis in prevalent patients followed over several years.
Our analysis demonstrated a significant association of high-flux membranes with increased risk of fistula thrombosis as compared with low-flux membranes. These results should be interpreted with caution because we did not adjust for multiple comparisons; moreover, the HEMO study showed no effect of dialyzer membrane flux on overall survival7 and a lower risk of cardiac death in subjects randomized to high-flux membranes.30 In addition, the Membrane Permeability Outcomes study, a randomized trial of high- versus low-flux membranes in incident hemodialysis patients, demonstrated superior survival of the high-flux membrane group.31 However, given that no studies have specifically examined the effect of dialyzer membrane flux on vascular access thrombosis, the effects of membrane flux on vascular access thrombosis may warrant further examination.
Our analysis has several limitations. First, because we only had information from a single monitored dialysis session to represent all of the dialysis sessions in a given month, there may have been inaccuracies in the BP measurements or assessment of intradialytic hypotension. We chose to aggregate the information on intradialytic hypotension over a 6-month period before the period in which access thrombosis was assessed to leverage more of the data, reduce the risk of exposure misclassification, and ensure that the exposure preceded the outcome. In so doing, we assumed that the frequency of intradialytic hypotension from the prior time period was a reasonable surrogate for episodes of intradialytic hypotension more proximal to the access thrombosis event but also lengthened the period of time between the exposure and outcome. However, the prolonged period of time would have weakened the association between intradialytic hypotension and vascular access thrombosis, and so our results are likely a conservative estimate of the true association. Second, we did not have information on the vascular access before randomization such as when it was created, baseline flow rates through the access, and if the access had already required prior intervention, which strongly predict future access failures.25,26 Lack of data on access flow and vascular access anatomy limited our ability to identify potential mechanisms by which intradialytic hypotension contributes to vascular access thrombosis, such as through progression of prior areas of stenosis or the development of de novo areas of stenosis. Third, we did not have data on the exact time to access thrombosis, thereby precluding survival analysis. However, we were able to use alternative statistical methods to incorporate all available information on vascular access thrombosis and examine the relative rates of access thrombosis over the entire follow-up period. Fourth, we did not have information regarding heparin or erythropoietin dosing, which may influence access thrombosis.32 However, we were able to adjust for time-varying predialysis hematocrit. Finally, information on access thrombosis was collected according to medical record or patient recall and may have been susceptible to recall bias.
In conclusion, our analysis shows that more frequent episodes of intradialytic hypotension are associated with increased relative rates of arteriovenous fistula thrombosis. Current clinical practice already dictates avoidance of intradialytic hypotension, and our results add another item to the growing list of adverse clinical outcomes associated with intradialytic hypotension, such as decreased quality of life, inadequate dialysis, myocardial dysfunction, and death.16,33–35 We also demonstrate that lower pre- and postdialysis SBP are associated with higher relative rates of fistula and graft thrombosis. Uncertainty regarding appropriate BP targets in hemodialysis continues, and whether higher BP targets are warranted in particular situations remains to be proven. The results of our observational analysis should be interpreted cautiously and cannot be used to justify less aggressive hemodialysis in the name of access preservation. Definitive answers can only be provided by randomized clinical trials, and our analysis underscores the importance of including vascular access patency as an outcome in future studies of BP management strategies in hemodialysis. Given the high costs and high prevalence of access thrombosis, finding modifiable risk factors and implementing strategies that might result in even small decreases in rates of access thrombosis may favorably influence patient outcomes and health care spending in ESRD.
CONCISE METHODS
Study Population
Details of the HEMO study have been published previously.7,8 Briefly, the HEMO study was a randomized clinical trial of 1846 subjects on thrice-weekly hemodialysis between 18 and 80 years of age from 15 U.S. centers. Subjects were enrolled between March 1995 and October 2000 and randomly assigned in a 2 × 2 factorial design to standard- or high-dose equilibrated Kt/Vurea and low- or high-flux dialyzers. Subjects were followed until death or December 2001 and censored at the time of kidney transplant. Subjects were excluded if they had serum albumin concentration ≤ 2.6 g/dl, residual urea clearance of ≥1.5 ml/min per 35 L of urea distribution volume, or if they were unable to achieve an equilibrated Kt/Vurea of >1.30 within 4.5 hours during two of three baseline urea kinetic modeling sessions.
For the analysis presented here, we excluded 91 (4.9%) subjects with catheter-based vascular access or vascular access classified as “other” at the time of randomization. Of the remaining 1755 subjects, we excluded 270 (15.4%) with <1 year of follow-up because these subjects would not have available information on the independent variables of interest in the preceding time period (further described in detail below). We also restricted our analysis to patients who had an assessment of their vascular access condition completed at the scheduled time, leaving a total of 1426 subjects in the analysis presented here.
Vascular Access Assessment
The type of vascular access (fistula, graft or catheter) used during the monitored hemodialysis session was recorded at baseline and monthly over the follow-up period. When patients had more than one type of vascular access recorded for a given time period, we categorized the patient on the basis of the access type that was recorded for most of the dialysis sessions. For example, if a patient had a fistula at months 12 to 16 and a catheter at months 17 to 18, we classified the patient as having a fistula for that time period. If patients had only a catheter used during a time period in which an access thrombosis episode was recorded, we classified the patient according to the type of permanent vascular access they had before having the catheter. Our reasoning was that patients might use a catheter while waiting for a permanent vascular access to become usable (such as a fistula to mature), and therefore it is feasible that they might have had a vascular access thrombosis episode recorded despite dialyzing with a catheter.
Our primary outcome was episodes of vascular access thrombosis, defined as an access that had clotted without blood flow. Information on vascular access thrombosis was obtained by the HEMO study coordinators from the dialysis facility medical records or directly from the study subject. Episodes of vascular access thrombosis were assessed in aggregate during the following time periods: baseline to 4 months, 5 to 12 months, and then every 6 months for the duration of the study. For example, at 12 months, the study coordinators recorded the number of episodes of vascular access thrombosis from 5 to 12 months. Similarly, at 18 months, the number of episodes of vascular access thrombosis since the previous assessment was recorded.
Independent Variables
During the monthly monitored hemodialysis sessions, the following yes/no question was asked: “Did the subject have hypotension requiring either saline infusion, lowering of the ultrafiltration rate, or reduced blood flow?” We counted each “yes” response to this question as an episode of intradialytic hypotension. Intradialytic hypotension information was obtained from the first three baseline prerandomization sessions and monthly during follow-up. To account for differences in length of follow-up, we divided the number of dialysis sessions with at least one episode of intradialytic hypotension by the total number of dialysis sessions during the following time periods: baseline to 4 months, 5 to 12 months, and then every 6 months for the duration of follow-up). We chose these time periods to correspond with the time at which episodes of vascular access thrombosis were assessed. Because the percentage of dialysis sessions with intradialytic hypotension was not normally distributed, we divided the exposure into quartiles (corresponding to 0%, 6.7% to 11.1%, 12.5% to 28.6%, and ≥30%) and analyzed it as a categorical variable, with the lowest quartile (0% of dialysis sessions with intradialytic hypotension) serving as the referent group.
SBP and DBP were recorded before (predialysis) and after (postdialysis) the monthly monitored dialysis sessions in a seated position using a sphygmomanometer as per the dialysis unit routine practice. MAP was calculated as follows: MAP = (SBP − DBP)/3 + DBP. BP parameters were defined as the mean values recorded during the same time periods used to identify episodes of intradialytic hypotension: baseline to 4 months, 5 to 12 months, then every 6 months for the duration of follow-up. We reported results of regression models relative to cross-sectional differences in BP parameters per SD as continuous variables. Pre- and postdialysis SBP, DBP, and MAP were analyzed separately. DBP > 150 mmHg was recorded as missing.
We defined large-volume ultrafiltration as a dialysis session with ≥10% loss of total body water calculated using Watson's formula.36 We used the Spearman's rank correlation coefficient (ρ) to describe the relation between intradialytic hypotension and large-volume ultrafiltration. To assess the relation of large-volume ultrafiltration to vascular access thrombosis, we divided the number of dialysis sessions with ≥10% loss of total body water by the total number of dialysis sessions during the same time periods described above. The percentage of dialysis sessions with large-volume ultrafiltration was then divided into quartiles (corresponding to 0%, 7.1% to 15.4%, 16.7% to 42.8%, and ≥44.4%) and analyzed as a categorical variable, with the lowest quartile (0% of sessions with large-volume ultrafiltration) serving as the referent group.
Covariates
In multivariable models, we adjusted for the following covariates assessed at baseline: age, sex, race (black versus nonblack), mean serum albumin concentration (averaged from two values obtained during the prerandomization period, in g/dl), current smoking status, diabetes mellitus, ischemic heart disease, congestive heart failure, peripheral vascular disease, vascular access type (fistula versus graft), intervention group (high- or standard-dose dialysis and high- or low-flux dialyzer membrane), and clinical center. We adjusted for hospitalization for any reason as a dichotomous (yes versus no) time-varying variable. We also included an adjustment for mean predialysis SBP and mean hematocrit (averaged over each 6-month assessment period) as time-varying variables. For missing hematocrit values (8.8% of observations), we used a last-value carried forward method of imputation. All time-varying variables were assessed during the concurrent time period as intradialytic hypotension or large-volume ultrafiltration. We chose these covariates because we hypothesized that they might have significant associations with vascular access thrombosis and/or intradialytic hypotension.
Statistical Methods
We compared baseline clinical characteristics by quartiles of intradialytic hypotension using the Cochran–Armitage trend test for categorical characteristics or linear regression for continuous characteristics.
We evaluated the association between episodes of vascular access thromboses for a given 6-month time period with the percentage of dialysis sessions with intradialytic hypotension occurring in the immediately preceding 6-month time period. We chose to include the 6-month lag period to ensure that episodes of intradialytic hypotension preceded episodes of vascular access thrombosis. Thus, at 24 months, the number of access thromboses that had occurred from 18 to 24 months was related to the percentage of dialysis sessions with intradialytic hypotension that had occurred from 12 to 18 months. The exception was at 12 months, when the number of vascular access thromboses from 5 to 12 months was related to the percentage of dialysis sessions with intradialytic hypotension that occurred from baseline to 4 months. We analyzed the BP parameters and large-volume ultrafiltration in the same manner as for intradialytic hypotension.
We assumed a negative binomial model and used a generalized estimating equations approach to estimate the parameters. An autoregressive correlation structure was assumed to account for the correlation expected among repeated measures within an individual. The latter allows the correlation to vary in strength by distance in time.37,38 We hypothesized a priori that the associations of intradialytic hypotension, BP, and large-volume ultrafiltration with vascular access thrombosis would be modified by the type of vascular access and therefore tested for effect modification by including a multiplicative interaction term of vascular access type with the independent variables of interest. When significant interactions were found, we present the results of analyses after stratifying by baseline vascular access type. We used the type 3 score test to assess for global significance of the categorical and interaction terms.
We also conducted two companion analyses. First, because patients may change the type of vascular access over the course of follow-up, we repeated our analyses with vascular access type as a time-varying variable, updating this information at the same vascular access thrombosis assessment time points. Second, although in our main analysis we did not include an adjustment for baseline aspirin, warfarin, erythropoietin, angiotensin converting enzyme inhibitor, or calcium channel blocker use given the problem of indication bias inherent to observational studies, we repeated our analyses after including these covariates to check the robustness of our results.
All tests were two-sided and conducted at the 0.05 level of significance. All analyses were conducted with SAS Enterprise Guide 4.2 (Cary, NC).
DISCLOSURES
None.
Acknowledgments
Dr. Chang is supported by a grant from the American Heart Association Pharmaceutical Round Table. Dr. Chertow and Dr. Desai are supported by K24 DK085446. The HEMO study was conducted by the HEMO investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIDDK Central Repositories supplied the data from the HEMO study reported here. This manuscript was not prepared in collaboration with investigators of the HEMO study and does not necessarily reflect the opinions or views of the HEMO study, the NIDDK Central Repositories, or the NIDDK. We are indebted to Ms. Jessica Kubo, M.S., for her assistance in preparing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Intradialytic Hypotension Strikes Again,” on pages 1396–1398.
REFERENCES
- 1. Fan PY, Schwab SJ: Vascular access: Concepts for the 1990s. J Am Soc Nephrol 3: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 2. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009 [Google Scholar]
- 3. Brenner L, Singh AK, Campbell D, Frei F, Winkelmayer WC: Associations between demographic factors and provider structures on cost and length of stay for hemodialysis patients with vascular access failure. Clin J Am Soc Nephrol 1: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Aman LC, Levin NW, Smith DW: Hemodialysis access site morbidity. Proc Clin Dial Transplant Forum 10: 277–284, 1980 [PubMed] [Google Scholar]
- 5. May RE, Himmelfarb J, Yenicesu M, Knights S, Ikizler TA, Schulman G, Hernanz-Schulman M, Shyr Y, Hakim RM: Predictive measures of vascular access thrombosis: A prospective study. Kidney Int 52: 1656–1662, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Brattich M: Vascular access thrombosis: Etiology and prevention. ANNA J 26: 537–540, 1999 [PubMed] [Google Scholar]
- 7. Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G: Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials 21: 502–525, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Culp K, Flanigan M, Taylor L, Rothstein M: Vascular access thrombosis in new hemodialysis patients. Am J Kidney Dis 26: 341–346, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Goldwasser P, Avram MM, Collier JT, Michel MA, Gusik SA, Mittman N: Correlates of vascular access occlusion in hemodialysis. Am J Kidney Dis 24: 785–794, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Davenport A, Cox C, Thuraisingham R: Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int 73: 759–764, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Chang TI, Friedman GD, Cheung AK, Greene T, Desai M, Chertow GM: Systolic blood pressure and mortality in prevalent haemodialysis patients in the HEMO study. J Hum Hypertens 25: 98–105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Shoji T, Tsubakihara Y, Fujii M, Imai E, Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Andrulli S, Colzani S, Mascia F, Lucchi L, Stipo L, Bigi MC, Crepaldi M, Redaelli B, Albertazzi A, Locatelli F: The role of blood volume reduction in the genesis of intradialytic hypotension. Am J Kidney Dis 40: 1244–1254, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Barth C, Boer W, Garzoni D, Kuenzi T, Ries W, Schaefer R, Schneditz D, Tsobanelis T, van der Sande F, Wojke R, Schilling H, Passlick-Deetjen J: Characteristics of hypotension-prone haemodialysis patients: Is there a critical relative blood volume? Nephrol Dial Transplant 18: 1353–1360, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Nette RW, van den Dorpel MA, Krepel HP, Ie EH, van den Meiracker AH, Poldermans D, Weimar W, Zietse R: Hypotension during hemodialysis results from an impairment of arteriolar tone and left ventricular function. Clin Nephrol 63: 276–283, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Yang N-I, Wang C-H, Hung M-J, Chen Y-C, Wu I-W, Lee C-C, Wu M-S, Kuo L-T, Cheng C-W, Cherng W-J: Real-time three-dimensional echocardiography provides advanced haemodynamic information associated with intra-dialytic hypotension in patients with autonomic dysfunction. Nephrol Dial Transplant 25: 249–254, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Sapoznikov D, Backenroth R, Rubinger D: Baroreflex sensitivity and sympatho-vagal balance during intradialytic hypotensive episodes. J Hypertens 28: 314–324, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ: Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis 36: 1126–1134, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Prischl F, Kirchgatterer A, Brandstatter E, Wallner M, Baldinger C, Roithinger F, Kramar R: Parameters of prognostic relevance to the patency of vascular access in hemodialysis patients. J Am Soc Nephrol 6: 1613–1618, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Saran R, Dykstra DM, Wolfe RA, Gillespie B, Held PJ, Young EW: Association between vascular access failure and the use of specific drugs: The dialysis outcomes and practice patterns study (DOPPS). Am J Kid Dis 40: 1255–1263, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hodges TC, Fillinger MF, Zwolak RM, Walsh DB, Bech F, Cronenwett JL: Longitudinal comparison of dialysis access methods: Risk factors for failure. J Vasc Surg 26: 1009–1019, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Woods JD, Turenne MN, Strawderman RL, Young EW, Hirth RA, Port FK, Held PJ: Vascular access survival among incident hemodialysis patients in the united states. Am J Kidney Dis 30: 50–57, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ: Hemodialysis arteriovenous fistula patency revisited: Results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3: 714–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vernaglione L, Mele G, Cristofano C, Distratis C, Perrone F, Frascina M, Pennacchiotti F, Chimienti S: Comorbid conditions and gender impact the primary survival of distal radio-cephalic arteriovenous fistula inpatients on long-term hemodialysis. J Nephrol 18: 276–281, 2005 [PubMed] [Google Scholar]
- 30. Cheung AK, Levin NW, Greene T, Agodoa L, Bailey J, Beck G, Clark W, Levey AS, Leypoldt JK, Ornt DB, Rocco MV, Schulman G, Schwab S, Teehan B, Eknoyan G: Effects of high-flux hemodialysis on clinical outcomes: Results of the HEMO study. J Am Soc Nephrol 14: 3251–3263, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R; Membrane Permeability Outcome (MPO) Study Group: Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian erythropoietin study group. BMJ 300: 573–578, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tisler A, Akocsi K, Borbas B, Fazakas L, Ferenczi S, Gorogh S, Kulcsar I, Nagy L, Samik J, Szegedi J, Toth E, Wagner G, Kiss I: The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant 18: 2601–2605, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Tisler A, Akocsi K, Harshegi I, Varga G, Ferenczi S, Grosz M, Kulcsar I, Locsey L, Samik J, Solt I, Szegedi J, Toth E, Wagner G, Kiss I: Comparison of dialysis and clinical characteristics of patients with frequent and occasional hemodialysis-associated hypotension. Kidney Blood Press Res 25: 97–102, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Watson PE, Watson ID, Batt RD: Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr 33: 27–39, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE: Generalized linear models. In: Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models, New York, Springer Science, 2005, pp 291–303 [Google Scholar]
- 38. SAS annotated output: Negative binomial regression. Available at: http://www.ats.ucla.edu/stat/sas/output/sas_negbin_output.htm Accessed March 3, 2010