Abstract
The relative risk of death for patients treated with peritoneal dialysis compared with those treated with hemodialysis appears to change with duration of dialysis therapy. Patients who start dialysis urgently are at high risk for mortality and are treated almost exclusively with hemodialysis, introducing bias to such mortality comparisons. To better isolate the association between dialysis treatment modality and patient mortality, we examined the relative risk for mortality for peritoneal dialysis compared with hemodialysis among individuals who received ≥4 months of predialysis care and who started dialysis electively as outpatients. From a total of 32,285 individuals who received dialysis in Ontario, Canada during a nearly 8-year period, 6573 patients met criteria for elective, outpatient initiation. We detected no difference in survival between peritoneal dialysis and hemodialysis after adjusting for relevant baseline characteristics. The relative risk of death did not change with duration of dialysis therapy in our primary analysis, but it did change with time when we defined our patient population using the more inclusive criteria typical of previous studies. These results suggest that peritoneal dialysis and hemodialysis associate with similar survival among incident dialysis patients who initiate dialysis electively, as outpatients, after at least 4 months of predialysis care. Selection bias, rather than an effect of the treatment itself, likely explains the previously described change in the relative risk of death over time between hemodialysis and peritoneal dialysis.
There is controversy about the effect of dialysis treatment modality on the survival of patients with ESRD.1–13 It has been consistently shown that the relative risk of death on peritoneal dialysis (PD) compared with on hemodialysis (HD) changes over time,1–4,7,14 with the relative risk on PD being lowest in the first 2 years of therapy and increasing thereafter.
The explanation proposed for this phenomenon is that the slower loss of residual renal function and urine output in PD patients may provide an early survival advantage.15–21 Furthermore, the loss of ultrafiltration capacity may complicate volume control and lead to an increased risk of death with time on therapy.22,23 This has led some to suggest an integrated model of care wherein ESRD patients are electively switched from PD to HD.2,7,24,25
However, patients who are acutely ill and start dialysis urgently are treated almost exclusively with HD.26 This may inflate the risk of death in HD patients early after the initiation of dialysis. This effect would be expected to dissipate over time and may explain why PD appears to have an advantage during the first 2 years of treatment in previous studies.
We examined the relative risk of mortality on PD compared with HD in individuals with at least 4 months of predialysis care, all of whom started dialysis electively, as outpatients. The analysis was then repeated using traditional cohort definitions. Our objective was to isolate the association between dialysis modality and patient mortality (independent of bias introduced by acute or unplanned dialysis starts). A secondary objective was to demonstrate the extent to which different analytical approaches can influence conclusions about the risk of mortality associated with PD as compared with HD.
RESULTS
A total of 32,285 individuals received dialysis treatment in Ontario, Canada between July 1, 1998 and March 31, 2006. Of these, 1172 were excluded from the analysis because they had incomplete information about baseline characteristics or important outcome events. Baseline characteristics of the cohorts are presented in Table 1 and Table 2. Race was not available in our datasets, but the vast majority of dialysis patients in Canada are Caucasian.
Table 1.
Baseline characteristics of Elective Outpatient Start cohort
| Variable | All Elective Outpatient Starts | PD | HD | Standardized Difference |
|---|---|---|---|---|
| Total number of patients | 6573 | 2035 | 4538 | |
| Mean age (SD; range) | 62.6 (15.3; 18 to 95) | 61.5 (15.2; 18 to 92) | 63.1 (15.3; 18 to 95) | 0.10 |
| Median age | 65 | 64 | 66 | 0.10 |
| Acute kidney injury to start dialysis (%) | 28 (0.4) | 0 (0) | 28 (0.6) | 0.09 |
| Predialysis care of >12 months (%) | 5621 (85.5) | 1736 (85.3) | 3885 (85.6) | 0.01 |
| Mean length of predialysis care, days (SD; range) | 1732.5 (1306.4; 121 to 5364) | 1751.5 (1322.5; 121 to 5297) | 1724.1 (1299.2; 121 to 5364) | 0.02 |
| Median length of predialysis care, days | 1421 | 1455 | 1400.5 | 0.02 |
| Mean number of predialysis visits in 2 years prior to start (SD; range) | 9.3 (5.3; 0 to 48) | 9.5 (4.8; 0 to 29) | 9.5 (5.5; 0 to 48) | 0.01 |
| Median number of predialysis visits | 9.0 | 9 | 9 | 0.01 |
| Hospitalized in past year (%) | 3176 (48.3) | 1067 (52.4) | 2109 (46.5) | 0.12 |
| Mean number of hospitalizations (SD; range) | 0.84 (1.2; 0 to 11) | 0.84 (1.1; 0 to 8) | 0.83 (0 to 11) | 0.01 |
| Median number of hospitalizations | 0 | 1 | 0 | 0.01 |
| Mean number of hospital days (SD; range) | 6.7 (13.9; 0 to 291) | 6.1 (13.1; 0 to 291) | 6.9 (14.2; 0 to 199) | 0.06 |
| Median number of hospital days | 0 | 2 | 0 | 0.06 |
| Congestive heart failure (%) | 1805 (27.5) | 483 (23.7) | 1322 (29.1) | 0.12 |
| Ischemic heart disease (%) | 2510 (38.2) | 733 (36.0) | 1777 (39.2) | 0.06 |
| Valvular disorder (%) | 220 (3.4) | 81 (4.0) | 139 (3.1) | 0.05 |
| Rhythm disturbance (%) | 1106 (16.8) | 316 (15.5) | 790 (17.4) | 0.05 |
| Cardiomyopathy (%) | 115 (1.8) | 31 (1.5) | 84 (1.9) | 0.02 |
| Hypertension | ||||
| no complications (%) | 4873 (74.1) | 1532 (75.3) | 3341 (73.6) | 0.04 |
| complications (%) | 2156 (32.8) | 732 (36.0) | 1424 (31.4) | 0.10 |
| Cerebrovascular disease (%) | 729 (11.1) | 192 (9.4) | 537 (11.8) | 0.08 |
| Chronic ulcer (%) | 222 (3.4) | 54 (2.7) | 168 (3.7) | 0.06 |
| Chronic obstructive Pulmonary disease (%) | 699 (10.6) | 166 (8.2) | 533 (11.8) | 0.12 |
| Disorder of lipoid metabolism (%) | 981 (14.9) | 318 (15.6) | 663 (14.6) | 0.03 |
| High-risk malignancy (%) | 296 (4.5) | 98 (4.8) | 198 (4.4) | 0.02 |
| Low-risk malignancy (%) | 336 (5.9) | 111 (5.5) | 275 (6.1) | 0.03 |
| Depression (%) | 76 (1.2) | 14 (0.7) | 62 (1.4) | 0.06 |
| Nutritional deficiency (%) | 95 (1.5) | 18 (0.9) | 77 (1.7) | 0.07 |
| Osteoporosis (%) | 309 (4.7) | 89 (4.4) | 220 (4.9) | 0.02 |
| Autoimmune disorder (%) | 702 (10.7) | 168 (8.3) | 534 (11.8) | 0.11 |
| Gastroesophageal reflux disease (%) | 269 (4.1) | 61 (3.0) | 208 (4.6) | 0.08 |
| Chronic liver disease (%) | 119 (1.8) | 30 (1.5) | 89 (2.0) | 0.04 |
| Diabetes mellitus, Ontario Diabetes Database (%) | 3255 (49.5) | 981 (48.2) | 2274 (50.1) | 0.04 |
PD, peritocal dialysis; HD, hemodialysis.
Table 2.
Baseline characteristics according to cohort definition
| Variable | All Outpatient Dialysis | 90-Day Cohort | All Elective Outpatient Starts |
|---|---|---|---|
| Total number of patients | 16,915 | 12,369 | 6573 |
| Started as outpatient (%) | 9178 (54.3) | 6613 (53.5) | 6573 (100) |
| First treatment modality (%) | |||
| PD | 3292 (19.5) | 2499 (20.2) | 2035 (31.0) |
| HD | 13,216 (78.1) | 9570 (77.4) | 4538 (69.0) |
| CRRT | 407 (2.4) | 293 (2.4) | 0 (0) |
| Mean age (SD; range) | 63.6 (15.7; 18 to 96) | 63.5 (15.5; 18 to 98) | 62.6 (15.3; 18 to 95) |
| Median age | 67 | 66 | 65 |
| AKT to start (%) | 5274 (31.2) | 3727 (30.1) | 28 (0.4) |
| History of CKD (%) | 14,877 (88.0) | 11,610 (93.9) | 6573 (100) |
| Predialysis care (%) | 13,489 (79.8) | 10,513 (85.0) | 6573 (100) |
| Predialysis care of >4 months (%) | 11,261 (66.6) | 8892 (71.9) | 6573 (100) |
| Predialysis care of >12 months (%) | 9520 (56.3) | 7496 (60.6) | 5621 (85.5) |
| Mean length of predialysis care, days (SD; range) | 1109.0 (1300; 0 to 5364) | 1180.4 (1292.8; 0 to 5288) | 1732.5 (1306.4; 121 to 5364) |
| Median length of predialysis care, days | 559 | 686 | 1421 |
| Mean number of predialysis visits in 2 years prior to start (SD; range) | 6.0 (5.8; 0 to 48) | 6.7 (5.9; 0 to 48) | 9.5 (5.3; 0 to 48) |
| Median number of predialysis visits | 5.0 | 6.0 | 9.0 |
| Hospitalized in past year (%) | 8688 (51.4) | 6577 (53.2) | 3176 (48.3) |
| Mean number of hospitalizations (range) | 0.96 (1.34; 0 to 18) | 0.98 (1.3; 0 to 15) | 0.84 (1.2; 0 to 11) |
| Median number of hospitalizations | 1.0 | 1.0 | 0.0 |
| Mean number of hospital days (range) | 11.8 (19.4; 0 to 327) | 11.8 (19.3; 0 to 291) | 6.7 (13.9; 0 to 291) |
| Median number of hospital days | 5.0 | 5.0 | 0.0 |
| Congestive heart failure (%) | 5957 (35.2) | 4449 (36.0) | 1805 (27.5) |
| Ischemic heart disease (%) | 7012 (41.5) | 5205 (42.1) | 2510 (38.2) |
| Valvular disorder (%) | 885 (5.2) | 610 (4.9) | 220 (3.4) |
| Rhythm disturbance (%) | 3739 (22.1) | 2683 (21.7) | 1106 (16.8) |
| Cardiomyopathy (%) | 503 (3.0) | 355 (2.9) | 115 (1.8) |
| Hypertension | |||
| no complications (%) | 11,818 (69.9) | 8979 (72.6) | 4873 (74.1) |
| complications (%) | 5845 (34.6) | 4648 (37.6) | 2156 (32.8) |
| Cerebrovascular disease (%) | 2241 (13.3) | 1666 (13.5) | 729 (11.1) |
| Chronic ulcer (%) | 952 (5.6) | 693 (5.6) | 222 (3.4) |
| Chronic obstructive pulmonary disease (%) | 2338 (13.8) | 1690 (13.7) | 699 (10.6) |
| Disorder of lipoid metabolism (%) | 2706 (16.0) | 2027 (16.4) | 981 (14.9) |
| High-risk malignancy (%) | 1036 (6.1) | 679 (5.5) | 296 (4.5) |
| Low-risk malignancy (%) | 1273 (7.5) | 883 (7.1) | 386 (5.9) |
| Depression (%) | 344 (2.0) | 244 (2.0) | 76 (1.2) |
| Nutritional deficiency (%) | 428 (2.5) | 314 (2.5) | 95 (1.5) |
| Osteoporosis (%) | 815 (4.8) | 564 (4.6) | 309 (4.7) |
| Autoimmune disorder (%) | 2059 (12.2) | 1502 (12.1) | 702 (10.7) |
| Gastroesophageal reflux disease (%) | 764 (4.5) | 564 (4.6) | 269 (4.1) |
| Chronic liver disease (%) | 527 (3.1) | 363 (2.9) | 119 (1.8) |
| Diabetes mellitus, Ontario Diabetes Database (%) | 8101 (47.9) | 6219 (50.3) | 3255 (49.5) |
CRRT, continuous renal replacement therapy; PD, peritoneal dialysis; HD, hemodialysis.
Primary Analysis
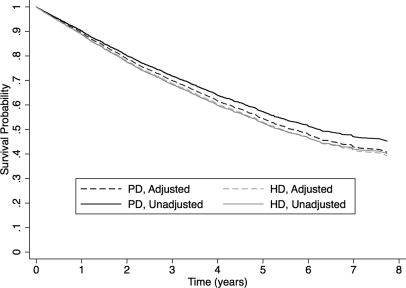
A total of 6573 patients met criteria for the “Elective Outpatient Start” cohort. In the unadjusted analysis, PD was associated with a significantly lower risk of death when compared with HD (unadjusted HRPD:HD = 0.87; 95% confidence limits, 0.79 to 0.96), but the effect disappeared after adjusting for important baseline characteristics (adjusted HRPD:HD = 0.96; 95% confidence limits, 0.88 to 1.06). On the basis of our model diagnostics, an interaction term between age and diabetes mellitus was retained in our final adjusted model, and the effect of diabetes on survival appeared to change over time, so a time-dependent covariate was included. Of note, the effect of modality on survival did not vary with follow-up time and therefore did not violate the proportional hazards assumption. In addition, there was no significant interaction between diabetes and treatment modality. The variables included in the final model, their hazard ratios, and accompanying 95% confidence limits are found in Table 3. Adjusted survival curves for the PD and HD groups are presented in Figure 1. When the analysis was repeated with censoring for renal transplantation, the results were very similar (adjusted HRPD:HD = 0.97; 95% confidence limits, 0.88 to 1.07).
Table 3.
Results of primary analysis comparing survival on PD and HD in the Elective Outpatient Start cohort
| Variable | Parameter Estimate | Adjusted Hazard Ratio | P |
|---|---|---|---|
| PD | −0.04 | 0.96 | 0.44 |
| Age | 0.06 | 1.06 | <0.01 |
| Male | 0.001 | 1.00 | 0.98 |
| Hospitalized in the past year | 0.21 | 1.23 | <0.01 |
| Diabetes mellitus | 1.84 | 6.28 | <0.01 |
| Ischemic heart disease | 0.13 | 1.14 | 0.01 |
| Congestive heart failure | 0.42 | 1.52 | <0.01 |
| High-risk malignancy | 0.56 | 1.75 | <0.01 |
| Hypertension, no complications | −0.24 | 0.78 | <0.01 |
| Cerebrovascular disease | 0.33 | 1.39 | <0.01 |
| Chronic ulcer | 0.48 | 1.62 | <0.01 |
| Chronic obstructive pulmonary disease | 0.21 | 1.23 | 0.00 |
| Lipid disorder | −0.19 | 083 | 0.00 |
| Cardiac rhythm disturbance | 0.13 | 1.13 | 0.02 |
| Chronic liver disease | 0.35 | 1.41 | 0.02 |
| Age × diabetes mellitus | −0.02 | 0.98 | <0.01 |
| Diabetes mellitus × time (years) | 0.06 | 1.06 | <0.01 |
PD, peritoneal dialysis. Bold type identifies PD as the main exposure variable.
Figure 1.
There was no statistically significant difference in adjusted survival between individuals treated with PD and HD who had received at least 4 months of predialysis care and started dialysis electively, as outpatients. Adjusted survival curves were generated using the corrected group-prognosis method.
Secondary Analyses
A significant interaction was found between diabetes and treatment modality in both the All Outpatient Dialysis cohort and the 90-day cohort, suggesting that patients with diabetes had a higher mortality rate on PD relative to individuals without diabetes (Figure 2). Treatment modality and diabetes also violated the proportional hazards assumption in both cohorts, suggesting that the risk of death rose faster over time in patients with diabetes and that the relative risk of death on PD increased over time. To present the results accounting for these interaction terms, hazard ratios were calculated according to diabetes status and follow-up time.
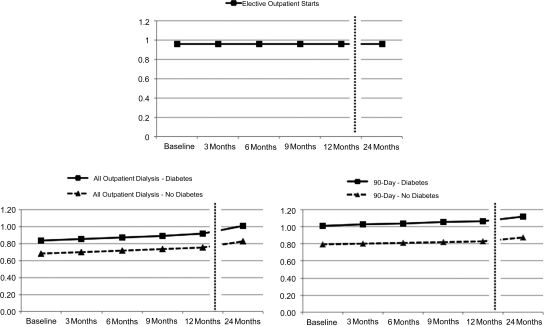
Figure 2.
Different definitions of chronic dialysis lead to different conclusions when mortality is compared among patients treated with PD compared with those treated with HD. This figure presents the relative hazard of death on PD versus HD over time, according to the presence or absence of diabetes mellitus, and the definition of chronic dialysis. In the Elective Outpatient Start cohort (top panel), there was no change in the relative hazard of death over time, and diabetes status did not influence the relationship between treatment modality and the risk of death. In both the All Outpatient Dialysis cohort (bottom left panel) and the 90-day cohort (bottom right panel), the risk of death on PD relative to HD rose over time and was higher in patients with diabetes compared with those without diabetes at any given time point.
In the All Outpatient Dialysis cohort, PD was associated with a lower, adjusted hazard of death in patients with diabetes until approximately 2 years. The benefit of PD appeared to extend well beyond 2 years in individuals without diabetes. In the 90-day cohort, PD was associated with a higher, adjusted hazard of death in patients with diabetes at all time points. Conversely, in patients without diabetes, PD was associated with a survival benefit that extended beyond 2 years of follow-up.
DISCUSSION
Our results suggest that PD and HD are associated with similar survival in incident dialysis patients who have at least 4 months of predialysis care and start therapy electively, as outpatients. In contrast, when definitions of chronic dialysis that have been traditionally used to study the effect of dialysis modality on mortality were used, the relative risk of death on PD compared with HD increased over time, which is consistent with previous studies. This association disappeared when patients who started dialysis therapy urgently were excluded, suggesting that the apparent increase in the relative risk of death over time on PD compared with HD is due to bias rather than a specific effect of dialysis modality. These findings do not support a strategy of electively switching patients to HD.
Several studies have suggested that the relative risk of death on PD as compared with HD increases over time.1–4,7,14 Historically, it has been felt that the slower loss of residual renal function and urine output in PD patients may provide an early survival advantage and that the loss of ultrafiltration capacity may complicate volume control in PD patients, leading to an increased risk of death with time on therapy.22,23 In the analyses performed to mirror traditional comparisons of PD and HD, we again showed that the relative risk of death on PD rose with time. However, if the loss of residual renal function and urine output were responsible for this phenomenon, one would have expected to see the same findings in the Elective Outpatient Start cohort. This was not observed in our study and suggests that it was the exclusion of acutely ill, urgent dialysis starts that led to the discrepant findings. It may be that including “sicker” patients treated almost exclusively with HD biases analyses against HD early in the treatment course and that this effect decreases over time. This hypothesis is supported by an analysis of the relationship between comorbidities and treatment choice by Couchoud et al.27 In their study, they found a significant difference in mortality between “unplanned” and “planned” HD starts and suggested that comparing PD and HD after removing unplanned HD starts would likely provide a more balanced estimate of the effect of modality choice on survival in elderly patients. It is important to note that this explanation does not necessarily mean that the loss of residual renal function is not slower in PD patients or that residual renal function is not a predictor of survival. However, it does suggest that these factors may not explain the change in the relative risk of death over time.
It has been argued that starting mortality comparisons after patients have been on therapy for at least 90 days will eliminate the effect of acuity and early deaths, giving a truer representation of the relative effects of treatment modality. However, studies have shown that acuity at presentation is a predictor of survival even when analyses begin at the start of outpatient dialysis28 or 3 months after the start of dialysis.26 We recently showed that individuals starting dialysis in hospital had a more than 50% increase in the odds of death, even if they survived to hospital discharge.28 Murphy et al.26 reported that an acute onset of renal failure was much more common in HD patients and was associated with a 55% increase in the relative hazard of death. In that analysis, they examined the effect of modality in use at 3 months and those patients that were still alive. These findings are consistent with the fact that acuity is a surrogate for a patient with a poorer prognosis and show that the effect is present even if an analysis starts 90 days after the initiation of dialysis.
Van Biesen et al.25 evaluated an “integrative care” approach to modality selection that has been subsequently supported by others.2,7,24 In their study, no difference was found in adjusted survival in patients treated with PD as compared with HD, using an intention-to-treat analysis. However, the authors also compared patients that switched from PD to HD with both vintage-matched patients who were already on HD and patients remaining on PD. Outcomes in individuals that switched from PD to HD were no different than matched HD patients but were superior to those that remained on PD. The authors suggested that an integrative-care model where patients are electively switched from PD to HD warranted further evaluation. However, this study had several limitations. First, the study was a retrospective, single-center study of 223 HD and 194 PD patients treated between 1979 and 1986. Second, there were only 32 patients in the integrative care group who switched from PD to HD. The characteristics of patients that remained on PD were compared with the integrative care group, but they were the characteristics of the patients at the initiation of dialysis, not at the time of the switch, and only an unadjusted analysis was presented. Finally, the magnitude of benefit was much larger than one would have expected, with a median survival that was nearly 5 years longer in the integrative care group.
Our study has limitations. First, we had no information about residual renal function. Although it would be interesting to know what happened to residual renal function in both groups over time, it would not change our conclusions. Second, our study used administrative data that were not collected for research purposes and may be subject to residual confounding. However, these data have been used for numerous previous studies, their validity is well known, and we have specifically validated renal variables that are importance for this study.29 Third, although we had considerable information about comorbidities, we did not have specific information about the severity of these comorbidities or data about patient eligibility for PD or HD at baseline. Ideally, outcomes should be compared in patients that are eligible for both therapies. Fourth, we were unable to reliably identify at what center a patient was being treated, making it impossible to see whether the center was an important modifier of the effect of treatment modality on outcomes. Finally, we could not present the results of an “as-treated” analysis where outcomes are attributed to the therapy that a patient was receiving at the time of the event, because our data cannot reliably identify changes in dialysis treatment.29
Our study also has important strengths. It was population based and included over 30,000 dialysis patients who were treated with modern dialysis techniques. The data we used have also been validated in a multi-center validation study, as mentioned above. Thus our results should be valid and generalizable. In addition, our analysis examines outcomes in patients starting dialysis electively, as outpatients. By restricting our analysis to this cohort, we removed the influence of urgent or unplanned starts who are treated preferentially with HD and examined outcomes in the group that is most likely to be eligible for both PD and HD and to be faced with a choice between the two modalities in clinical practice.
In summary, we have shown that PD and HD are associated with similar survival in incident dialysis patients starting therapy electively, as outpatients. Removing acutely ill patients and unplanned starts from the analysis eliminates the change in the relative risk of death between PD and HD over time that is observed using traditional methods of cohort formation. This challenges the rationale for electively switching PD patients to HD.
CONCISE METHODS
The study protocol was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre.
Data Sources
The Province of Ontario, Canada has a population of approximately 12 million residents and provides universal hospital and physician coverage. We used anonymized administrative health data housed at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Canada to conduct this study. The following datasets were linked using encrypted versions of patients' unique health insurance numbers: the Registered Persons Database, the Ontario Health Insurance Plan (OHIP) database, the Canadian Institute of Health Information Discharge Abstracts Database (CIHI-DAD), the Same Day Surgery database, and the National Ambulatory Care Reporting System database. A multi-center study was recently conducted to validate these databases for the identification of key dialysis-related variables.29
Patient Population
All individuals 18 years of age or older who had at least one OHIP billing claim for any form of dialysis therapy between July 1, 1998 and March 31, 2006 were identified. The cohort was then restricted to those who were eligible for OHIP coverage for at least 2 years before starting dialysis. This allowed a 2-year look-back period for comorbidities and other important baseline information. Any individuals residing in the Kingston-Quinte-Rideau region were excluded, because dialysis providers in that area are reimbursed on an alternative funding arrangement and may not reliably submit dialysis billing claims to OHIP.
From this population of incident dialysis patients, we constructed three different cohorts for analysis. For our primary analysis, we restricted the cohort to patients who had documented chronic kidney disease before starting dialysis, who had more than 4 months of predialysis care (as defined by a visit to a nephrologist more than 4 months before the start of dialysis), and who started dialysis electively as outpatients (Elective Outpatient Start cohort). This was done to exclude acutely ill and/or urgent dialysis starts that were preferentially treated with HD, to ensure that all patients had “optimal” predialysis care, to increase the likelihood that individuals started on their intended modality, and to thereby allow us to focus on the effect of dialysis modality on outcomes.
Secondary analyses compared survival in two cohorts of PD and HD patients selected to reflect traditional definitions of chronic dialysis: all patients starting outpatient dialysis, with patients that start in hospital being eligible for this cohort upon discharge (All Outpatient Dialysis cohort), and all patients that are alive on PD or HD 90 days after their first dialysis treatment (90-day cohort).
Identification of Comorbidities
To identify baseline comorbidities, the Johns Hopkins Adjusted Clinical Groups® case-mix system algorithm was used to generate expanded diagnosis clusters (EDCs). EDCs are groups of International Classification of Diseases codes that represent the same disease process.30 EDCS have been used to study the epidemiology and distribution of specific diseases within and across populations and can be generated for ambulatory patients.30 We used a 2-year look-back before the start of dialysis to capture comorbidities using EDCs as outlined previously.28 Diabetes status was taken from the Ontario Diabetes Database because this method has been previously validated.31
Statistical Analyses
Baseline demographic, comorbidity, and treatment variables were described for the PD and HD cohorts, and baseline characteristics were compared using standardized differences. The standardized difference is the difference in the mean of a variable between two groups, divided by an estimate of the SD, and is helpful when assessing the balance in baseline characteristics between groups if sample sizes are large. A standardized difference of <10% is considered to represent negligible imbalance between groups.32–34
Follow-up began at the time of the first dialysis treatment in the Elective Outpatient Start cohort, from the start of outpatient dialysis in the All Outpatient Dialysis cohort, and starting 90 days after the first dialysis treatment in the 90-day cohort. We used an intention-to-treat approach on the basis of treatment modality in use at baseline. All of the patients were followed until death, loss to follow-up, or the end of the study period. Because most previous studies have censored for the occurrence of transplantation, a sensitivity analysis was done in which patients who received a transplant were censored, but the results were unchanged. We did not censor for changes in treatment status, because prior work suggested that the dates of such events are not reliably captured.29 For the same reason, an as-treated analysis was not performed.
The primary exposure of interest was initial treatment modality, and the outcome was all-cause mortality. A list of candidate variables for risk adjustment was generated from a review of the literature and was informed by previous work.28 Candidate covariates included demographic variables (age, gender, and socioeconomic status), comorbidities (congestive heart failure [CHF], ischemic heart disease [IHD], cardiac valvular disorders, rhythm disturbance, cardiomyopathy, hypertension, cerebrovascular disease, chronic ulcer, chronic obstructive pulmonary disease, lipid disorder, malignancy, depression, nutritional deficiency, osteoporosis, autoimmune disorder, gastroesophageal reflux disease, chronic liver disease, and diabetes mellitus), a history of hospitalization, and the number of days spent in hospital in the year before starting dialysis.
For the primary analysis, a full Cox proportional hazards model was constructed containing treatment modality, all of the candidate covariates, and several interaction terms containing variables that have previously been identified as important modifiers of the relationship between modality and survival (age × modality, diabetes × modality, IHD × modality, CHF × modality, and gender × modality).35 We also screened for interactions between diabetes status and age, as well as diabetes status and gender.36 Age, gender, diabetes, IHD, CHF, and treatment modality were forced into the model. Remaining covariates were retained if they were significant at a P value of 0.05 or less. Once the complete model was constructed, we tested the proportional hazards assumption using the test proposed by Harrel and Lee,37 graphical plots of log(−log) survival, and using time-dependent covariates for all covariates. Adjusted survival curves were generated using the corrected group-prognosis method.38 Similar methods were used for secondary analyses, but for multivariate models we used the same predictor variables that were included in the primary analysis, regardless of significance.
DISCLOSURES
None.
Acknowledgments
This study was supported by the ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. The MOHLTC had no role in the design and conduct of the study, collection, management, analysis or interpretation of the data, preparation, review, or approval of the manuscript.
An abstract of this work has been submitted to the World Congress of Nephrology in Vancouver, Canada (April 2011).
R.R.Q. was supported by a Canadian Institutes for Health Research-Institute for Health Services and Policy Research Fellowship award.
R.R.Q. was involved in conception, design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision for important intellectual content, and statistical analysis. R.R.Q. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
J.E.H., M.J.O., M.T., and A.L. were involved in conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision for important intellectual content, and provided supervision. M.J.O. received salary support from the Department of Medicine, Sunnybrook Health Sciences Centre.
P.C.A. was involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision for important intellectual content, statistical analysis, and provided supervision. P.C.A. was supported by a Career Investigator Award from the Heart and Stroke Foundation of Ontario.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Achilles Heel of Mortality Risk by Dialysis Modality is Selection Bias,” on pages 1398–1400.
REFERENCES
- 1. Fenton SS, Schaubel DE, Desmeules M, Morrison HI, Mao Y, Copleston P, Jeffery JR, Kjellstrand CM: Hemodialysis versus peritoneal dialysis: A comparison of adjusted mortality rates. Am J Kidney Dis 30: 334–342, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Heaf JG, Lokkegaard H, Madsen M: Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17: 112–117, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Collins AJ, Hao W, Xia H, Ebben JP, Everson SE, Constantini EG, Ma JZ: Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis 34: 1065–1074, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bloembergen WE, Port FK, Mauger EA, Wolfe RA: A comparison of mortality between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 6: 177–183, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG: Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14: 415–424, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B: Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 64: 1071–1079, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, Levin NW, Sadler JH, Kliger A, Powe NR: Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med 143: 174–183, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J: Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis: A propensity score approach. J Am Soc Nephrol 13: 2353–2362, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Collins AJ, Weinhandl E, Snyder JJ, Chen SC, Gilbertson D: Comparison and survival of hemodialysis and peritoneal dialysis in the elderly. Semin Dial 15: 98–102, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Vonesh EF, Moran J: Mortality in end-stage renal disease: A reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 10: 354–365, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Serkes KD, Blagg CR, Nolph KD, Vonesh EF, Shapiro F: Comparison of patient and technique survival in continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis: A multicenter study. Perit Dial Int 10: 15–19, 1990 [PubMed] [Google Scholar]
- 12. Frimat L, Durand PY, Loos-Ayav C, Villar E, Panescu V, Briancon S, Kessler M: Impact of first dialysis modality on outcome of patients contraindicated for kidney transplant. Perit Dial Int 26: 231–239, 2006 [PubMed] [Google Scholar]
- 13. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Termorshuizen F, Korevaar JC, Dekker FW, Van Manen JG, Boeschoten EW, Krediet RT: The Netherlands Cooperative Study on the Adequacy of Dialysis Study Group: Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis: Analysis of the Netherlands cooperative study on the adequacy of dialysis 2. J Am Soc Nephrol 14: 2851–2860, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, Movilli E, Pola A, d'Avolio G, Gelatti U: Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients: A longitudinal study. Nephrol Dial Transplant 10: 2295–2305, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM: Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 33: 523–534, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, Li PK: Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int 58: 400–407, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT: NECOSAD Study Group: Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 62: 1046–1053, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Rocco M, Soucie JM, Pastan S, McClellan WM: Peritoneal dialysis adequacy and risk of death. Kidney Int 58: 446–457, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Churchill D, Taylor D, Keshaviah P: Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J Am Soc Nephrol 7: 198–207, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Bargman JM, Thorpe KE, Churchill DN: CANUSA Peritoneal Dialysis Study Group: Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D: Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) peritoneal dialysis study group. J Am Soc Nephrol 9: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Menon MK, Naimark DM, Bargman JM, Vas SI, Oreopoulos DG: Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol Dial Transplant 16: 2207–2213, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Thodis E, Passadakis P, Vargemezis V, Oreopoulos DG: Peritoneal dialysis: Better than, equal to, or worse than hemodialysis? Data worth knowing before choosing a dialysis modality. Perit Dial Int 21: 25–35, 2001 [PubMed] [Google Scholar]
- 25. Van Biesen W, Vanholder RC, Veys N, Dhondt A, Lameire NH: An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol 11: 116–125, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Murphy SW, Foley RN, Barrett BJ, Kent GM, Morgan J, Barre P, Campbell P, Fine A, Goldstein MB, Handa SP, Jindal KK, Levin A, Mandin H, Muirhead N, Richardson RM, Parfrey PS: Comparative mortality of hemodialysis and peritoneal dialysis in Canada. Kidney Int 57: 1720–1726, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Couchoud C, Moranne O, Frimat L, Labeeuw M, Allot V, Stengel B: Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant 22: 3246–3254, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Quinn RR, Laupacis A, Hux JE, Oliver MJ, Austin PC: Predicting the risk of 1-year mortality in incident dialysis patients: accounting for case-mix severity in studies using administrative data. Med Care 49: 257–266, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Quinn RR, Laupacis A, Austin PC, Hux JE, Garg AX, Hemmelgarn BR, Oliver MJ: Using administrative datasets to study outcomes in dialysis patients: A validation study. Med Care 48: 745–750, 2010 [DOI] [PubMed] [Google Scholar]
- 30. The Johns Hopkins ACG® Case-Mix System, reference manual, version 7.0.2005.
- 31. Hux JE, Ivis F, Flintoft V, Bica A: Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25: 512–516, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Austin PC: Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 134: 1128–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Austin PC: A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 27: 2037–2049, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ: Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol 54: 387–398, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Vonesh EF, Snyder JJ, Foley RN, Collins AJ: Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int Suppl 103: S3–S11, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC: Comparison of hemodialysis and peritoneal dialysis survival in the Netherlands. Kidney Int 71: 153–158, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Harrell FE, Lee KL, Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML: APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease) Investigators: Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 286: 1494–1497, 2001 [DOI] [PubMed] [Google Scholar]