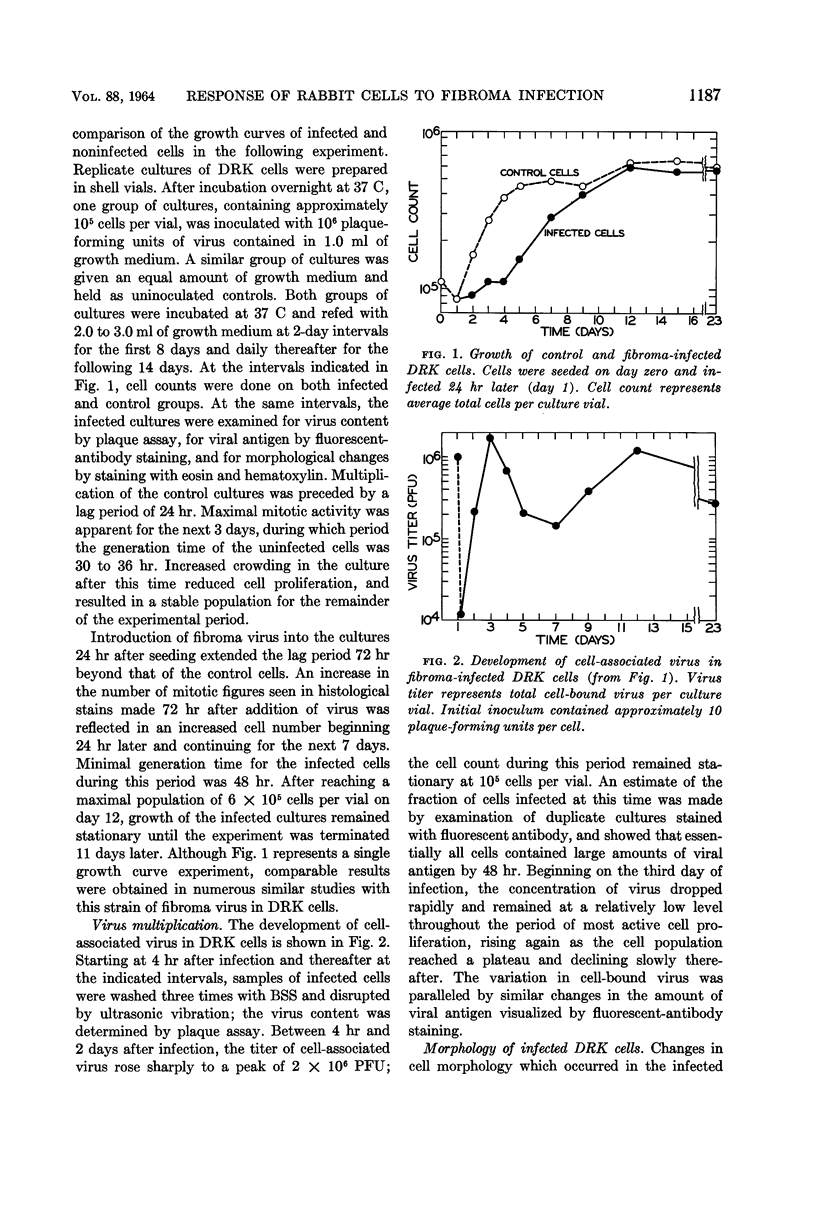
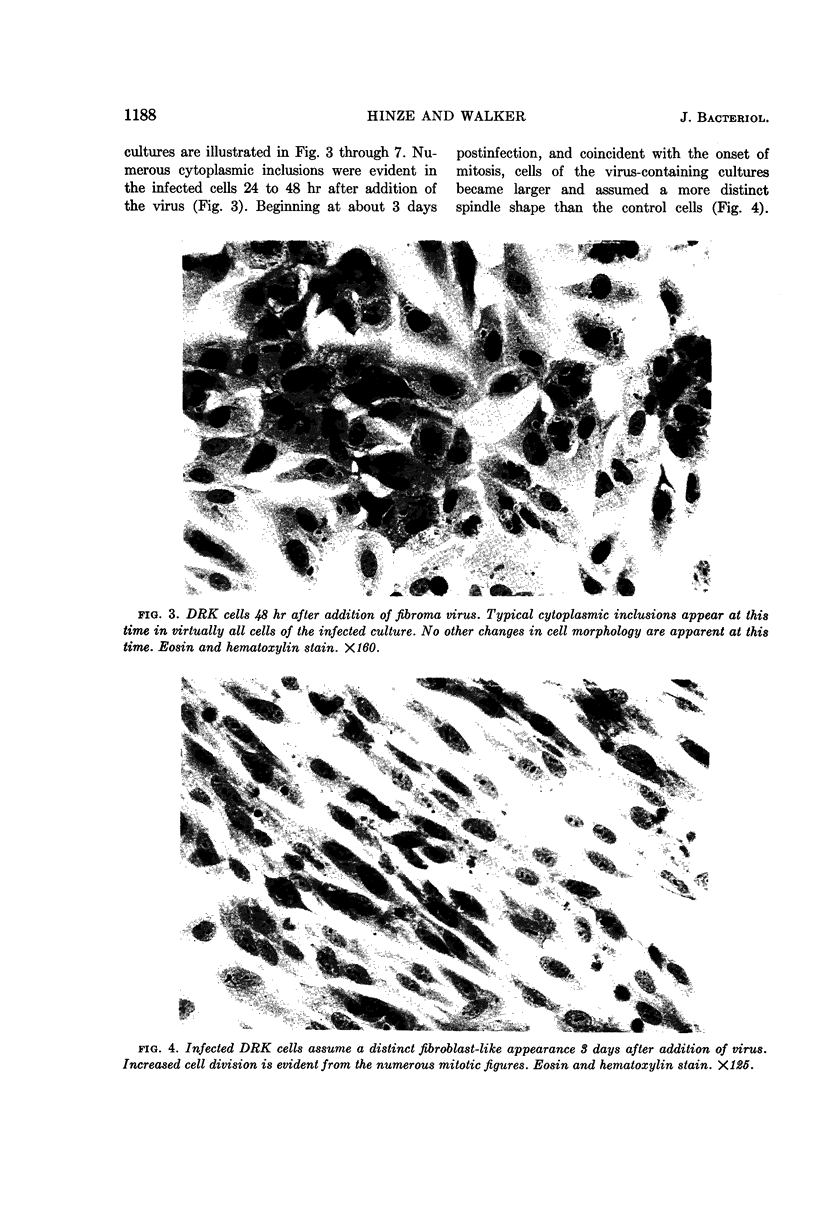
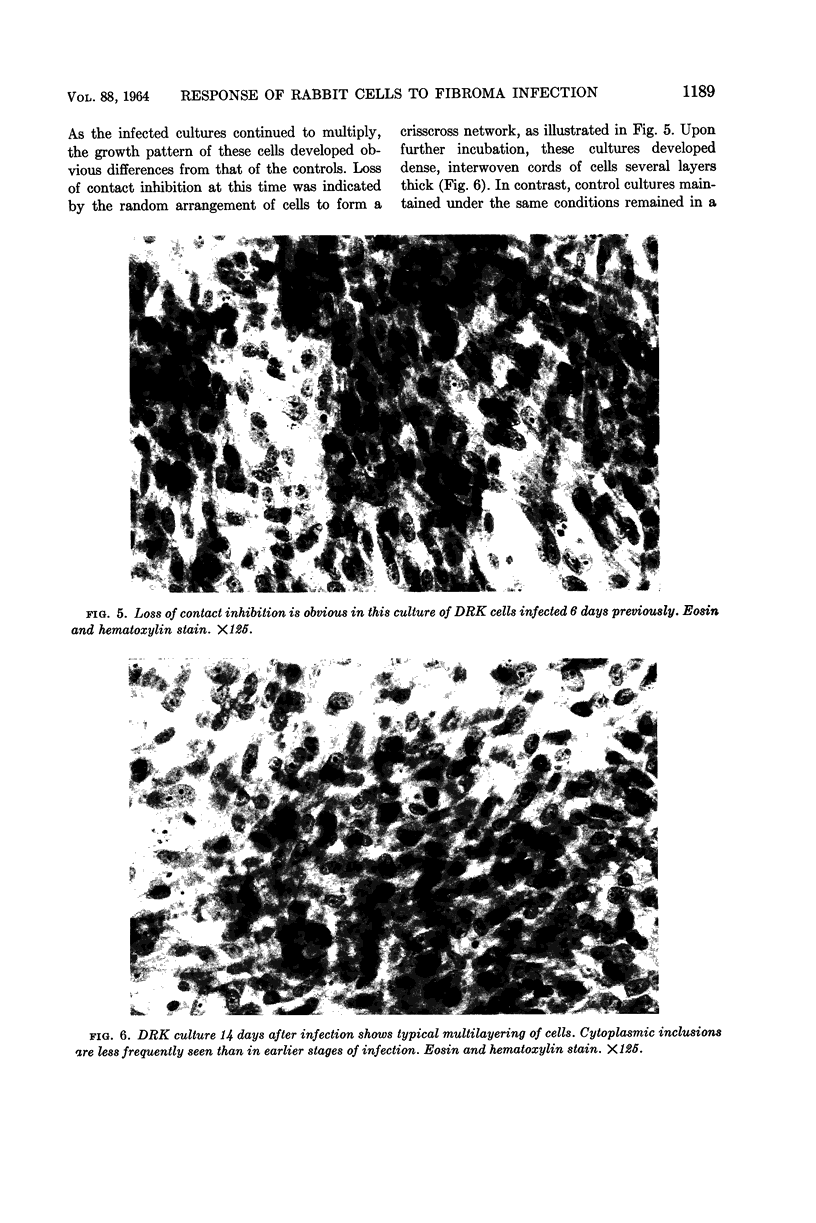
Abstract
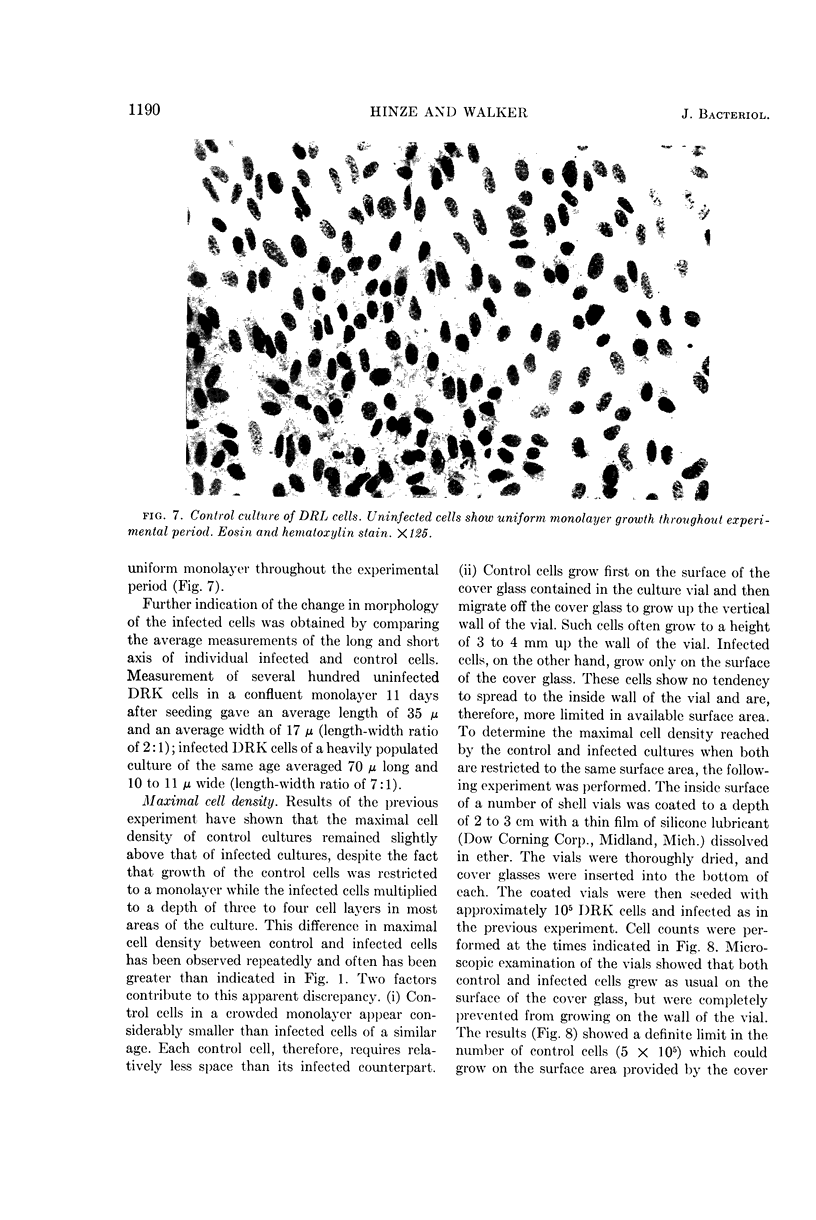
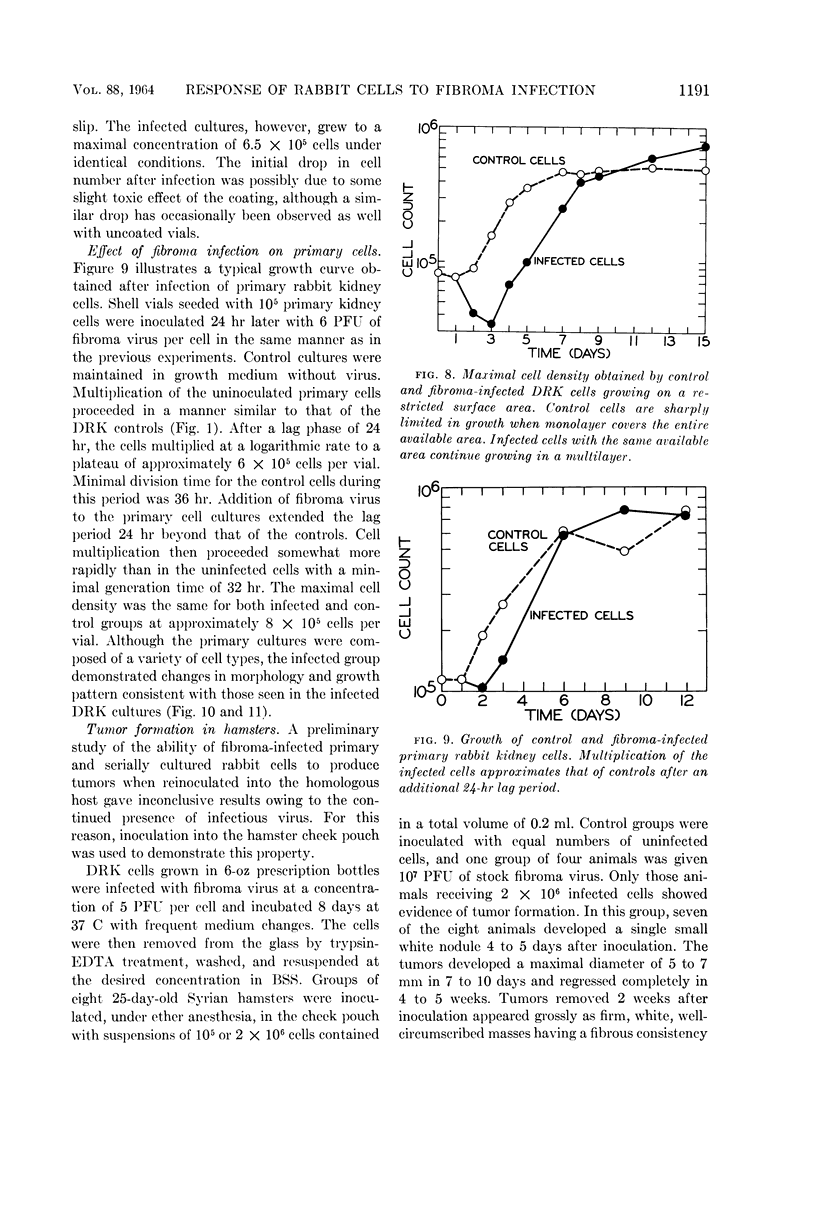
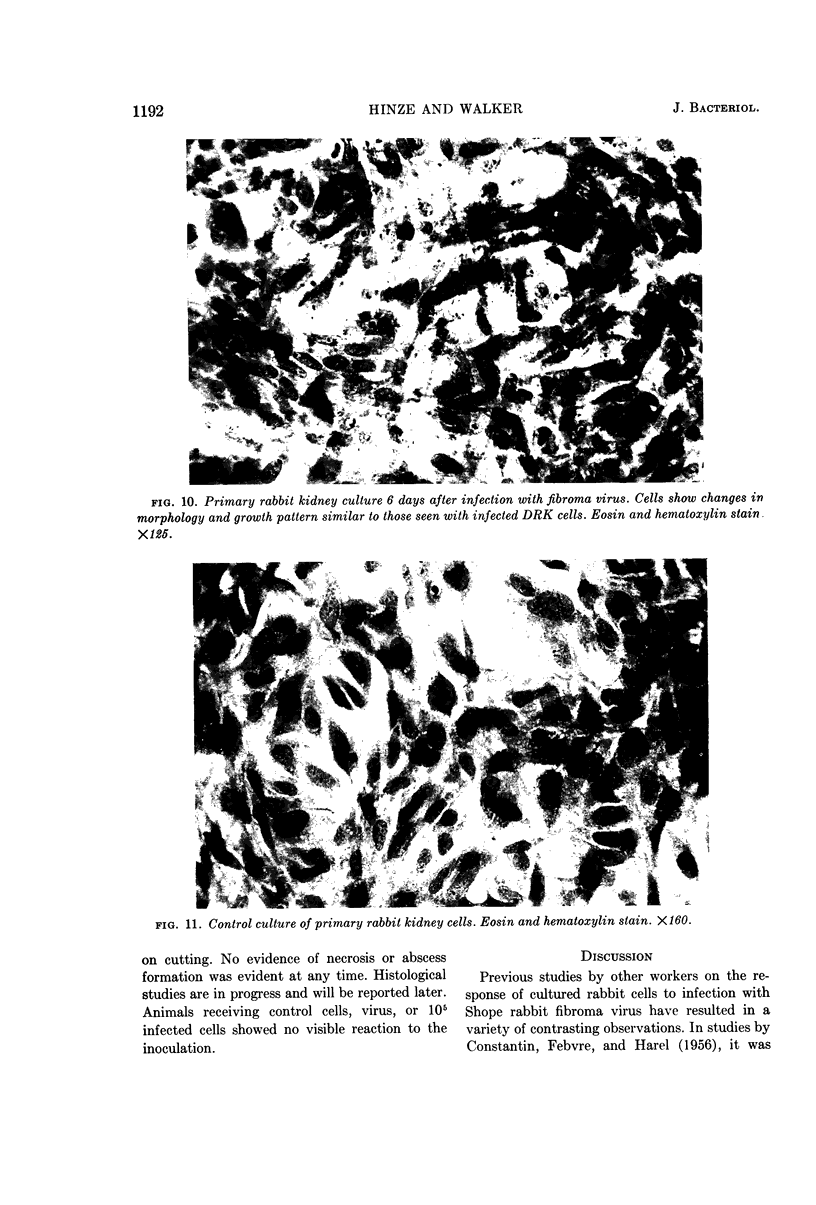
Hinze, Harry C. (University of Wisconsin, Madison), and Duard L. Walker. Response of cultured rabbit cells to infection with the Shope fibroma virus. I. Proliferation and morphological alteration of the infected cells. J. Bacteriol. 88:1185–1194. 1964.—Primary and serially cultured rabbit kidney cells were grown under conditions promoting rapid cell multiplication. When such cultures were infected with 5 to 10 plaque-forming units of Shope fibroma virus, cell multiplication was inhibited for a period of 2 to 3 days. After this stationary period, cell multiplication in the infected cultures was resumed at a rate approximating that of the uninfected controls. With the resumption of cell multiplication in the infected cultures, concurrent changes were observed in cell morphology and growth pattern. Cells showing such alterations also possessed the ability to form tumors when inoculated into the hamster cheek pouch.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., GOETZ I. E. Morphological conversion of cell cultures by avian myeloblastosis virus. Virology. 1961 Oct;15:185–199. doi: 10.1016/0042-6822(61)90234-3. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A. Properties of cells infected with avian myeloblastosis virus. Cold Spring Harb Symp Quant Biol. 1962;27:415–425. doi: 10.1101/sqb.1962.027.001.039. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., HARTLEY J. W., ROWE W. P., HUEBNER R. J. TRANSFORMATION OF BOVINE TISSUE CULTURE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1963 Sep 7;199:1016–1018. doi: 10.1038/1991016a0. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P. Transformation in hamster kidney monolayers by vacuolating virus, SV-40. Virology. 1963 Jan;19:107–109. doi: 10.1016/0042-6822(63)90031-x. [DOI] [PubMed] [Google Scholar]
- CHAPRONIERE D. M., ANDREWES C. H. Cultivation of rabbit myxoma and fibroma viruses in tissues of nonsusceptible hosts. Virology. 1957 Oct;4(2):351–365. doi: 10.1016/0042-6822(57)90069-7. [DOI] [PubMed] [Google Scholar]
- CIECIURA S. J., MARCUS P. I., PUCK T. T. Clonal growth in vitro of epithelial cells from normal human tissues. J Exp Med. 1956 Oct 1;104(4):615–628. doi: 10.1084/jem.104.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTIN T., FEBVRE H., HAREL J. Cycle de multiplication du virus du fibrome de Shope in vitro (souche OA). C R Seances Soc Biol Fil. 1956;150(2):347–348. [PubMed] [Google Scholar]
- GROUPE V., MANAKER R. A. Discrete foci of altered chicken embryo cells associated with Rous sarcoma virus in tissue culture. Virology. 1956 Dec;2(6):838–840. doi: 10.1016/0042-6822(56)90064-2. [DOI] [PubMed] [Google Scholar]
- KILHAM L. Propagation of fibroma virus in tissue cultures of cottontail testes. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):739–742. doi: 10.3181/00379727-92-22598. [DOI] [PubMed] [Google Scholar]
- KILHAM L. Relation of thermoresistance to virulence among fibroma and myxoma viruses. Virology. 1959 Nov;9:486–487. doi: 10.1016/0042-6822(59)90140-0. [DOI] [PubMed] [Google Scholar]
- PADGETT B. L., MOORE M. S., WALKER D. L. Plaque assays for myxoma and fibroma viruses and differentiation of the viruses by plaque form. Virology. 1962 Jul;17:462–469. doi: 10.1016/0042-6822(62)90141-1. [DOI] [PubMed] [Google Scholar]
- SACHS L., MEDINA D. In vitro transformation of normal cells by polyoma virus. Nature. 1961 Feb 11;189:457–458. doi: 10.1038/189457a0. [DOI] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Transformation induced by simian virus 40 in human renal cell cultures. I. Morphology and growth characteristics. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1164–1172. doi: 10.1073/pnas.48.7.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMIN H. M., RUBIN H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958 Dec;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:407–414. doi: 10.1101/sqb.1962.027.001.038. [DOI] [PubMed] [Google Scholar]
- VERNA J. E., EYLAR O. R. Rabbit fibroma virus plaque assay and in vitro studies. Virology. 1962 Oct;18:266–273. doi: 10.1016/0042-6822(62)90013-2. [DOI] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of cells transformed by polyma virus. Cold Spring Harb Symp Quant Biol. 1962;27:367–374. doi: 10.1101/sqb.1962.027.001.034. [DOI] [PubMed] [Google Scholar]
- VOGT P. K., RUBIN H. The cytology of Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:395–405. doi: 10.1101/sqb.1962.027.001.037. [DOI] [PubMed] [Google Scholar]